Abstract
Key message
Beside temperature, soil moisture was found as the most important environmental factor influencing the relationship between stem CO2 efflux and sap flow.
Abstract
Stem CO2 efflux is an important component of the forest carbon balance. Even after several studies on this issue, there is still uncertainty about the influence of the sap flux on stem CO2 efflux. This study analyses stem CO2 efflux and sap flow measured on Norway spruce [Picea abies (L.) Karst] trees and environmental factors influencing this relationship during the growing seasons of 2010 and 2011. Stem CO2 efflux measurements were performed using an automatic dynamic closed gasometrical system, whilst sap flow measurements were carried out by applying a sap flow method heat pulse velocity. Stem CO2 efflux was positively correlated with stem temperature; sap flow was positively correlated with incident global radiation. During optimal soil moisture conditions, stem CO2 efflux and sap flow were positively correlated while during dry conditions, stem CO2 efflux and sap flow were not positively correlated. Almost all significant correlations between stem CO2 efflux and sap flow were not controlled by any investigated environmental factor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The net carbon budget of a forest is a fine balance between the carbon assimilation rate and efficiency, carbon redistribution and allocation and processes of carbon release (respiration of living biomass, tree mortality, microbial decomposition of litter, oxidation of soil carbon, degradation and disturbance) (Malhi et al. 1999). Respiration activity in plants differs from one organ to another, changing in relation to the availability of respirable substrates, the state of seasonal ontogeny and influencing environmental factors; therefore, they should be represented separately if possible (Cannell and Thornley 2000).
Respiration is an important component of forest carbon balance, releasing up to 80 % of fixed carbon by the autotrophic and heterotrophic pathway (Janssens et al. 2001; Ryan et al. 1997; Sprugel and Benecke 1991) stem respiration is regarded as a significant element of this balance due to the fraction of assimilates used and the dramatic increase in wood with stand development. The magnitude and temporal pattern of stem respiration have been quantified by measuring the efflux of CO2 from the stem, although it has been known for a long time that these two processes are not strictly equal to each other (Acosta et al. 2008; Teskey and McGuire 2002).
It is widely accepted that temperature is a key environmental factor influencing stem CO2 efflux, affecting enzymatic processes (biological effect) and the rate of diffusion (physical effect). The rate of stem respiration generally increases exponentially with increasing temperature (Stockfors 2000; Zha et al. 2004). On the other hand, some studies showed that bark photosynthesis can reduce stem CO2 efflux by re-fixation of CO2 (Pfanz and Aschan 2000; Pfanz et al. 2002). Wittmann et al. (2006) in a study of young birch trees (Betula pendula Roth.) under illumination estimated that corticular photosynthesis was able to refix up to 97 % of the CO2 produced by branch respiration. Despite several studies having been performed over the past decade on stem CO2 efflux and factors influencing it (Acosta et al. 2010; Rayment et al. 2002; Wieser and Bahn 2004), there is still some uncertainty about the influence of the sap flow on stem CO2 efflux. CO2 produced by the respiration of roots and parenchyma cells within the woody tissues inside the stems diffuses radially out through the bark, but is also simultaneously transported upward within the xylem by the sap flux and partly consumed by photosynthesis in the leaves (Levy et al. 1999; Martin et al. 1994; Teskey and McGuire 2002). Different relationships between stem CO2 efflux and sap flow rate have been observed in various studies: an increase in CO2 diffusion with decreasing sap flow rate (e.g. Levy et al. 1999) a decrease in CO2 diffusion with increasing sap flow rate (Bowman et al. 2005, Teskey and McGuire 2002) and no relationship (Cerasoli et al. 2009, Maier and Clinton 2006) have all been noted. An important feature that also influences the relationship between stem CO2 efflux and sap flow is the large resistance to CO2 diffusion from the stem interior to ambient air (Eklund and Lavigne 1995) because of the inner wood structure and outer bark resistance. Ryan et al. (1995) reported that CO2 efflux lagged behind air temperature by up to 5 h, and the lag was longer for species with thicker bark (ponderosa pine and slash pine) than for species with thin bark (red pine and western hemlock). In addition, the diffusivity of gases is closely correlated to the water content in woody tissue (Sorz and Hietz 2006) and thus may influence stem respiration during periods of water stress. However, information regarding the relationship between the stem CO2 efflux and sap flux rate is still required. Another interesting issue related to this relationship is the environmental factors which affect both processes.
The aims of the this study were (1) to quantify stem CO2 efflux and sap flow rate under two different conditions of soil water availability, (2) to estimate the effect of seasonal course of temperature on stem CO2 efflux and sap flow, (3) to improve our understanding of the relationship between sap flux rate and CO2 efflux from stem of Norway spruce trees and (4) to determine possible environmental factors affecting this relationship.
