Abstract
Key message
Fine roots of orange trees reached a depth of approx. 6 m in a sandy tropical soil. Root area index was more responsive to irrigation than leaf area index.
Abstract
Although the crucial role of deep rooting on the hydraulic functioning of tropical trees has been pointed out, studies dealing with root development below 2 m are still scarce. Our study aimed to gain insight into the fine root traits of rainfed and irrigated orange trees down to the root front in deep tropical soils. Irrigation was applied during dry periods, only 3–15% more than the annual amounts of water supplied by rain. Fine roots were sampled down to a depth of 8 m on four dates in a randomized block design. The effects of soil depth and irrigation on major fine root traits, total fine root length and the relationship between leaf area index (LAI) and root area index (RAI) were studied. The total fine root mass was 728 g m−2 in rainfed plots and 536 g m−2 in irrigated plots during the driest period (Sept/Oct 2012). Across the four sampling dates, the mean depth of the root front was 6.1 m in rainfed plots and 5.5 m in irrigated plots close to the trees, and approximately 4.5 m in the inter-row covered by Brachiaria decumbens plants. LAI was little influenced by irrigation (about 4.5 m2 m−2), but mean RAI was 19.1 m2 m−2 in rainfed plots and 13.7 m2 m−2 in irrigated plots. Small irrigation rates reduced root development in very deep soil layers, and increased fruit production by 9% during the dry year. Deep rooting provides access to water stored in deep soil layers during the rainy season, and thus might have an important functional role during dry periods in tropical orange orchards.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Meta-analyses show that trees tend to have deeper roots than shrub and herbs and those roots reach on average a higher depth in tropical regions than in temperate and boreal regions (Jackson et al. 1997; Schenk and Jackson 2002). Although deep roots are commonly defined as roots growing below a depth of 1 m (Maeght et al. 2013), fine roots have been found very deeply in some forest ecosystems, down to depths of 50–60 m (Stone and Kalisz 1991). Roots at depths >5 m can play an important role to supply water to trees during dry periods (Harper et al. 2009; Bleby et al. 2010; Battie-Laclau et al. 2014; Christina et al. 2016). However, studies measuring fine root distributions down to the root front are scarce in tropical ecosystems due to sampling difficulties (Maeght et al. 2013). Recent studies in sugarcane and eucalypt plantations growing on very deep Ferralsol soils in Brazil showed that the fine roots at depths >1 m represented about 50% of the total fine root biomass down to the root front (Battie-Laclau and Laclau 2009; Pinheiro et al. 2016). Maximum rooting depths have probably been under estimated in many tropical terrestrial ecosystems and the role of very deep roots in taking up water and nutrients remains poorly understood (Böhm 1979; Jackson et al. 1997; Freycon et al. 2015). Tree vertical extension was roughly symmetric above- and below-ground for seedlings and clones of various species in Brazilian eucalypt plantations (Christina et al. 2011; Pinheiro et al. 2016). Small fine root densities can be sufficient to withdraw substantial amounts of water and the root front depth can explain the survival of trees during drought periods (Oliveira et al. 2005; Harper et al. 2009; Ma et al. 2013). Trees adjust their area of water uptake to their area of water loss which constrains leaf and fine root areas (Magnani et al. 2002). The root area to leaf area ratio and the root length to leaf area ratio depend on genotype (Costa e Silva et al. 2004; Pinheiro et al. 2016) and tree age (O’Grady et al. 2006). An increase in soil water availability led to a sharp decrease in the root area index (RAI) to leaf area index (LAI) ratio both for a drought-tolerant and a drought-sensitive Eucalyptus globulus clone (Costa e Silva et al. 2004).
Although a plant’s capacity to take up water and nutrients is highly dependent on fine root architecture (Guo et al. 2008; Pagès 2011), changes in fine root traits with soil depth are poorly documented for very deep soil profiles (Prieto et al. 2015). Roots of different sizes and orders have different functions (absorption of nutrients, transport, support) and therefore different metabolic activities (Pregitzer et al. 1998; Anderson et al. 2007). Common fine root traits (mean diameter, specific root length and specific root area) provide indirect information on fine root architecture, and therefore on plant plasticity that may permit to explore resource-rich soil patches (Roumet et al. 2006; Freschet et al. 2015). Changes in fine root traits did not exhibit a clear pattern between the topsoil and the root front at a depth of approx. 10 m for four genotypes of eucalypt trees in Brazil (Pinheiro et al. 2016). Some root traits differed between shallow and deep fine roots across 20 plant communities studied in tropical, Mediterranean and montane regions, but absolute differences were small for most of the traits measured (Prieto et al. 2015).
Studies dealing with fine root distributions are highly relevant in intensively managed tree crops to supply water and nutrients in soil patches where the fine root densities are the highest and therefore to minimize the risk of resource losses by deep drainage. The area of orange orchards harvested in 2012 was 782,041 ha in Brazil, of which 525,514 ha were located in the São Paulo State. Brazil is the first exporter of orange juice with about 60% of the global production (Agrianual 2013). Citrus plants transpire all along the year, and water requirements range from 900 to 1300 mm per year, depending on weather conditions, stocking density, and leaf area index (Parsons et al. 2000). The area of irrigated Citrus orchards in the São Paulo State is approximately 20% of the total cultivated area and drip irrigation is widely used (Coelho 2010; Salomão et al. 2012). Irrigation is likely to improve the profitability of orange orchards as a result of better plant growth and fruit quality (Pires et al. 2005; Bremer Neto et al. 2013). Even in regions where the annual rainfall is relatively high, uneven distributions along the year and/or inter-annual variability can justify irrigation (Pires et al. 2005). Climate changes should increase the length of dry periods in many Brazilian regions (IPCC 2013), which could make irrigation compulsory for orange production. Irrigation and fertilization are the most important management practices influencing fruit quality in Citrus orchards and efficient use of water is a priority in regions where water is scarce (Martínez-Alcântara et al. 2012). Although detailed information on plant hydraulic traits is needed to accurately manage irrigation, very deep roots have only been studied by Ford (1954) in orange orchards (Whitney et al. 1991; Souza et al. 2008). Ford (1954) showed fine roots down to a depth of 5 m.
