Abstract
The photosynthetic development of pedunculate oak (Quercus robur L.) sun leaves in a mature woodland canopy in Oxfordshire, southern England, was investigated in situ during 3 years with contrasting weather conditions. Development of full photosynthetic capacity (indicated by light-saturated net assimilation rates, A max, typical of the summer period) took between approximately 50 and 70 days after budbreak in different years. This slow development means that these leaves do not utilise a substantial fraction of the seasonal peak of solar irradiance. During the late autumn senscence period the photosynthetic capacity declined over a 2-week period, but as this is a time of low irradiance, the loss of potential photosynthesis was relatively small. The consequences of these developmental changes and differences in bud break dates for daily and seasonal leaf carbon balance were investigated through a simple light-response photosynthetic model. Seasonal changes in photosynthetic capacity would decrease annual carbon uptake per unit leaf area by about 23% compared to that potentially possible if leaves photosynthesised at peak rates throughout the growing season. This difference is likely to be up to 30% larger in years with late budburst and as low as 18% in years with early budburst.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There has been a resurgence of interest in phenology with the increasing concern about the potential impacts of global climate change. The onset of leafing and flowering is temperature-dependent in many species and there are well-documented examples of these phenological events occurring earlier in recent decades (e.g. Sparks and Carey 1995; Menzel 2000; Menzel et al. 2001). In some of these cases there has also been an extension of the potential growing season in the autumn with leaf senescence and abscission delayed.
Satellite remote sensing provides supporting evidence for an extension of the growing season, particularly in the spring (e.g. Myneni et al. 1997; Tucker et al. 2001; Shabanov et al. 2002). What, however, is the significance of these findings for growth and productivity? Can one assume that the emergence of a leaf is closely followed by the development of photosynthetic capacity? Earlier work by Morecroft and Roberts (1999) on the deciduous oak Quercus robur has shown that, in one particular year (1996), trees took approximately 50 days from budburst (which was at the end of April) to reach the high light-saturated assimilation rates (A max) typical of the rest of the summer. This is particularly curious as Q. robur is a late-leafing species (Sparks and Carey 1995) and very little carbon would be assimilated during May and early June when day length is long and solar radiation intensity is high (average daily total radiation in southern England is approximately 16 MJ m–2 day–1). In the autumn some photosynthetic activity persisted in Q. robur right up to abscission in November, when day length is short and total daily solar radiation is low (average daily totals for this time of year are approximately 5 MJ m–2 day–1). However, a fall in A max was measured from 16 days prior to abscission. In this paper we examine whether this pattern of slow development in the spring and late senescence in the autumn was also seen in subsequent years in Q. robur and whether year-to-year differences in climate were likely to have affected them. We examined the early stages of leaf photosynthetic development using chlorophyll fluorescence techniques when the leaves were too small and delicate for use with a leaf gas-exchange chamber. Finally we have attempted to quantify the effect of low photosynthetic rates in spring and autumn on the carbon balance of oak sun leaves over the whole growing season.
Materials and methods
Site description and phenological observations
The study was carried out at Wytham Woods, Oxfordshire, UK (51°46′N 1°20′W; UK National Grid: SP 46 08), where a scaffolding walkway, approximately 12 m above the ground, gives access to the canopies of oak trees in ancient woodland. The oak trees are approximately 150–200 years old. The site is described in more detail by Morecroft and Roberts (1999) and Roberts et al. (1999). Standard climate data were collected by an automatic weather station 500 m away at an open site; long-term temperature measurements were also made at the walkway itself (Morecroft et al. 1998).
Bud break was recorded in three different ways:
-
1.
The first sign of green leaves seen from general observations of trees around Wytham Woods at ground level (analogous to most phenology records)
-
2.
Date of first budbreak in oak trees observed from the walkway (L. Cole, personal communication 2001) (mean of six trees).
-
3.
Date on which budbreak was estimated to have occurred in 50% of leaves on trees observed from the walkway (mean of six trees).