Materials and methods
Site description
The experiment was carried out in a Norway spruce [Picea abies (L.) Karst] forest stand at the Ecological Experimental Study Site (ES) Bily Kriz (49°30´ N, 18°32´ E, 890 m a.s.l.) situated in Moravian-Silesian Beskydy Mts., the Czech Republic. ES Bily Kriz is characterised by a mean annual temperature of 6.8 °C and a mean annual amount of precipitation of 1306 mm (1998–2011). In 1981, the Norway spruce stand was established by 4-year-old seedlings planted in density of 5,000 trees per ha on the slope terrain (13.5°) with SSW exposure in 1981. The main descriptive characteristics of the forest stand are shown in Table 1.
Stem CO2 efflux and sap flow measurements
Measurements of stem CO2 efflux and sap flow were carried out on three representative trees within the forest stand for each year of the experiment (2010 and 2011). In total, six trees were investigated over the whole experiment (Table 2). The investigated trees were selected with respect to the diameter at breast height (DBH), i.e. at 1.3 m above the ground distribution, crown dimensions and tree dominance classes. Stem CO2 efflux measurements were made using an automatic closed dynamic system (non-steady-state through-flow) based on Pavelka et al. 2004. The system consists of maximum eight stem chambers (in our experiment we used only six chamber due to the experimental set up) with pneumatic closing connected to an infrared gas analyser (Li-840, Li-Cor, USA) and a control unit with control software (Inris s.r.o., CZ). The stem chambers are closed only during measurements (300 s); within the remaining time they are left opened. When closing, the chamber reaches an aluminium frame sealed with neoprene (to be airtight closed) and permanently installed on the northern surface part of the stem (to exclude warming caused by the sun radiation) of the sample tree by two textile bels. The stem chamber (half cylinder shape made of stainless steel, height 12 cm and diameter 6.5 cm) was installed on the northern surface part of the stem (to exclude warming caused by the sun radiation) at a height of about 1.3 m on each investigated tree. Each stem chamber measured CO2 efflux every 2 h. Moreover, stem temperature was measured in five-minute intervals using thermocouples (PT 100, CZ) installed approximately 3 cm below the chambers in cambium layer in each investigated tree.
Sap flow measurements were carried out by applying a sap flow system (model SF 300, Greenspan Technology, Australia) based on the heat pulse velocity method (Köstner et al. 1998). Two probes per tree were inserted into 20 and 60 % depths of sapwood (counted inward from cambium) of each sample stem. The probes were installed about 1.5 m and 1.6 heights from the soil surface, with west–east orientation against each other, respectively. The Probe sets were reinserted every 3 weeks. An accurate quantification of sap flow was based on previous site-specific analysis of sap flow radial velocity profile (Čermák and Nadezhdina 1998). Self-made calibration was performed according to Cohen et al. (1993) on representative Norway spruce trees with a stem diameter at breast height (DBH) ranging from 5 to 8 cm. The sapwood area (SA) of trees was estimated by allometric relation obtained during harvest experiments in the same study site, \( {\text{SA}} = 0.537\; \times \;{\text{DBH}}^{2.0791} \) (Pokorný and Tomášková 2007).
Measurements of stem CO2 efflux were carried out from 3 May until 31 October in 2010 and from 13th of May until 8 November in 2011. Sap flow was measured from 30 June until 5 October in 2010 and from 1 July until 13 October in 2011. Simultaneous measurements of the stem CO2 efflux and sap flow were performed on three trees (T1, T2 and T3) from 1 July until 30 September in 2010. During the growing season of 2011, one tree (T4) was measured from 1 August until 13 October and two trees (T5 and T6) were measured from 1 July until 13 October. In our analysis, data from tree T3 were not taken into account due to malfunction of the sap flow sensors in the middle of the experiment period.
Ancillary data
Environmental parameters such as air temperature at 2 m height (EMS 33 Rotronics, EMS, CZ), incident global radiation (CNR1 Net Radiometer, Kipp-Zonen, Delft, NL) and precipitation (MetOne 386, Met One Instruments, Inc., Oregon, USA) were measured over the whole experimental period. The volumetric soil water content (Theta Probe ML2x, Delta-T Devices Ltd., Cambridge, UK) was also measured at depths of 5 and 20 cm in a soil profile among investigated trees during the experimental period. Moreover, dendrometrical parameters, such as the stem diameter at the breast height (DBH 1.3 m) and tree height by Forestor Vertex (Hagflöf, SE) were measured at the end of each growing period. Changes in the tree trunk diameter were measured by automatic dendrometers (DRL 26, EMS, CZ) in 1 h time steps throughout the entire growing seasons.