Our study aimed to gain insight into the exploration of very deep soil layers by fine roots of Citrus trees in tropical regions. We hypothesized that: (1) approximately 50% of the fine roots explore the soil at depths >1 m, (2) the order of magnitude of morphological root traits (mean diameter, specific root length and specific root area) is similar in superficial and deep soil layers, and (3) irrigation decreases the RAI/LAI ratio.
Materials and methods
Study site
The study site was located in South-Eastern Brazil (state of São Paulo, 23°17′S; 48°40′W) at 630 m above sea level. Over the last 15 years, the mean annual rainfall was 1360 mm and the mean monthly temperature ranged from 15 °C to 25 °C. The climate is characterized by two distinct seasons: the summer, from October to May, with high rainfall, temperature and global radiation, and the winter with low rainfall and temperature. The distribution of rainfall throughout the study period, from January 2012 to May 2014, followed the usual pattern in region with rainfall events concentrated from November to June (Fig. 1). In comparison with historical rainfall patterns, our study period was characterized by high rainfall amounts in winter 2013 and low rainfall amounts in summer 2014 (without any rainfall event >60 mm likely to replenish deep soil layers). The soil was a very deep Ferralic Arenosol (FAO classification) with a high textual uniformity (Table 1). The pH was acidic (about 4.5) and mean concentrations of exchangeable K+, Ca2+ and Mg2+ in the 0–25 cm soil layer were 0.5, 14.3 and 5.8 mmolc kg−1, respectively, and decreased down to less than 0.2, 3.6 and 1.2 mmolc kg−1 below a depth of 100 cm.
Experimental design
Sweet orange [Citrus sinensis (L.) Osbeck] plants of the Valencia variety grafted on Rangpur lime (Citrus limonia Osbeck) were planted in January 2006 at a spacing of 6.5 m between rows and 2.0 m between plants in the planting rows (769 plants per ha). A complete randomized block design was set-up in July 2012 on 1.4 ha in a flat area with two treatments (rainfed vs irrigated) replicated in four blocks. Each experimental plot contained 132 trees distributed in six rows. All the measurements were performed in the inner plots, excluding two buffer rows. Drip irrigation applied in the planting rows of the irrigated plots (four emitters per tree) over the dry periods amounted to a total of 146 mm over the study period, from July 2012 to May 2014 (Fig. 1).
Repeated applications of dolomitic lime were carried out to maintain the base saturation close to 70% in the 0–40 cm soil layer, 180 kg N ha−1, 90 kg P2O5 ha−1 and 170 kg K2O ha−1 were applied annually after planting. Orange productions in the studied orchard amounted to 15.7 t ha−1 in 2008 (first harvest after planting), then ranged from 34 to 68 t ha−1 between 2009 and 2013 depending on climatic conditions and management practices.
Aboveground biomass and leaf area
The trunk circumference at a height of 25 cm (just above the grafting) was measured for 12 trees in each plot (48 trees per treatment). A total of 12 trees distributed over the range of circumferences in each treatment were sampled in May 2013 to estimate the aboveground biomass. The crown of the trees was divided into three parts (upper, medium and lower). The trees were separated into four components: leaves, twigs (diameter <2 cm), branches and trunk. Subsamples of each component were dried at 65 °C to constant weight. Thirty leaves randomly selected were scanned immediately after collection in each crown third and dried at 65 °C to estimate the leaf water content and the specific leaf area (SLA, estimated as the ratio between the area and the dry mass of the sampled leaves, cm2 g−1). Leaf dry matter in each third of the crown of the sampled trees was estimated from the fresh leaf weight measured in the field and the water content measured for 30 leaves. Leaf area was estimated multiplying leaf dry matter by the SLA in the same third of the crown. Aboveground biomass and leaf area index (LAI) were calculated in each treatment applying allometric equations to the square diameter of the trunks at 25 cm above the soil in each plot (R 2 > 0.89, except for LAI in the rainfed treatment with R 2 = 0.75).
Root sampling
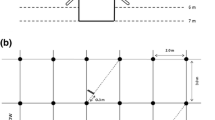
The stump (cut at the soil surface) and all the coarse roots (diameter >1 cm) of four trees per treatment covering the range of trunk circumferences were excavated and weighed in the field. Sub-samples were oven dried at 65 °C to estimate the dry weight of stumps and coarse roots. Three trenches (3 m × 1 m × 1 m) were dug close to one tree of average diameter in each plot (in three blocks) to sample medium-sized roots (2–10 mm in diameter) (Fig. 2). The roots were separated from the soil, dried at 65 °C and weighed.
Sampling grid scheme for fine root density measurements. The four sampled positions (P1, P2, P3 and P4, grey-filled circles) were distributed at different distances of four trees (black-filled circles) in each plot. The total number of sampled positions in our study was 128 (4 positions × 4 blocks × 2 treatments × 4 dates). P2 and P3 were sampled down to a depth of 1 m and P1 and P4 were sampled down to a depth of 8 m. The hatched rectangle indicates the position of the trench dug to sample medium-sized roots. The shaded area in each inter-row indicates the presence of the Brachiaria decumbens graminea
A mechanized soil corer (internal diameter of 7 cm) was used to sample fine roots at the end of the winter (September/October 2012 and 2013) and at the end of the summer (March/April 2013 and 2014). The fine roots were sampled at four distances from the trees down to a depth of 1 m in each plot (Fig. 2) for a total of 64 sampled positions down to 1 m in each treatment (4 positions × 4 blocks × 4 times). Among the four positions sampled in each plot, the nearest and the farthest of the trees were sampled down to a depth of 8 m (for a total of 32 sampled positions down to 8 m in each treatment over the study period). The 0–25 cm soil layer was collected, then the 25–50 cm layer, the 50–100 cm layer, and then every 50 cm down to a depth of 8 m. FLORAGRO (2011) prepared a short video showing the methodology used to sample very deep fine roots in eucalypt plantations. This method briefly described by Christina et al. (2011) has been used here in an orange orchard. We were especially careful to avoid contamination of the soil samples collected in deep soil layers by roots from the upper layers. After sampling the soils down to a depth of 2 m, we dug further to enlarge the diameter of the hole and we installed a 2-m long plastic tube with a diameter of 20 cm to avoid surface soil falling further down. Moreover, we only considered soil blocks from the inner part of the auger and all fragmented soil pieces likely to come from upper soil layers were systematically discarded. Therefore, only a fraction of the soil sampled at each depth was collected and the fine root mass density in each soil sample was computed dividing the fine root dry weight by the soil dry weight. The root front was defined in each plot as the depth of the layer where the deepest root was observed. Soil samples were collected approximately 2 m beyond the last root found at each sampling position to check that the root front was not underestimated (maximum soil coring down to 9.5 m at the position where fine roots were found down to 7.5 m).