Photosynthesis measurements
In this paper we present only data from sun leaves (i.e. those that developed in mainly unshaded positions in the top canopy). Other measurements have suggested that shade leaves contribute little or nothing to the carbon balance of the canopy in these trees (Morecroft and Roberts 1999; Stokes 2002). In 1996, gas-exchange measurements were made in situ on leaves of five Q. robur trees approximately once a week during the period when trees were in leaf, so long as the leaves were dry, with five leaves sampled from each tree on each occasion. An LCA 2 infrared gas analyser with PLC(B) leaf chamber (ADC Ltd., Hoddesdon, Herts, UK) was used; full details are given by Morecroft and Roberts (1999). Measurements were also collected throughout the growing seasons of 1999 and 2000 on updated equipment [CIRAS 1, with PLC(B) leaf chamber, PP Systems, Hitchin, Herts, UK]. Measurements were made on seven trees in 1999 and five in 2000, and in each case measurements were made on five leaves per tree. Measurements were made between 0900 hours and 1400 hours GMT (c.f. 1000–1400 hours in 1996: other data show that maximum rates were attained by 0900 hours) and the mean of five measurements taken in 1 min was used (rather than a single spot measurement as in 1996). In order to estimate maximum net photosynthetic rates (A max), on each occasion, data were used only when the photosynthetic photon flux density (PPFD) received by the leaves exceeded 1,000 μmol m–2 s–1, as measured by the leaf chamber.
Chlorophyll fluorescence measurements were taken between 0900 hours and 1400 hours GMT, using a Plant Efficiency Analyser (Hansatech, Herts, UK) with leaf clips. After 30 min dark adaptation, F v/F m was measured, using a 5-s flash of saturating light (>3,000 μmol m–2 s–1). Dark-adapted F v/F m represents the intrinsic maximum efficiency of photosystem II (the quantum efficiency if all reaction centres were open) and is a sensitive indicator of plant photosynthetic performance (Bolhàr-Nordenkampf and Öquist 1993).
Carbon balance calculations
In order to test the impact of the slow development of photosynthetic capacity on annual carbon balance, we first estimated the potential photosynthetic carbon gain per unit leaf area for each day from the long-term mean irradiance on that day. Daily average solar radiation totals were estimated for the 24 years from 1968 to 1991 (inclusive) by using sunshine hour data from the nearby climatological station at Whiteknights, Reading, (approx. 40 km south east of Wytham) and a modified Angstrom relationship (Cowley 1978). Long-term mean irradiance data, rather than actual values for particular years were used to standardise comparisons between years with different dates of budbreak.
The response of photosynthesis to irradiance was derived from measurements of photosynthetic rate at experimentally manipulated irradiances using standard equipment for the infrared gas analyser (PP Systems, Hitchin, Herts, UK) on several different dates in mid to late summer of 1999 and 2000. The relationship between photosynthesis and solar irradiance for sun leaves was described by a non-rectangular hyperbola (see, e.g. Marshall and Biscoe 1980):
Where A is the net CO2 assimilation rate, I is the mean irradiance (PPFD), α is the quantum yield (0.045), P max is the maximum gross photosynthetic rate (15.0 μmol m–2 s–1) (c.f. assimilation rate), θ is a curvature factor (0.70) and R d is the dark respiration rate (1.6 μmol m–2 s–1) Although this empirical model does not explicitly account for seasonal variations in the dark respiration rate, effects of variations in vapour pressure deficit or temperature (for example), we assume these factors are averaged-out across all the different occasions used to derive the parameters for Eq. (1). Many previous studies have shown that photosynthesis has a rather small response to temperature within the normal range encountered, (e.g. Hollinger 1992, for Q. lobata) and, although a simplification, ignoring temperature effects is probably even less of a problem if account is taken of slow seasonal acclimation as well.
Potential daily CO2 assimilation rates were calculated from mean irradiances, using Eq. 1 with a three-point Gaussian integration of an assumed half-sine wave diurnal time course of incident radiation (e.g. Goudriaan 1986). Potential carbon uptake for sun leaves was compared with that calculated using a correction for the observed reduced photosynthetic capacity in the spring and autumn, based on the assumption of a linear daily rate of increase in P max between bud break and an estimated date of full photosynthetic capacity 63 days later (see Results) and a linear daily decline in P max in the 16 days prior to the date of abscission of most leaves (about the time of the last measurements taken in the 3 years).