Stem CO2 efflux—data processing
The natural logarithm of the stem CO2 efflux rate and the woody-tissue temperature were regressed using a linear model:
where α and β are the regression coefficients. The Q 10 parameter (the proportional change in CO2 efflux from 10 °C increase in temperature) was calculated according to the following equation:
where α is the regression coefficient obtained from the previous Eq. ( 1 ). Then, CO2 efflux was normalised to the temperature of 10 °C according to the following equation:
where R i is the measured CO2 efflux rate (μmol CO2 m−2 s−1) and T i (°C) the woody tissue measured temperature.
Sap flow—data processing
Raw data of the temperature response in time from the probes were processed by SAPCAL software (Greenspan Technology, Australia). Mean sap flow velocity was obtained in half-hour time intervals.
Dataset treatment
Daily sums of the sap flow and mean stem CO2 efflux were calculated, as well as mean daily temperatures at 2 m height and volumetric soil water contents at a depth of 5 cm. Days with precipitation events were excluded from the analysis. The dataset was divided into two groups of data according to the mean volumetric soil water content at the depth of 5 cm. The threshold value was set to 12 % according to the permanent wilting point. When soil volumetric water content was above this threshold, the days were considered moist days; when below the threshold, the days were called dry days.
Statistical analysis
Evaluation of the potential relationship between stem temperature and stem CO2 efflux was performed by the correlation analysis on a daily time step for the entire growing seasons. Descriptive statistics were carried out for normalised stem CO2 efflux (R 10) and sap flow. The daily means of the R 10 and daily sums of sap flow were compared to each other by independent two-sample t test for the comparison of two trees (2010) and ANOVA analysis for the comparison of three trees (2011). When significant differences occurred in t tests or ANOVA analysis, one-sided variant of the t test and multiple comparisons by Tukey test were performed to determine the prevailing variable values. Potential differences of sap flow and stem CO2 efflux between moist and dry days at the individual tree level were examined by an independent two-sample t test.
All correlation analyses were performed by the Pearson correlation coefficient test. Correlation analyses of R 10 and sap flow were performed on all of the individual trees investigated. Cross correlations were performed within the trees to examine the potential effects of individual trees on the correlation significance. The incident global radiation, volumetric soil water content at depths of 5 and 20 cm, air temperature at a height of 2 m, stem temperature and cumulative and non-cumulative volumetric changes of the tree stem were used as possible control factors influencing the correlation between R 10 and sap flow when applying partial correlations. When we included control factors individually into the previously significant correlation between R 10 and sap flow and the correlation was not significant afterwards, the significant effect of the factor was validated. The threshold of significance was set to p < 0.05. Analyses were performed in STATISTICA 9.0 software (StatSoft, Inc., Tulsa, USA) and SPSS 17.0 (IBM, New York, USA) software. One-sided independent two sample t tests were performed in R Commander 1.4-7.
Results
Climate conditions
The mean air temperature at the experimental site at a height of 2 m was 11.4 °C during the growing season of 2010 with maximum values in July; in 2011, it was 11.8 °C with maximum values in August. The highest monthly sum of incident photosynthetically active radiation (PAR) was 231 MJ m−2 month−1 in July 2010 and 246 MJ m−2month−1 in May 2011. The seasonal amounts of precipitation (1 May–31 November) were 1046 and 664 mm in 2010 and 2011, respectively. The mean annual relative humidity was 91 % in 2010 and 87 % in 2011. The mean seasonal volumetric soil moisture measured at a depth of 5 cm was 28.5 % in 2010, with the lowest soil moisture in the middle of July, while the mean soil moisture in 2011 was 23.7 %, with low soil moisture also in the middle of July, as well as in September and October. The year 2011 was specific with very low amounts of precipitation at the end of the growing season, which caused an elongation of the dry period (Fig. 1).
Stem CO2 efflux
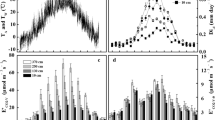
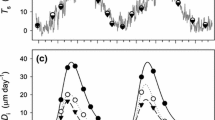
Daily stem CO2 efflux was highly correlated with the mean daily stem temperature during both growing seasons (mean r = 0.866, P ≪ 0.001). The comparison of correlation coefficients on daily time series showed that values of the correlation coefficient show seasonality or other trends, tight correlation between stem CO2 efflux and temperature of woody tissues resulted in high variability of stem CO2 efflux during the growing season (Fig. 2, example for tree T4 in 2011) due to the stem temperature changes. The obtained slopes of the linear regression for the tree T4 per individual months were of 0.0895 in May, 0.162 in June, 0.1791 in July, 0.1748 in August, 0.1549 September, 0.059 for October and 0.0288 for December (Fig. 2). At low temperatures during October and November, an increase of temperature up to 8–10 °C did not cause much of a change in the stem CO2 efflux of the studied trees. After this stem temperature threshold, stem CO2 efflux was exponentially dependent on the temperature of woody tissues. There were obvious diurnal changes in stem CO2 efflux corresponding to the diurnal variation of stem temperature (Fig. 3a), with the highest stem CO2 efflux rates close to noon. The maximum stem CO2 efflux rate occurred close the maximum stem temperature (about two hours’ lag time). The stem CO2 efflux rate presented a small fluctuation despite changes in temperature at the end of the growing season (Fig. 3b).