Soil samples were gently washed and fine roots (diameter <2 mm) were carefully separated by hand. Living and dead roots were distinguished using criteria of colour, living stele and elasticity. While living roots were yellow and flexible, dead roots were grey and breakable. Only living roots were studied, dead roots were discarded. A sub-sample (10% by weight of each sample) was separated and washed through sieves with a mesh size of 300 µm to separate the roots <1 cm in length and the remaining sample was washed through sieves with a mesh size of 500 µm to separate all the fine roots with a length ≥1 cm. At the first sampling dates (September/October 2012 and March/April 2013), a sub-sample of fine roots of each soil sample was scanned (400 dpi) and the images were analyzed with the WinRHIZO 2.0 software (Régent Instruments, QC, Canada). Subsequently, the roots were dried at 65 °C for 72 h to determine their dry mass. Fine root mass in each soil sample was estimated by summing the mass of the entire roots ≥1 cm in length with ten times the mass of the roots <1 cm in length (separated in 10% of the soil sample mass). Root densities per unit of soil dry weight were converted into root densities per unit of soil volume using soil bulk densities measured in three pits per treatment down to a depth of 2 m. Soil texture varied little between the depths of 2 and 8 m (Table 1), and the bulk density of the 1.5–2.0 m layer was considered constant below 2 m. Fine root mass density (FRMD, g dm−3) was estimated for each soil sample as the ratio between the dry mass of fine roots and the soil volume sampled. Individual values per soil layer (four values for 0–1 m and two values for 1–8 m) were averaged arithmetically to estimate FRMDs in each plot. Mean FRMDs per treatment were estimated in each soil layer from four replicates (in the four blocks). Total belowground biomass was estimated in each plot summing the mean values of fine, medium-sized and coarse root dry masses.
Specific root length (SRL, m g−1) was estimated as the ratio between the length and the dry mass of the fine roots in each sub-sample, and specific root area (SRA, cm2 g−1) as the ratio between the area and the dry mass of the fine roots in each sub-sample. Fine root length density and fine root area density were estimated at each sampling date in each soil sample multiplying FRMD by SRL and SRA, respectively. We used SRL and SRA measured at the same position at the same depth in the same plot in September/October 2012 and March/April 2013 to estimated fine root length density and fine root area density in September/October 2013 and March/April 2014, respectively. Fine root mass per soil layer in each plot (FRM, g root mass m−2 ground area) and fine root length per soil layer in each plot (FRL, km root length m−2 ground area) were estimated multiplying mean FRMD and mean fine root length density in each soil layer, respectively, with the corresponding soil volume. Root area index (RAI, m2 root surface m−2 ground area) was estimated per soil layer in each plot by multiplying FRM with the mean SRA. Soil moisture in each layer was measured from 20 g of soil dried at 105 °C for 72 h. Roots of Brachiaria decumbens Stapf, the main plant species in the inter-row of orange orchards in Brazil, were separated in all the soil samples collected at position 4 (Fig. 2). The methodology shown above to estimate fine root biomass for orange plants was used. However, B. decumbens roots were not scanned and fine root lengths and areas were not estimated.
Data analyses
Effects of treatments and blocks on trunk diameter, LAI, aboveground biomass, belowground biomass, and fruit yield were assessed using two-way analyses of variance (proc glm in SAS). Sampling positions were located close to different trees in each plot. Therefore, individual root biomass measurements within a given soil layer were considered independent. Three-way ANOVAs were made to test the effects of treatments, blocks, and sampling dates, as well as the interaction between sampling dates and treatments on FRM, FRL and RAI in each soil layer (0–25, 25–50, then every 50 cm-thick layer down to 750–800), as well in the whole soil profile (0–800 cm). Homogeneity of variances was tested by Levene’s test and original values were log-transformed when variances were unequal. Mixed-effect models were used to test the effects of treatments, soil depth, season, sampling position, and interaction between treatments and season, treatments and sampling position, treatments and soil depth, sampling position and soil depth, sampling position and season, soil depth and season, soil depth and sampling position, as well as season and sampling position (as fixed effects) on fine root mass density (FRMD), fine root diameter, SRL and SRA. Blocks were considered as random effects. Residues were modelled by a first-order autoregressive correlation model to account for the correlations between soil depths. A significance level of 5% was used in all the analyses. When significant differences were detected between treatments, the Tukey’s test was used to compare treatment means. All the data were processed using the software package SAS v.9.2 (SAS, Cary, NC, USA).
Results
Growth and fruit production of orange plants
Irrigation did not significantly increase tree height, tree biomass, LAI and fruit yield of orange trees at our study site (Table 2). However, a trend toward a slight enhancement (less than 10%) of fruit yields was observed in response to irrigation over three successive years. LAI at the end of winter 2012 was about 4.5 m2 m−2 for the two water supply regimes. The belowground biomass accounted for 35–38% of the total biomass in the two treatments. The root area to leaf area ratio was 4.3 ± 1.0 m2 m−2 in rainfed plots and 3.0 ± 0.1 m2 m−2 in irrigated plots.