Results
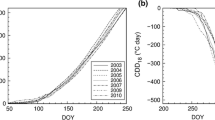
Summer and autumn temperatures were broadly similar in each of the 3 years (Fig. 1), but differences were seen in spring and late winter. The mean temperature for the period January to May was over 2 °C colder in 1996 than in both other years, and the spring (March–May) of 1999 was slightly warmer (0.5 °C) than that of 2000. Temperatures declined more rapidly from October in 1996. The spring patterns in temperature were reflected in the data for budbreak (Table 1): the latest dates for bud break were recorded in 1996 and the earliest in 1999. Consistent trends between years were found with the different methods of recording. Observation of buds from close proximity on the walkway detected leafing at an earlier stage than traditional ground level observations in 2 of the 3 years, though the differences were small and both preceded 50% bud break, as would be expected.
In each of the 3 years, A max rose from initially low levels to approximately constant values over the summer period before declining in the autumn (Fig. 2). The autumn decline started in early October, and was a few days later in 1996 than in 1999. By early November, A max rates were 30% of the summer values. There was some variation in A max over the summer, which may be attributed to differences in factors such as light climate immediately prior to measurement, temperature and leaf-air vapour pressure difference. In 1996 the mid-growing season values of A max were lower than in the 2 later years. This is best explained by differences in soil water content, which was unusually low in 1996, following drought in the summer of 1995 (Morecroft et al. 2000). It is also possible that there was an effect of differences in sampling methodology or equipment between years (this would not prevent comparisons of seasonal trends between years).
In the early part of the growing season, late May and early June, A max values were significantly higher in 1999 than 1996 (there are insufficient data to examine 2000 in this period). This is consistent with the earlier leafing which was observed in that, warmer, year; in particular May was 4 °C warmer. However, the date on which mean A max first exceeded 10 μmol m–2 s–1 was remarkably similar between years (Table 1). This threshold was chosen, after inspection of Fig. 2, to provide an approximate, but objective, measure of when full photosynthetic capacity had been reached. There was therefore a substantial difference (up to 20 days) in the time required to develop full photosynthetic capacity between years. The mean time from bud burst to full photosynthetic capacity was, however, 63 days and this was used in modelling studies.
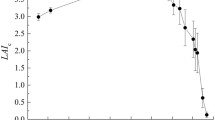
Measurements of F v/F m by chlorophyll fluorescence (Fig. 3) also showed that maximum photosynthetic capacity developed slowly. The rate of increase in F v/F m was approximately linear, including the period before day of year (DOY) 150, when it was not possible to use an infrared gas analyser. An autumn decline in F v/F m was also found.
Estimates of daily potential photosynthetic carbon uptake per unit leaf area for Q. robur sun leaves in average cloud conditions in a typical year (Fig. 4) show a broad peak centred on the summer solstice in late June, when irradiance is high and the photoperiod is long. However, calculations using the average date of budbreak (DOY 109) show that a large part of this potential is not attained (Fig. 4). Firstly, it takes some 11 days for the leaves to have a positive carbon balance. Secondly the average daily difference in carbon assimilation between a hypothetical leaf at maximum capacity and one developing over 63 days from bud break to the attainment of full capacity is 3.2 gC m–2 day–1. The estimated total carbon assimilation for Q. robur at Wytham was only 46% of the potential in this period (Table 2). In the autumn the decline in photosynthetic capacity also has an effect on net daily carbon uptake. The average difference between the potential carbon uptake and that attained is 1.5 gC m–2 day–1, or 77% for this period; however, the effect on annual carbon balance is small in comparison to the effect of the slow development in the spring. By the time the leaves start to senesce and fall, solar radiation levels are so low that the potential carbon gain is very small (Fig. 4). Over the growing season as a whole, developmental changes in photosynthetic capacity reduce the carbon uptake per unit leaf area by approximately 23% (Table 2) compared to the potential uptake.