Stem CO2 efflux also presented seasonal changes (Fig. 4a); it increased in the beginning of the growing season and peaked in summer months with maxima of 3.2 and 3.8 µmol m−2 s−1 in 2010 and 2011, respectively, before decreasing gradually until the end of the growing season. These changes were mainly caused by changes in stem temperature. To eliminate the effect of temperature, the measured stem CO2 efflux was normalised to a temperature of 10 °C (R 10). The R 10 increased at the beginning of the growing season from values of approximately 0.5 µmol m−2 s−1 up to the maxima in July and August (1.3 and 1.7 µmol m−2 s−1 in 2010 and 2011, respectively); then, it gradually decreased until the end of the season (Fig. 4b).
Sap flow
Sap flow was correlated with incident global radiation during both growing seasons (2010 r = 0.883, P ≪ 0.001; 2011 r = 0.836, P ≪ 0.001). Diurnal courses of sap flow corresponded well with the diurnal variation of incident global radiation (Fig. 5). The lag time between maximum of incident global radiation and sap flow was about 1.5 h. Sap flow during precipitation events was close to zero.
Seasonal changes in the sap flow were observed (Fig. 6). During summer days, sap flow reached its maxima of 34.0 l day−1 in 2010 and 39.3 l day−1 in 2011, respectively.
As described above, stem CO2 efflux and sap flow had their maxima during daytime when stem temperature and incident global radiation were highest, respectively. When the stem CO2 efflux was normalised to 10 °C (R 10), it had the opposite trend compared to sap flow, with minima in the midday and an inverse fluctuation (Fig. 7).
R 10 and sap flow versus soil moisture conditions
The average R 10 was about 1.35 µmol m−2 s−1 during the moist conditions for both years (2010 and 2011), while the average sap flow was 20.35 and 18.54 l day−1 in 2010 and 2011, respectively (Table 2). During the dry conditions, the averaged R10 and sap flow were 1.05 µmol m−2 s−1 and 19.40 l day−1 in 2010, and 1.04 µmol m−2 s−1 and 12.21 l day−1 in 2011, respectively (Table 3).
There was a significant difference in normalised stem CO2 efflux (R 10) between moist and dry days only for tree T 2 (t = 3.491, P = 0.002) (Fig. 8a). R 10 was reduced in dry days by about one-third (33.6 %) in comparison with moist days in 2010. During 2011, under moist days, stem CO2 efflux was measured only in two trees (T5 and T6) and there was no significant difference in R10 between these trees (Fig. 8b). In dry days in 2011, three trees were measured and there was a significant difference between the R 10 of the investigated trees (F = 7.89, P = 0.001); R 10 of T5 was significantly lower than T4 and T6.
Concerning the sap flow measurements, there was no significant difference in sap flow between investigated trees during either moist or dry days in 2010 (Fig. 9a). The mean sap flow through both years and both soil moisture conditions was 19.88 ± 0.88 (SD) l day−1. In 2011, during moist days, the sap flow of T5 was significantly higher compared to T6
(t = −6.76, P = 0.005). During dry conditions, a significant difference in sap flow was seen between the investigated trees (F = 7.075, P ≪ 0.001); the sap flow of T5 was significantly lower than that of T4 and T6 (Fig. 9b). When comparing sap flow of the investigated trees under different soil moisture conditions, we found significantly lower sap flow during dry days compared to moist days for tree T5 (t = 8.962, P = 0.000).
R 10 versus control factors
During moist conditions in 2010, normalised stem CO2 efflux (R 10) of tree T1 and T2 was significantly correlated with incident global radiation (r = 0.65, 0.69), cumulative changes of DBH (r = −0.79, r = −0.81), stem temperature (r = 0.71, r = 0.80) and air temperature at a height of 2 m (r = 0.71, r = 0.82), respectively. In contrast, during the same conditions in 2011, R 10 of both trees did not correlate with any of the tested control factors.
During dry conditions in 2010, normalised stem CO2 efflux (R 10) of tree T1 correlated with volumetric soil moisture content at depths of 5 cm (r = 0.87) and 20 cm (R = 0.90). In 2011, R 10 of tree T4 was significantly correlated with incident global radiation (r = 0.48), non-cumulative increment (r = 0.48) and stem temperature (r = 0.57), R 10 of tree T5 was correlated with incident global radiation (r = 0.48) and with non-cumulative changes in stem volume (r = 0.38), and R 10 of tree T6 was correlated with incident global radiation (r = 0.47), non-cumulative stem volume changes (r = 0.47) and cumulative stem volume changes (r = 0.40). In both soil moisture conditions, all correlations identified during the comparison of R 10 and control factors were significant.