The soil water content changes were relatively low among the four sampling dates in our sandy soil (ranging from 5 to 10% down to a depth of 7 m), despite a strong seasonal variability of rainfall (Fig. 3). The sampled period with the lowest soil water contents was Sept/Oct 2012. The effect of irrigation on soil water contents was low, whatever the soil layer.
Gravimetric water contents (%) in soil layers 0–25 cm (a), 25–100 cm (b), 100–200 cm (c), 200–300 cm (d), 300–500 cm (e) and 500–700 cm (f) in the rainfed (open symbols) and irrigated (filled symbols) treatments. The sampling periods were September–October 2012, March–April 2013, September–October 2013 and March–April 2014. Mean values and standard errors between blocks are indicated (n = 4)
Spatial variability of fine root mass densities
While the densities of orange fine roots were not influenced by the water supply regime and the sampling season, they were highly dependent on the sampling depth, the position relative to trees as well as their interaction (Table 3). Fine root densities, and maximum rooting depths sharply declined from the vicinity of the trunks to mid inter-rows (Fig. 4a, b). Densities of orange fine roots at the P1 sampling position were approximately 70% higher than at the P4 position in both treatments across the four sampling periods. The density of B. decumbens fine roots sampled in the inter-row was 56% lower than the density of orange fine roots at the same position in the 0–25 cm soil layer, and much lower in all the soil layers below a depth of 25 cm (Fig. 4b, c). Orange and B. decumbens FRMDs dropped below the upper soil layer (0–25 cm) in both treatments. The deepest B. decumbens roots were found in the 350–400 cm soil layer in the rainfed treatment and in the 300–350 cm soil layer in the irrigated treatment (Fig. 4c).
Fine root distributions (g dm−3) down to the root front in rainfed (filled bars) and irrigated (open bars) treatments. Orange roots were sampled at position 1 (75 cm from the nearest tree) (a) and at position 4 (255 cm from the nearest tree) (b), and Brachiaria decumbens roots were sampled at position 4 (c). Mean values and standard errors between the sampling dates are indicated (n = 4), from September 2012 to May 2014 (see Fig. 2 for a description of sampling positions). In the rainfed treatment, fine roots were found in 100% of the soil samples collected at depths <5 m, and in 81, 56, 37, 25, 12 and 0% of the samples collected at the depths of 5.0–5.5, 5.5–6.0, 6.0–6.5, 6.5–7.0, 7.0–7.5 and 7.5–8.0 m, respectively. In the irrigated treatment, fine roots were found in 100% of the soil samples collected at depths <4 m, and in 94, 75, 69, 44, 6 and 0% of the samples collected at the depths of 4.0–4.5, 4.5–5.0, 5.0–5.5, 5.5–6.0, 6.0–6.5 m, and >6.5 m, respectively
Fine root distributions throughout the soil profiles
Total FRM over the study period was 711 g m−2 in the irrigated treatment and 826 g m−2 in the rainfed treatment on average across the four sampling dates (Fig. 5a). However, total FRM was not significantly different between the two treatments as a result of high spatial and temporal variability (Table 2). While FRM was the highest in the rainfed treatment in summer 2014 (1210 g m−2), it was the lowest in the irrigated treatment in winter 2012 (536 g m−2). FRM in the 0–1 m soil layer significantly differed between treatments and sampling dates. In this soil layer, FRM ranged from about 400 g m−2 in both treatments at the first three sampling dates to 840 g m−2 in the rainfed treatment in summer 2014.
Fine root mass (FRM) (a), fine root length (FRL) (b), and root area index (RAI) (c) in rainfed and irrigated orange orchards, from September 2012 to March 2014. Roots were sampled at the end of the winter (September/October) and at the end of the summer (March/April). Standard errors between blocks are indicated (n = 4). Significant differences between sampling dates and treatments (P < 0.05) are indicated by different letters, in lower case for an individual soil layer and in upper case for the whole soil profile
Total FRL ranged from 8.2 to 17.7 km m−2 over the study period, and RAI ranged from 13.7 to 26.1 m2 m−2 (Fig. 5b, c). FRL below a depth of 5 m was generally higher in the rainfed than in the irrigated treatment, but differences were not significant. In summer 2014, FRL below a depth of 5 m accounted for 14.3% of total FRL in the rainfed treatment and only 3.4% in the irrigated treatment. The proportion of FRL in the 0–1 m soil layer was higher in the irrigated treatment than in the rainfed treatment, except in winter 2013. Total RAI across all the sampling dates was 20.7 m2 m−2 in the rainfed treatment and 16.9 m2 m−2 in the irrigated treatment. However, differences between treatments were only significant in the 2–3 m soil layer, where RAI was higher in the rainfed (3.8 m2 m−2) than in the irrigated treatment (0.8 m2 m−2) in winter 2012.
While mean tree height was approx. 4 m in November 2012, the depth of the root front was approximately 6 m across the sampling periods in both treatments. However, orange trees are frequently pruned in the orchards and the height of trees growing in natural conditions would be higher. Some fine roots were observed more deeply in the rainfed treatment (down to a depth 7.0–7.5 m) than in the irrigated treatment (down to 6.0–6.5 m) but the effect of irrigation on root front depth was not significant across the four sampling periods (Table 2). About 50% of the total fine root mass was in the 0–1 m soil layer, whatever the treatment and the season (Fig. 6). The highest difference between treatments occurred in winter 2012, with 52% and 68% of the total fine root mass in the 0–1 m soil layer in the rainfed treatment and the irrigated treatment, respectively.