Seasonal time course of estimated daily total carbon gain per unit leaf area for oak sun leaves, using average solar radiation data for Reading. Thin line The potential carbon assimilation, if leaves were at full photosynthetic capacity. Thick line The attained carbon assimilation of leaves, taking into account development of photosynthetic capacity in the spring and decline in the autumn. Vertical lines Assumed dates of bud burst (average data from Table 1) and abscission. See Materials and methods for calculation details. Potential carbon assimilation rates smoothed for clarity using a 7-day running mean
Using these comparisons of potential and attained photosynthesis we can also make a preliminary estimate of the effect on carbon balance of the wide interannual range of dates of budbreak that have been reported for Q. robur. In the Marsham (Norfolk, eastern England) phenological record, which extends from 1736 to 1947 (Sparks and Carey 1995) the mean oak budbreak date was DOY 114, only 5 days later than the average date observed here. However, the earliest and latest dates in the Marsham record are DOY 84 and 140. The effect of these dates on carbon balance were investigated using the modelled assimilation data for Wytham, assuming 63 days between budbreak and full photosynthetic capacity and 16 days for the sensescence period. Table 2 shows that the calculated difference between potential and attained daily carbon balance during the period of development of photosynthetic capacity varies from 2.8 to 3.5 gC m–2 day–1. The ratio of attained to potential leaf carbon assimilation during this development period is nearly constant across these different bud break dates at around 45% (Table 2). However, the attained seasonal total varies from 82% to 70% of the potential (1.11 – 0.83 kgC m–2 day–1, Table 2). This is because the summer period makes a proportionally larger contribution to the annual carbon balance in years with early leafing. (This would not necessarily hold true if the development time had not been held constant.)
Discussion
The measurements in 1999 and 2000 indicate that the delay of 50 or more days between leaf bud break and the development of full photosynthetic capacity, found in 1996 (Morecroft and Roberts 1999) is typical of Q. robur. Full photosynthetic capacity is attained around the time when rapid decreases in specific leaf area cease, and specific leaf area settles to a minimum summer value (Stokes 2002). Fluorescence measurements in the early growing season suggest an approximately linear rate of development of photosynthetic capacity. This is, however, almost certainly modified by variations in meteorological conditions as development times differed between years. It is curious that the attainment of the highest A max values occurred around the same time in all 3 years (Table 1); this may well be coincidental, but it warrants further research.
The long development period prevents the leaves from fully exploiting the period of long day length and high irradiance in May and early June. This clearly has a major effect on the carbon balance of leaves over the whole growing season. In contrast, the autumn decline in photosynthetic capacity has very little effect on the annual carbon balance because the short days and low irradiance make the potential carbon gain very small. The modelling exercise has allowed us to quantify the impact of slow development of photosynthetic capacity on the annual carbon balance and also serves as a warning that this factor should not be ignored in modelling studies of tree growth and carbon sequestration. Assuming full photosynthetic capacity from budbreak would lead to an over-estimation of annual carbon gain by approximately 28% in an average year. In addition to the change in photosynthetic capacity per unit leaf area, estimating the whole-canopy carbon balance would require information on the development of leaf area, as well as any seasonal changes in respiration of non-photosynthetic tissues. Two different estimates for temperate oak forests indicate that it can take approximately 60–80 days to reach the peak leaf area index after bud burst (Breda et al. 1993; Ogink-Hendriks 1995). This will compound the effects of low productivity on a leaf area basis and further reduce annual carbon uptake per unit ground area. It will, however, mean that the net carbon losses in the first few days after budburst have a relatively small impact on the annual carbon balance at the canopy scale.