Sap flow versus control factors
During moist conditions in 2010, the sap flow of tree T1 correlated with incident global radiation (r = 0.97), cumulative changes of DBH (r = −0.90), volumetric soil water content at depths of 5 cm (r = −0.62) and 20 cm (r = −0.61), stem temperature (r = 0.85) and air temperature at a height of 2 m (r = 0.85). The sap flow of tree T2 correlated significantly with incident global radiation (r = 0.82), cumulative changes of DBH (r = −0.60), volumetric soil water content at depths of 5 cm (r = −0.60) and 20 cm (r = −0.45), stem temperature (r = 0.82) and air temperature at a height of 2 m (r = 0.83). In 2011, during moist conditions, the sap flow of tree T5 significantly correlated with incident global radiation (r = 0.93), stem temperature (r = 0.73) and air temperature at 2 m (r = 0.79), while the sap flow of tree T6 also significantly correlated with incident global radiation (r = 0.90) and non-cumulative changes of stem volume (r = −0.75).
During dry conditions in 2010, the sap flow of tree T1 correlated significantly with cumulative changes of DBH (r = −0.87), and the sap flow of tree T2 correlated with volumetric soil water content at depths of 5 cm (r = 0.85) and 20 cm (r = 0.88) and air temperature at a height of 2 m (r = −0.84). In 2011, during dry conditions, the sap flow of tree T4 correlated with soil moisture at depths of 5 cm (r = 0.46) and 20 cm (r = 0.43), the sap flow of tree T5 correlated with incident global radiation (r = 0.49), cumulative changes of stem (r = 0.43) and air temperature at 2 m (r = 0.35), and the sap flow of the tree T6 correlated with incident global radiation (r = 0.61), cumulative changes of stem volume (r = 0.42), stem temperature (r = 0.53) and air temperature at 2 m (r = 0.48). In both soil moisture conditions, all correlations identified during the comparison of sap flow and control factors were significant.
Relationship between R 10 and sap flow
The correlation between R 10 and sap flux within the investigated trees was significant for moist days 2010, but not during dry days, with the exception of tree T6 (Table 4a). The opposite situation occurred in 2011, when correlations on dry days were significant, but were not on moist days (Table 4b). The highest correlation between R 10 and sap flow was found during dry days for tree T6 (r = 0.95) in 2010, while the lowest significant correlation was found for dry days in tree T5 (r = 0.50) in 2011. In our analysis of partial correlation during moist conditions, significant control factors for tree T1 were incident global radiation (r = 0.32), stem temperature (r = 0.21) and air temperature at a height of 2 m (r = 0.27) in 2010. No other significant control factors were observed in 2010 or 2011. During dry conditions, no control factor showed any influence on the correlations between R 10 and sap flow for growing seasons in 2010 and 2011. However, all correlations calculated during the comparison of R10 and control factors were significant.
Discussion
The results of stem CO2 efflux and sap flow rates presented in this study are in the range reported in previous studies for coniferous species (Acosta et al. 2008; Čermák et al. 1992; Lagergren and Lindroth 2002; Pokorný 2000; Zha et al. 2004). In our results, stem CO2 efflux presented diurnal and seasonal patterns that were positively significantly correlated with stem temperature in both studied growing seasons; the mean correlation coefficient of stem CO2 and stem temperature was r = 0.87 in both growing seasons. The obtained value of correlation coefficient showed no seasonal trend during growing seasons; however, the functional relationship between stem CO2 efflux and stem temperature showed great differences in month time steps due to the tight correlation of the variables.
It is widely accepted that temperature is a key environmental factor, in that the rate of CO2 efflux generally increases exponentially with an increase in temperature (Amthor 1984; Stockfors 2000). However, in our study, this fact was only valid when volumetric soil water content conditions were over the threshold (12 %) according to the permanent wilting point. In our experiment, stem CO2 efflux during dry conditions also presented a diurnal pattern that strongly correlated with soil water content at depths of 5 cm (r = 0.87) and 20 cm (r = 0.90) instead of air or stem temperature. This indicates that soil water content has an influence on stem CO2 efflux during dry conditions that superimpose the direct effect of air and stem temperature. Generally, lower volumetric soil water content is likely to induce a restriction in tree growth and transpiration through stomatal closure (Bonal and Guehl 2001) as well as a decrease in autotrophic respiration (Epron et al. 2004; Sotta et al. 2006), including stem CO2 efflux and/or heterotrophic respiration through decreased microbial activity in the soil (Li et al. 2006). During moist days, we determined higher stem CO2 efflux in comparison with dry days. We consider that these differences in stem CO2 efflux could be explained by higher substrate supplies (Xu et al. 2004) and sap flow in the moist period compared to the dry period. Moreover, during dry conditions, short-term soil water deficit was observed. The above-mentioned observations suggest that some of the CO2 produced in the stem that is not diffused via the bark due to slow diffusion could be transported upward by the transpiration stream and released by the upper branches (Acosta et al. 2011).