Fine root traits
The water supply regime did not significantly influence fine root diameter, SRL and SRA (Table 3). These root traits were significantly influenced by the sampling depth and the sampling position, as well as their interaction (except for fine root diameter). Fine root diameters were also significantly influenced by the sampling season, with a mean value of 0.58 mm at the end of the winter and 0.65 mm at the end of the summer across the sampling depths and treatments (Fig. 7). Mean fine root diameters ranged from 0.36 to 0.85 mm at the end of the winter, depending on the soil layer, and from 0.56 to 0.81 mm at the end of the summer, with a trend toward an increase with soil depth (Fig. 7). Mean SRL was 15 m g−1 and mean SRA was 279 cm2 g−1 across the sampling seasons, depths and treatments. SRL and SRA changes were low between the upper and the lower soil layers. While mean SRL was not affected by the season, mean SRA was 16% higher at the end of winter 2012 than at the end of summer 2013 (Table 3). SRL was 14.9 and 15.0 m g−1 on average across the sampling depths in the rainfed treatment, and 15.0 and 15.8 m g−1 in the irrigated treatment at the end of the winter and the summer, respectively. SRA was 268 and 295 cm2 g−1 on average across the sampling depths in the rainfed treatment, and 247 and 305 cm2 g−1 in the irrigated treatment at the end of the winter and the summer, respectively.
Discussion
Fine root biomass
As far as we are aware, our study is the first to quantify the fine root mass down to the root front, as well as the fine root length and fine root area in tropical orange orchards. Fine root biomass ranged from 528 g m−2 in the irrigated treatment in winter 2012–1200 g m−2 in summer 2014 in the rainfed treatment, which was higher than the range from 100 to 500 g m−2 reported for terrestrial ecosystems (Jackson et al., 1997). Mandarin fine root biomass ranged from 220 to 620 g m−2 down to a depth of 0.3 m in India, depending on the season (Dhyani and Tripathi 2000), which is consistent with our estimates for orange orchards down to a depth of 8 m in Brazil. While drip irrigation also decreased fine root biomass down to the root front in sugar-cane crops (Battie-Laclau and Laclau 2009), the opposite was observed in jujube plantations, with less fine root biomass in the rainfed treatment than in the irrigated treatment (Ma et al. 2013). Total fine root biomass in our study was highly dependent on the rainfall regime the months before each sampling date. Fine root biomass was higher in summer 2014 than at the other sampling dates, as a result of higher fine root densities in the 0–1 m soil layer. We speculate that this pattern might reflect a concentration of extractible water in the topsoil as a result of low-intensity rainfall events the months before root sampling (Fig. 1), which might have favoured root growth in the upper soil layers. The adjustments in belowground biomass allocation play a critical role to explain the responses of plants to different conditions of light and nutrient supply (Freschet et al. 2015).
Fine root distributions
Drip irrigation did not modify the main characteristics of the spatial distribution of fine roots. Indeed, the maximum rooting depth and the fine root densities were higher in the planting row than in the inter-row for the two water supply regimes, whatever the sampling date. The sharp decrease in fine root densities with soil depth observed in our study is common for tropical tree species (e.g. Oliveira et al. 2005; Freycon et al. 2015). Fine roots in the topsoil play an essential role to take up water and nutrients after rainfall events. The moisture of the soil layers influences fine root vertical distributions, and a shift of fine root growth between superficial and deep layers may contribute to improving the survival of tropical orange trees during drought periods.
In agreement with our first hypothesis, about 50% of the total FRM was found below a depth of 1 m in our rainfed and irrigated orange orchards, which is a similar proportion as in eucalypt and sugar cane plantations in deep Brazilian Ferralsol soils (Battie-Laclau and Laclau 2009; Laclau et al. 2013). The maximum depth reached by fine roots was 30% lower in the inter-row than close to the orange plants across all the sampling dates, regardless of the water supply regime. This pattern might reflect the architecture of tree roots with a decrease in root densities with the distance from the trunk commonly observed (e.g. Sudmeyer et al. 2004). In addition, the availability of water and nutrients for orange plants was probably lower in the inter-row than close to the planting row, as a result of competition between B. decumbens and orange plants.
The root front was only slightly deeper in the rainfed treatment than in the irrigated treatment, which might reflect the low rainfall deficit at our study site suggested by a non-significant effect of drip irrigation on LAI and fruit production. A similar pattern was shown for deep roots of sugar cane in the same region with a root front reaching 4.7 and 4.2 m in treatments rainfed and irrigated, respectively (Battie-Laclau and Laclau 2009). In a semi-arid environment, roots of jujube plantations reached 10 m in rainfed plots and only 4 m in plots where drip irrigation was applied (Ma et al. 2013). A concentration of fine roots in resource-rich soil patches is well documented (e.g. Hodge 2004), and recent studies carried out in deep tropical soils suggest that plant plasticity makes an adjustment possible of root front depth to the water availability in the top soil (Abouatallah et al. 2012; Ma et al. 2013). Plants with high water requirements growing under climates with prolonged dry periods commonly explore very deep soil layers. In a review, Canadell et al. (1996) showed that tropical savanna is the biome with the deepest rooting depth (15 ± 5 m on average) and among the greatest depths reached by roots (down to 68 m). Despite the scarcity of fine roots in very deep soil layers, eddy-covariance measurements of actual evapotranspiration show that they have a crucial role to supply plant water requirements during dry periods in tropical crops (Cabral et al. 2012), savannas and forests (Rocha et al. 2009). Modeling approaches show that the Amazonian climate is dependent on water withdrawal by trees at depths >10 m over dry seasons (Kleidon and Heimann 2000; Saleska et al. 2007), which highlights that the role of deep roots in tropical terrestrial ecosystems deserves more attention.