Slow photosynthetic development has also been found for other canopy trees, particularly Quercus species. Reich et al. (1991) found that light saturated photosynthetic rates took some 40 days to reach maximum values in Quercus ellipsoidalis in Wisconsin, coinciding with the attainment of minimum specific leaf area. Similar patterns were seen in Acer rubrum and Acer saccharum. Wilson et al. (2000) report that Q. prinus and Q. alba leaves in a mixed deciduous forest in Tennessee took approximately 40–50 days to reach the minimum specific leaf area and maximum rate of carboxylation, another measure of photosynthetic capacity; this was slightly slower than for A. rubrum and Nyssa sylvatica. Bassow and Bazzaz (1998) found that Q. rubra at Harvard Forest, Massachusetts, USA, only reached maximal photosynthetic rates in August. In contrast the birch species, Betula alleghaniensis and Betula papyrifera reached near-maximal photosynthetic rates in early June in the same study; red maple, A. rubrum, was intermediate. Other work at our study site has shown that the sycamore, Acer pseudoplatanus, develops photosynthetic capacity more quickly than Q. robur in some (but not all) years (Morecroft and Roberts 1999, Stokes 2002). There is, however, reason to suppose that there are differences between sites as well as between species, in contrast to Bassow and Bazzaz, Jurik (1986) found that it took only approximately 1 month for Q. rubra to develop full photosynthetic capacity at field sites in Michigan, USA. It should be borne in mind that access to the canopy of mature trees is available in very few places and that it is generally limited to just a few trees. Much further work is needed to quantify both inter- and intraspecific differences.
The reason why it can take a substantial part of the growing season for the leaves of some trees to develop photosynthetically is not known, but it is interesting that trees in the genus Quercus are ring-porous: a ring of large pores develops in the early growth of the annual ring, followed by smaller ones in the late wood. Rapid new wood formation is started 2–3 weeks before budbreak, as the previous year's xylem is not fully functional, unlike the situation in diffuse porous trees (Thomas 2000). This may reduce the availability of resources for leaf development in the spring or cause leaf development to be restricted by the developing capacity of the vascular tissue. Oak leaves also have a high content of phenolic compounds; the synthesis of these may divert some resources away from photosynthetic development. Late budbreak and slow development could also be postulated to be an adaptive response to the occurrence of late frosts. Between 1994 and 2001, the latest sub-zero air temperature recorded within the canopy itself at Wytham was DOY 127 (7 May) and the mean date was DOY 98 (8 April), which is prior to bud break. This suggests that, whilst late budbreak may have adaptive significance in frost avoidance, full photosynthetic capacity is reached long after the frost danger has gone. However, later frosts may well have been experienced over the course of the evolutionary history of Quercus and slow development may have conferred some selective advantage.
Phenology has already been identified as an important factor to be included in the modelling of climate change impacts on the carbon balances of trees and forests (Kramer et al. 2000). It is clear that it is not sufficient to base this simply on dates of budburst and development of leaf area index, particularly as there is evidence of substantial interspecific variation in photosynthetic development. Wilson et al. (2001) demonstrated that incorporating realistic patterns of seasonal development of photosynthetic capacity into stand-scale models substantially improved the agreement of a modelled net ecosystem exchange of carbon with results from eddy correlation measurements for a mixed deciduous forest at Oak Ridge, Tennessee (USA). Unlike our study, they also demonstrated decreases in the photosynthetic rate of several species, including Quercus species over the course of the summer, before a final steep decline during sensescence. Further studies are clearly required to investigate the development of photosynthetic capacity and its impact on carbon balance across a range of species and sites.
References
Bassow SL, Bazzaz FA (1998) How environmental conditions affect canopy leaf-level photosynthesis in four deciduous tree species. Ecology 79:2660–2675
Bolhàr-Nordenkampf GO, Öquist HR (1993) Chlorophyll fluorescence as a tool in photosynthesis research In: Hall DO, Scurlock JMO, Bolhàr-Nordenkampf GO, Leegood RC, Long SP (eds) Photosynthesis and production in a changing environment: a field and laboratory manual. Chapman & Hall, London, pp 193–206