As we expected, the diurnal and seasonal patterns of sap flow in our experiment were highly correlated with incident global radiation (r = 0.88). The pattern of sap flow was caused mainly by the dynamics of incident global radiation. On the other hand, when the volumetric soil water content was lower than evapotranspiration atmospheric demands, we observed a steep decrease in sap flow, which occurred at the end of the growing season of 2011. Köstner et al. (1998) pointed out that variation in sap flow rates can also be explained on the basis of variation in the leaf area index of individual trees, heterogeneity in soil conditions and methodological differences in sap flow measurements. In previous studies on sap flow and influencing factors at our experimental site, Pokorný and Šalanská (2001) demonstrated that sap flow rate was influenced not only by tree size but also by the climatic conditions course (including incident global radiation and soil water availability). Moreover, they pointed out that low soil water content values limited stand transpiration, particularly under high radiation conditions.
Obviously, in our experiment, soil water content showed a significant influence on sap flow dynamics. We found significantly lower sap flow dynamics during dry days compared to moist days (Kurjak et al. 2012; Lagergren and Lindroth 2002). Some studies pointed out that when sap flow increased, a decrease of stem CO2 efflux occurred, which was mainly due to the fact that CO2 at the measured position was moved upward with the transpiration stream (Acosta et al. 2008; Gansert and Burgdorf 2005; McGuire et al. 2007). In our experiment, a different trend was observed; maxima of stem CO2 efflux and sap flow were the highest during the culmination of stem temperature and incident global radiation. However, when R 10 and sap flow were considered separately, the lowest values of R 10 were observed during daytime, which was the inverse of the sap flow dynamics.
It is well known that CO2 within the stem originates from respiring cells in the stem and roots, and is transported in the transpiration stream, representing the aqueous transport of CO2 (Levy et al. 1999). Other studies of this issue (Bowman et al. 2005, McGuire and Teskey 2004) assumed a mass balance describing CO2 external and internal fluxes of stem respiration and pointed out that sap flow would transport both the dissolved CO2 in the xylem stream and the CO2 produced by stem live tissue upwards into other locations of the stem or leaves in the canopy. Furthermore, Sorz and Hietz (2006) pointed out that slow diffusion through coniferous wood is probably related to the length of the conducting elements, i.e. the frequency at which gas has to diffuse through the cell wall, rather than to tracheid diameter. Thus, this slow diffusion positively influenced CO2 enrichment of the transpiration stream. Moreover, a recent study by Bloemen et al. (2012) with 13CO2 labelling demonstrated that the internal transport of root-respired CO2 can be assimilated in stems, branches and leaves of large trees in the field and confirmed the transport of CO2 via the transpiration stream and xylem sap. However, it is less clear which environmental factors affect this process. The results from our correlation analysis between R 10 and sap flow versus environmental control factors confirmed that the impact of stem temperature, incident global radiation and soil water content were the most important factors (Kurjak et al. 2012; Lagergren and Lindroth 2002; Stockfors 2000).
The cross-correlation analysis between R 10 and sap flow showed a significant correlation between them on moist days but not dry days during the first year of the experiment, while the opposite situation occurred in the second year of the experiment (significant correlation on dry days, but not moist days). On the other hand, Cerasoli et al. (2009) reported no significant relationship between stem CO2 efflux and sap flow and explained this as a result of great barriers between the wood and the inner bark that restrict the diffusion of CO2 from the transpiration stream. Our results showed the inconsistent correlation between stem CO2 efflux and sap flow indicating the complexity of the effect of sap flow on the stem CO2 efflux between growing seasons. The influence of microclimatic and biotic effects on the studied correlation seems to be more complex. Nevertheless, our results reflected the importance of performing additional and deeper studies of the relationship between the stem CO2 efflux and the sap flow, as well as environmental factors influencing both processes, to better understand the overall forest carbon budget. Moreover, the identification of drivers of this relationship could be used to improve models of carbon budget.