Fine root traits
In agreement with our second hypothesis, the order of magnitude of morphological root traits (mean diameter, SRL and SRA) was similar in superficial and deep soil layers. A low effect of drip irrigation on fine root traits might be partly explained by a low water deficit at our study site shown by non-significant effects of drip irrigation on LAI and fruit production. The increase in fine root diameter and SRA in summer 2013 relative to winter 2012 might be a result of the increase in soil water contents throughout the soil profile. A meta-analysis suggested that plants generally have more flexibility to alter their morphology than their biomass allocation (Poorter et al. 2012) which was confirmed for leaf area adjustment to light availability in a recent experimental study carried out for 12 herbaceous species (Freschet et al. 2015). However, changes in fine root length in response to nutrient stress in the latter study was mainly driven by changes in dry matter allocation and minor changes in SRL suggest that allocation to roots could be more important for plants to achieve an increase in root length than root morphology changes (Freschet et al. 2015). Consistently with low root morphology changes in response to environmental changes, 9 years of irrigation did not significantly change SRL and fine root diameter in Quercus pubescens and Pinus sylvestris stands (Herzog et al. 2014). SRL values down to a depth of 1 m for orange trees in our study (from 12 to 18 m g−1) were slightly higher than the range from 8 to 14 m g−1 observed for apple trees (Zhuo-Ting et al. 2010). Root traits measured at the community level in 20 plant communities across three climatic zones (tropical, mediterranean and montane) showed that roots had different suites of traits between the topsoil and the 100–150 cm soil layers, which suggested that root exploitation capacities were dependent on soil depth (Prieto et al. 2015). However, the effect of soil depth on fine root traits was low. While mean fine root diameter was 10% higher in the 100–150 cm layer than in the 0–20 cm layer, SRL did not significantly differ between the two layers. A recent study carried out for four eucalypt genotypes in Brazil also showed a low effect of the sampling depth in the soil (down to 11 m) on fine root traits (Pinheiro et al. 2016). Further investigations are needed to assess if the low changes in fine root traits across very deep soil profiles are common in tropical regions.
Biomass production and consequences for the management of orange orchards
Our third hypothesis was not fully validated since the decrease in RAI/LAI ratio in response to irrigation was only marginally significant (at P < 0.10). However, this trend suggests a plasticity of orange trees making it possible to adjust the area of water uptake (fine roots) to the availability of water in the soil, as reported for other tree species (Magnani et al. 2002; Costa e Silva et al. 2004; Pinheiro et al. 2016). Although RAI was 39% higher in rainfed plots than in irrigated plots in our study, the RAI/LAI ratios were not significantly different between the two treatments as a result of a large variability in the rainfed plots. Surprisingly, for an important fruit crop in tropical and Mediterranean regions, total tree biomass estimates are scarce in the literature for Citrus orchards. Self-shading is high in the crown of Citrus trees and LAI can reach up to 11 m2 m−2, even though values between 3 and 5 m2 m−2 are much more common (Castro Neto 2013; Consoli et al. 2014).
While the total biomass of 7-year-old orange plants in our study was 75–80 kg tree−1, it was 43 kg tree−1 at age 8 years for Navelina scion Citrus sinensis (L.) Osbeck grafted onto Carrizo citrange rootstock C. sinensis × Poncirus trifoliata L. (Raf.), which represents >70% of the orange production in Spain (Quiñones et al. 2013). The tree biomass measured in our study at age 7 years was only reached at age 11 years in Spain, where trees are considered adult from age 8 years onwards. The rates of biomass accumulation were slower in the Spanish orchards sampled by Quiñones et al. (2013) than in our study and only 27% of the total biomass was found belowground in 8-year-old trees (<20% from age 10 years onwards), while root biomass accounted for 35–38% of the total biomass in our Brazilian orchard.
Fruit yield in our study was representative of intensively managed orchards in Brazil (CONAB 2011). The mean productivity of orange orchards in the São Paulo state is lower, on average about 27 Mg ha−1 year−1 (CONAB 2011; EMBRAPA 2011). About 20% of the orange orchards in the São Paulo state are currently irrigated (Coelho 2010; Salomão et al. 2012). A study comparing fruit yields in rainfed, irrigated and fertirrigated orange orchards close to our study site showed a mean production of 48.8 Mg ha−1 of fruit. The fruit production increased by 8.3 and 4.0% in fertirrigated and irrigated plots relative to rainfed plots, respectively (Duenhas et al. 2005).
The lack of significant effect of drip irrigation on fruit yields in our deep Ferralsol suggest that deep fine roots have an important functional role, providing access to large amounts of water stored in deep soil layers during the rainy season. This role is likely to increase under climate changes since the intensity and frequency of future droughts should be exacerbated in tropical and subtropical regions (Solomon et al. 2009; Hawkins and Sutton 2012; IPCC 2013). Although the positive effect of irrigation on fruit yield from 2012 to 2014 was insignificant in our orange orchard, irrigation might become essential in the future to reduce the inter-annual variability of fruit production under extreme climatic events.
Conclusion
The total fine root biomass ranged from approximately 500 to 800 g m−2 in rainfed and irrigated orange orchards at most of the sampling dates. The root front reached about 6 m close to the trees and 4.5 m in the inter-row, and about 40% of the total fine root mass was found below a depth of 1 m. The effect of drip irrigation on fine root distribution was weak, with a trend toward a higher exploration of very deep soil layers in rainfed plots than in irrigated plots. Morphological root traits (mean diameter, SRL and SRA) were little affected by the water supply regime and the changes with soil depth were unclear. Root area index was more responsive to irrigation than leaf area index. Recent studies show that small densities of very deep roots can have an important functional role to supply the water demand of tropical trees during dry periods, providing access to water stored in deep soil layers during the rainy season. Our study suggests that improving our understanding of fine root ecology in very deep soil layers could help manage the irrigation of tropical orange orchards under a more variable future climate.
Author contribution statement
EA conducted the field work, prepared the figures and tables and wrote the manuscript. JPL proposed the experimental design, analysed the data and wrote the manuscript. JDR wrote the manuscript.