Breda N, Cochard H, Dreyer E, Granier A (1993) Water transfer in a mature oak stand (Quercus petrea): seasonal evolution and effects of a severe drought. Can J For Res 23:1136–1143
Cowley JP (1978) The distribution over Great Britain of global solar irradiation on a horizontal surface. Meteorol Mag 107:357–373
Goudriaan J (1986) A simple and fast numerical method for the computation of daily totals of crop photosynthesis. Agric For Meteorol 38:249–254
Hollinger DY (1992) Leaf and simulated whole-canopy photosynthesis in 2 co-occurring tree species. Ecology 73:1–14
Jurik TW (1986) Seasonal patterns of leaf photosynthetic capacity in successional northern hardwood tree species. Am J Bot 73:131–138
Kramer K, Leinonen I, Loustau D (2000) The importance of phenology for the evaluation of impact of climate change on growth of boreal, temperate and Mediterranean forests ecosystems: an overview. Int J Biometeorol 44:67–75
Marshall B, Biscoe PV (1980) A model for C3 leaves describing the dependence of net photosynthesis on irradiance. I. Derivation. J Exp Bot 31:29–39
Menzel A (2000) Trends in phenological phases in Europe between 1951 and 1996. Int J Biometeorol 44:76–81
Menzel A, Estrella N, Fabian P (2001) Spatial and temporal variability of the phenological seasons in Germany from 1951 to 1996. Global Change Biol 7:657–666
Morecroft MD, Roberts JM (1999) Photosynthesis and stomatal conductance of mature canopy oak (Quercus robur) and sycamore (Acer pseudoplatanus) trees throughout the growing season. Funct Ecol 13:332–342
Morecroft MD, Taylor ME, Oliver HR (1998) Air and soil microclimates of deciduous woodland compared to an open site. Agric For Meteorol 90:141–156
Morecroft MD, Burt TP, Taylor ME, Rowland AP (2000) Effects of the 1995–1997 drought on nitrate leaching in lowland England. Soil Use Manage 16:117–123
Myneni RB, Keeling CD, Tucker CJ, Asrar G, Nemani RR (1997) Increased plant growth in the northern high latitudes from 1981 to 1991. Nature 386:698–702
Ogink-Hendriks MJ (1995) Modelling surface conductance and transpiration of an oak forest in The Netherlands. Agric For Meteorol 74:99–118
Reich PB, Walters MB, Ellsworth DS (1991) Leaf age and season influence the relationship between leaf nitrogen, leaf mass per area and photosynthesis in maple and oak trees. Plant Cell Environ 14:251–259
Roberts J, Hopkins R, Morecroft M (1999) Towards a predictive description of forest canopies from litter properties. Funct Ecol 13:265–272
Shabanov NV, Zhou LM, Knyazikhin Y, Myneni RB, Tucker CJ (2002) Analysis of interannual changes in northern vegetation activity observed in AVHRR data from 1981 to 1994. IEEE Geosci Remote 40:115–130
Sparks TH, Carey PD (1995) The responses of species to climate over two centuries: an analysis of the Marsham phenological record, 1736–1947. J Ecol 83:321–329
Stokes VJ (2002) The impact of microenvironment, leaf development and phenology on annual carbon gain and water loss of two deciduous tree species. PhD Thesis, University of Essex, Colchester, UK
Thomas PT (2000) Trees: their natural history. Cambridge University Press, Cambridge
Tucker CJ, Slayback DA, Pinzon JE, Los SO, Myneni RB, Taylor MG (2001) Higher northern latitude normalized difference vegetation index and growing season trends from 1982 to 1999. Int J Biometeorol 45:184–190
Wilson KB, Baldocchi DD, Hanson PJ (2000) Spatial and seasonal variability of photosynthetic parameters and their relationship to leaf nitrogen in a deciduous forest. Tree Physiol 20:565–578
Wilson KB, Baldocchi DD, Hanson PJ (2001) Leaf age affects the seasonal pattern of photosynthetic capacity and net ecosystem exchange of carbon in a deciduous forest. Plant Cell Environ 24:571–583
Acknowledgements
We thank Dr. Lionel Cole for providing some of the phenological data. We are grateful to the Natural Environment Research Council for a studentship to V.J.S. and for funding monitoring and research at Wytham under the UK Environmental Change Network.
Author information
Authors and Affiliations
Corresponding author
Additional information
Prepared in conjunction with the International Conference "The times they are a-changin'". Climate change, phenological responses and their consequences for biodiversity, agriculture, forestry and human health, held in Wageningen, The Netherlands, 5–7 Dec 2001
Rights and permissions
About this article
Cite this article
Morecroft, M.D., Stokes, V.J. & Morison, J.I.L. Seasonal changes in the photosynthetic capacity of canopy oak (Quercus robur) leaves: the impact of slow development on annual carbon uptake. Int J Biometeorol 47, 221–226 (2003). https://doi.org/10.1007/s00484-003-0173-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-003-0173-3