References
Acosta M, Pavelka M, Pokorný R, Janouš D, Marek MV (2008) Seasonal variation in CO2 efflux of stems and branches of norway spruce trees. Ann Bot 101:469–477
Acosta M, Pokorny R, Janous D, Marek MV (2010) Stem respiration of Norway spruce trees under elevated CO2 concentration. Biol Plant 54:773–776
Acosta M, Pavelka M, Tomášková I, Janouš D (2011) Branch CO2 efflux in vertical profile of Norway spruce tree. Eur J Forest Res 4:649–656
Amthor JS (1984) The role of maintenance respiration in plant growth. Plant, Cell Environ 7:561–569
Bloemen J, McGuire MA, Aubrey DP, Teskey RO, Steppe, K (2012) Transport of root-derived CO2 via the transpiration stream affects aboveground tree physiology. In EGU General Assembly Conference Abstracts. Vol. 14:4391
Bonal D, Guehl J (2001) Contrasting patterns of leaf water potential and gas exchange responses to drought in seedlings of tropical rainforest species. Funct Ecol 15:490–496
Bowman WP, Barbour MM, Turnbull MH, Tissue DT, Whitehead D, Griffin KL (2005) Sap flow rates and sapwood density are critical factors in within- and between-tree variation in CO2 efflux from stems of mature Dacrydium cupressinum trees. New Phytol 167:815–828
Cannell MGR, Thornley JHM (2000) Modelling the components of plant respiration: some guiding principles. Ann Bot 85:45–54
Cerasoli S, McGuire MA, Faria J, Mourato M, Schmidt M, Pereira JS, Chaves MM, Teskey RO (2009) CO2 efflux, CO2 concentration and photosynthetic refixation in stems of Eucalyptus globulus (Labill.). J Exp Bot 60:99–105
Čermák J, Nadezhdina N (1998) Sapwood as the scaling parameter- defining according to xylem water content or radial pattern of sap flow? Ann For Sci 55:509–521
Čermák J, Cienciala E, Kučera J, Hallgren JE (1992) Radial velocity profiles of water flow in trunks of Norway spruce and oak and the response of spruce to severing. Tree Physiol 10:367–380
Cohen Y, Takeuchi S, Nozaka J, Yano T (1993) Accuracy of sap flow measurement using heat-balance and heat pulse methods. Agron J 85:1080–1086
Eklund L, Lavigne MB (1995) Restricted lateral gas movement in Pinus strobus branches. Trees-Struct Funct 10:83–85
Epron D, Nouvellon Y, Roupsard O, Mouvondy W, Mabiala A, Saint-André L, Joffre R, Jourdan C, Bonnefond JM, Berbigier P (2004) Spatial and temporal variations of soil respiration in a Eucalyptus plantation in Congo. For Ecol Manage 202:149–160
Gansert D, Burgdorf M (2005) Effects of xylem sap flow on carbon dioxide efflux from stems of birch Betula pendula Roth. Flora-Morphol, Distrib, Funct Ecol Plant 200:444–455
Janssens IA, Lankreijer H, Matteucci G, Kowalski AS, Buchmann N, Epron D, Pilegaard K, Kutsch W, Longdoz B, Grunwald T, Montagnani L, Dore S, Rebmann C, Moors EJ, Grelle A, Rannik U, Morgenstern K, Oltchev S, Clement R, Gudmundsson J, Minerbi S, Berbigier P, Ibrom A, Moncrieff J, Aubinet M, Bernhofer C, Jensen NO, Vesala T, Granier A, Schulze ED, Lindroth A, Dolman AJ, Jarvis PG, Ceulemans R, Valentini R (2001) Productivity overshadows temperature in determining soil and ecosystem respiration across European forests. Glob Change Biol 7:269–278
Köstner B, Granier A, Cermak J (1998) Sapflow measurements in forest stands: methods and uncertainties. Ann For Sci 55:13–27
Kurjak K, Střelcová L, Ditmarová T, Priwitzer J, Kmet´ M, Homolák V, Pichler D (2012) Physiological response of irrigated and non-irrigated Norway spruce trees as a consequence of drought in field conditions. Eur J For Res 131:1737–1746
Lagergren F, Lindroth A (2002) Transpiration response to soil moisture in pine and spruce trees in Sweden. Agric For Meteorol 112:67–85
Levy PE, Meir P, Allen SJ, Jarvis PG (1999) The effect of aqueous transport of CO2 in xylem sap on gas exchange in woody plants. Tree Physiol 19:53–58
Li Y, Xu M, Zou X (2006) Heterotrophic soil respiration in relation to environmental factors and microbial biomass in two wet tropical forests. Plant Soil 281:193–201
Maier CA, Clinton BD (2006) Relationship between stem CO2 efflux, stem sap velocity and xylem CO2 concentration in young loblolly pine trees. Plant, Cell Environ 29:1471–1483
Malhi Y, Baldocchi DD, Jarvis PG (1999) The carbon balance of tropical, temperate and boreal forests. Plant, Cell Environ 22:715–740
Martin TA, Teskey RO, Dougherty PM (1994) Movement of respiratory CO2 in stems of loblolly pine (Pinus taeda L.) seedlings. Tree Physiol 14:481–495