References
Abouatallah A, Salghi R, El Fadl A, Affi N, Ghnizar Y, Zarrouk A, Hammouti B (2012) Impact assessment of drippers distribution around the tree on soil moisture, roots and fruits growth of Citrus. Der Pharma Chemica 4:1969–1981
Agrianual (2013) Anuário da Agricultura Brasileira. FNP, São Paulo
Anderson TM, Starmer WT, Thorne M (2007) Bimodal diameter distributions in Serengeti grasses exhibit plasticity in response to defoliation and soil texture: implications for nutrient uptake. Funct Ecol 21:50–60
Battie-Laclau P, Laclau J-P (2009) Growth of the whole root system for a plant crop of sugarcane under rainfed and irrigated environments in Brazil. Field Crops Res 114:351–360
Battie-Laclau P, Laclau J-P, Domec J-C, Christina M, Bouillet J-P, Piccolo MC, Gonçalves JLM, Moreira RM, Krusche AV, Bouvet J-M, Nouvellon Y (2014) Effects of potassium and sodium supply on drought-adaptive mechanisms in Eucalyptus grandis plantations. New Phytol 203:401–413
Bleby TM, McElrone AJ, Jackson RB (2010) Water uptake and hydraulic redistribution across large woody root systems to 20 m depth. Plant Cell Environ 33:2132–2148
Böhm W (1979) Methods of studying root systems. Springer, Berlin
Bremer Neto H, Mourão Filho AA, Stuchi ES, Espinosa-Núñez E, Cantuarias-Avilés T (2013) The horticultural performance of five ‘Tahiti’ lime selections grafted onto ‘Swingle’ citrumelo under irrigated and non-irrigated conditions. Sci Hortic 150:181–186
Cabral OMR, Rocha HR, Gash JH, Ligo MAV, Tatsch JD, Freitas HC, Brasilio E (2012) Water use in a sugarcane plantation. GCB Bioenergy 4:555–565
Canadell J, Jackson RB, Ehleringer JR, Mooney HA, Sala OE, Shulze ED (1996) Maximum rooting depth of vegetation types at the global scale. Oecologia 108:583–595
Castro Neto MT (2013) Fisiologia. In: Cunha Sobrinho AP, Magalhães AFJ, Souza AS, Passos O, Soares Filho WS et al (eds) Cultura dos citros. EMBRAPA, Brazil, pp 173–193
Christina M, Laclau J-P, Gonçalves JLM, Jourdan C, Nouvellon Y, Bouillet J-P (2011) Almost symmetrical vertical growth rates above and below ground in one of the world’s most productive forests. Ecosphere 2:1–10
Christina M, Nouvellon Y, Laclau J-P, Stape JL, Bouillet J-P, Lambais GR, le Maire G (2016) Importance of deep water uptake in tropical eucalypt forest. Funct Ecol. doi:10.1111/1365-2435.12727 (online)
Coelho RB (2010) Irrigação por gotejo: problemas e soluções. Citric Atual 76:12–14
CONAB (2011) Laranja: safra 2011. http://www.agricultura.gov.br/arqeditor/file/camarassetoriais/Citricultura/27RO/AppSafra.pdf. Accessed 22 Apr 2015
Consoli S, Stagno F, Rocuzzo G, Cirelli GL, Intrigliolo F (2014) Sustainable management of limited water resources in a young orange orchard. Agric Water Manag 132:60–68
Costa e Silva F, Shvaleva A, Maroco JP, Almeida MH, Chaves MM, Pereira JS (2004) Responses to water stress in two Eucalyptus globulus clones differing in drought tolerance. Tree Physiol 24:1165–1172
Dhyani SK, Tripathi RS (2000) Biomass and production of fine and coarse roots of trees under agrisilvicultural practices in North-east India. Agrofor Syst 50:107–121
Duenhas LH, Villas Bôas RL, Souza CMP, Oliveira MVAM, Dalri AB (2005) Produção, qualidade dos frutos e estado nutricional da laranjaValência sob fertirrigação e adubação convencional. Eng Agrícola 25:154–160
EMBRAPA (2011) Considerações sobre a produção de laranja no Estado de São Paulo. http://pt.slideshare.net/marqueslms/35-ro-citricultura-embrapa-parte-3. Accessed 22 Apr 2015
FLORAGRO (2011) Fine root sampling using a mechanized soil auger. https://www.youtube.com/watch?v=tVdsiFKhnJ4. Accessed 11 Jul 2016
Ford HW (1954) The influence of rootstock and tree age on root distribution of Citrus. Proc Am Soc Hortic Sci 63:137–142
Freschet GT, Swart EM, Cornelissen JHC (2015) Integrated plant phenotypic responses to contrasting above-and below-ground resources: key roles of specific leaf area and root mass fraction. New Phytol 206:1247–1260
Freycon V, Wonkam C, Fayolle A, Laclau J-P, Lucot E, Jourdan C, Cornu G, Gourlet-Fleury S (2015) Tree roots can penetrate deeply in African semi-deciduous rain forests: evidence from two common soil types. J Trop Ecol 31:13–23
Guo D, Mengxue X, Xing W, Wenjing C, Ying L, Zhengquan W (2008) Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol 180:673–683
Harper RJ, Smettem KRJ, Carter JO, McGrath JF (2009) Drought deaths in Eucalyptus globulus (Labill.) plantations in relation to soils, geomorphology and climate. Plant Soil 324:199–207
Hawkins E, Sutton R (2012) Time of emergence of climate signals. Geophys Res Lett 39:L01702
Herzog C, Steffen J, Pannatier EG, Hajdas I, Brunner I (2014) Nine years of irrigation cause vegetation and fine root shifts in a water-limited pine forest. PLoS One 9:1–11
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24
IPCC (2013) Summary for Policymakers. In: Stocker TF, Qin D, Plattner G-K, Tignor MMB, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate change 2013: the physical science basis Contribution of Working Group I to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, p 27
Jackson RB, Mooney HA, Schulze ED (1997) A global budget for fine root biomass, surface area, and nutrient contents. Ecology 94:7362–7366
Kleidon A, Heimann M (2000) Assessing the role of deep rooted vegetation in the climate system with model simulations: mechanism, comparison to observations and implications for Amazonian deforestation. Clim Dyn 16:183–199