McGuire MA, Teskey RO (2004) Estimating stem respiration in trees by a mass balance approach that accounts for internal and external fluxes of CO2. Tree Physiol 24:571–578
McGuire MA, Cerasoli S, Teskey RO (2007) CO2 fluxes and respiration of branch segments of sycamore (Platanus occidentalis L.) examined at different sap velocities, branch diameters and temperatures. J Exp Bot 58:2159–2168
Pavelka M, Acosta M, Janousˇ D (2004) A new device for continuous CO2 flux measurement in forest stand. Ekologia-Bratislava 23(Suppl. 2):88–100
Pfanz H, Aschan G (2000) The existence of bark and stem photosynthesis and its significance for the overall carbon gain: an eco-physiological and ecological approach. Prog Bot 62:477–510
Pfanz H, Aschan G, Langenfeld-Heyser R, Wittmann C, Loose M (2002) Ecology and ecophysiology of tree stems: corticular and wood photosynthesis. Naturwissenschaften 89:147–162
Pokorný R (2000) Sap Flux Simulation and Tree Transpiration Depending on Tree Position within Stand of Different Densities. Phyton 40:157–162
Pokorný R, Šalanská P (2001) Sap flux of dominant trees under low soil water availability. Beskydy 14:99–106
Pokorný R, Tomášková I (2007) Allometric relationships for surface area and dry mass of young Norway spruce aboveground organs. J For Sci 53:548–554
Rayment MB, Loustau D, Jarvis PG (2002) Photosynthesis and respiration of black spruce at three organizational scales: shoot, branch and canopy. Tree Physiol 22:219–229
Ryan MG, Gower ST, Hubbard RM, Waring RH, Gholz HL, Cropper WP, Running SW (1995) Woody tissue maintenance respiration of 4 conifers in contrasting climates. Oecologia 101:133–140
Ryan MG, Lavigne MB, Gower ST (1997) Annual carbon cost of autotrophic respiration in boreal forest ecosystems in relation to species and climate. J Geophysic Res-Atmos 102:28871–28883
Sorz J, Hietz P (2006) Gas diffusion through wood: implications for oxygen supply. Trees-Struct Funct 20:34–41
Sotta ED, Veldkamp E, Guimaraes BR, Paixao RK, Ruivo MLP, Almeida SS (2006) Landscape and climatic controls on spatial and temporal variation in soil CO2 efflux in an Eastern Amazonian Rainforest, Caxiuana, Brazil. For Ecol Manage 237:57–64
Sprugel DG, Benecke U (1991) Measuring woody-tissue respiration and photosynthesis. Tech Approach For Tree Ecophysiol 1:329–355
Stockfors J (2000) Temperature variations and distribution of living cells within tree stems: implications for stem respiration modeling and scale-up. Tree Physiol 20:1057–1062
Teskey RO, McGuire MA (2002) Carbon dioxide transport in xylem causes errors in estimation of rates of respiration in stems and branches of trees. Plant, Cell Environ 25:1571–1577
Wieser G, Bahn M (2004) Seasonal and spatial variation of woody tissue respiration in a Pinus cembra tree at the alpine timberline in the central Austrian Alps. Trees-Struct Funct 18:576–580
Wittmann C, Pfanz H, Loreto F, Centritto M, Pietrini F, Alessio G (2006) Stem CO2 release under illumination: corticular photosynthesis, photorespiration or inhibition of mitochondrial respiration? Plant, Cell Environ 29:1149–1158
Xu L, Baldocchi DD, Tang J (2004) How soil moisture, rain pulses, and growth alter the response of ecosystem respiration to temperature. Glob Biogeochem Cycle 18:GB4002
Zha TS, Kellomaki Z, Wang KY, Ryyppo A, Niinisto S (2004) Seasonal and annual stem respiration of Scots pine trees under boreal conditions. Ann Bot 94:889–896
Author contribution statement
R.B. and E.D. carried out the field manual measurements, M.A., R.B. and E.D. contributed with writing of manuscript, data processing and analysis and P.R. and M.P. contributed with evaluation and analysis of the data.
Acknowledgments
This work was supported by projects CzechGlobe (CZ.1.05/1.1.00/02.0073), CzeCOS/ICOS (LM2010007) and COST CZ LD 14063.
Conflict of interest
The authors declare that they have no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Pfanz.
Rights and permissions
About this article
Cite this article
Bužková, R., Acosta, M., Dařenová, E. et al. Environmental factors influencing the relationship between stem CO2 efflux and sap flow. Trees 29, 333–343 (2015). https://doi.org/10.1007/s00468-014-1113-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-014-1113-z