Laclau J-P, Silva EA, Lambais GR, Bernoux M, Maire G, Stape JL, Bouillet J-P, Gonçalves JLM, Jourdan C, Nouvellon Y (2013) Dynamics of soil exploration by fine roots down to a depth of 10 m throughout the entire rotation in Eucalyptus grandis plantations. Front Plant Sci 4:243
Ma LH, Lui XL, Wang YK, Wu PT (2013) Effects of drip irrigation on the deep root distribution, rooting depth, and soil water profile of jujube in a semiarid region. Plant Soil 373:995–1006
Maeght JL, Rewald B, Pierret A (2013) How to study deep roots—and why it matters. Front Plant Sci 4:299
Magnani F, Grace J, Borghetti M (2002) Adjustment of tree structure in response to the environment under hydraulic constraints. Funct Ecol 16:385–393
Martínez-Alcântara B, Quiñones A, Forner-Giner MA, Iglesias DJ, Primo-Millo E, Legaz F (2012) Impact of fertilizer-water management on nitrogen use efficiency and potential nitrate leaching in citrus trees. Soil Sci Plant Nutr 58:659–669
O’Grady AP, Worledge D, Battaglia M (2006) Above- and below-ground relationships, with particular reference to fine roots, in a young Eucalyptus globulus (Labill.) stand in southern Tasmania. Trees 20:531–538
Oliveira RS, Bezerra L, Davidson EA, Pinto F, Klink CA, Nepstad DC, Moreira A (2005) Deep root function in soil water dynamics in savannas of central Brazil. Funct Ecol 19:574–581
Pagès L (2011) Links between root developmental traits and foraging performance. Plant Cell Environ 34:1749–1760
Parsons LR, Morgan KT, Wheaton TA (2000) Using soil water measurements to Schedule irrigation. Citrus Industry 81:21–22
Pinheiro RC, Deus JC Jr, Nouvellon Y, Campoe OC, Stape JL, Aló LL, Guerrini IA, Jourdan C, Laclau J-P (2016) A similar pattern of very deep rooting for Eucalyptus seedlings and clones in Brazilian planted forests. For Ecol Manag 366:143–152
Pires RCM, Luchiari DJF, Arruda FB, Mossak I (2005) Irrigação. In: Mattos Junior D, De Negri JD, Pio RM, Pompeu Junior J et al (eds) Citros. 369, Brazil, pp 369–408
Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L (2012) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol 193:30–50
Pregitzer KS, Laskowiski MJ, Burton AJ, Lessard VC, Zac DR (1998) Variation in sugar maple root respiration with root diameter and soil depth. Tree Physiol 18:665–670
Prieto I, Roumet C, Cardinael R, Dupraz C, Jourdan C, Kim JH, Maeght JL, Mao Z, Pierret A, Portillo N, Roupsard O, Thammahacksa C, Stokes A (2015) Root community traits along a land use gradient: evidence of a community-level economics spectrum. J Ecol 103:361–373
Quiñones A, Martínez-Alcántara B, Font A, Forner-Giner MA, Legaz F, Primo-Millo E, Iglesias DJ (2013) Allometric models for estimating carbon fixation in Citrus trees. Agron J 105:1355–1366
Rocha HR, Manzi AO, Cabral OM, Miller SD, Goulden ML, Saleska SR, Coupe NR, Wofsy SC, Borma LS, Artaxo P, Vourlitis G, Nogueira JS, Cardoso FL, Nobre AD, Kruijt B, Freitas HC, Randow C, Aguiar RG, Maia JF (2009) Patterns of water and heat flux across a biome gradient from tropical forest to savanna in Brazil. J Geophys Res 114:1–8
Roumet C, Urcelay C, Díaz S (2006) Suites of root traits differ between annual and perennial species growing in the field. New Phytol 170:357–368
Saleska SR, Didan K, Huete AR, Rocha HR (2007) Amazon forests green-up during 2005 drought. Science 318:612
Salomão LC, Souza TR, Villas Bôas RL, Andrade TF, Foratto LC, Santos AJM (2012) Posicionamento de extratores de cápsula porosa em solo arenoso na citricultura fertirrigada por gotejamento. Irriga 17:469–480
Schenk HJ, Jackson RB (2002) Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. J Ecol 90:480–494
Solomon S, Plattner GK, Knutti R, Friedlingstein P (2009) Irreversible climate change due to carbon dioxide emissions. Proc Natl Acad Sci 106:1704–1709
Souza LS, Souza LD, Paiva AQ, Rodrigues ACV, Ribeiro LS (2008) Distribuição do sistema radicular de citros em uma toposseqüência de solos de tabuleiro costeiro do estado da Bahia. Revista Brasileira de Ciência do Solo 32:503–513
Stone EL, Kalisz PJ (1991) On the maximum extent of tree roots. For Ecol Manag. 46:59–102
Sudmeyer RA, Speijers J, Nicholas BD (2004) Root distribution of Pinus pinaster, P. radiata, Eucalyptus globulus and E. kochii and associated soil chemistry in agricultural land adjacent to tree lines. Tree Physiol 24:1333–1346
van Raij B, Andrade JC, Cantarella H, Quaggio JA (2001) Análise química para avaliação da fertilidade de solos tropicais. Instituto Agronômico Campinas, Brazil, p 285
Whitney JD, Elizaby A, Castle WS, Wheaton TA, Littell RC (1991) Citrus tree spacing effects on soil water use, root density, and fruit yields. Am Soc Agric Eng 34:129–134
Zhuo-Ting G, Zheng-Chao Z, Wen-Zhao L (2010) Vertical distribution and seasonal dynamics of fine root parameters for apple trees of different ages on the Loess Plateau of China. Agric Sci China 9:46–55
Acknowledgements
We acknowledge the staff of the Itatinga Experimental Station (ESALQ-USP), in particular Rildo Moreira e Moreira, the staff of the Real Farm, as well as Floragro Apoio à Pesquisa (http://www.floragroapoio.com.br) for their technical support. The study was funded by FAPESP—Fundação de Amparo à Pesquisa do Estado de São Paulo (http://www.fapesp.br, 2012/03342-7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by K. Noguchi.
Rights and permissions
About this article
Cite this article
Adriano, E., Laclau, JP. & Rodrigues, J.D. Deep rooting of rainfed and irrigated orange trees in Brazil. Trees 31, 285–297 (2017). https://doi.org/10.1007/s00468-016-1483-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-016-1483-5