Abstract
Key message
Neck cells in Ginkgo biloba contribute to archegonial opening through morphological changes and might be involved in the production of fertilization liquid to attract spermatozoids toward archegonia.
Abstract
Neck cells are an essential part of the archegonium in archegoniate gymnosperms, but their function in the sexual reproductive process remains unclear, particularly in zoidogamous gymnosperms. To clarify the structural characteristics of neck cells and their role in fertilization, we examined the neck cells of Ginkgo biloba L. by means of scanning electron microscopy and transmission electron microscopy. The two curved inner neck cells, which are covered imbricately by the two turgid outer neck cells, were pushed to two sides during fertilization, which indicated that morphological changes in these cells contribute to archegonial opening. The neck cells contained many secretory organelles with some material accumulated outside the cell wall, thus the neck cells might be involved in the production of fertilization liquid to attract spermatozoids toward the archegonium. In addition, the surrounding surface cells of the female gametophyte also cooperate to produce the liquid. Taken together, these results indicate that the neck cells provide an effective mechanism by which zoidogamous gymnosperms achieve reproductive success through altering the morphology and cellular physiology of the neck cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neck cells, which are present in all archegoniate plants, are the only efficacious portion of the archegonium through which the male gametophyte enters the archegonium and affects fertilization (Fernando et al. 1998; Ma et al. 1998). Archegoniate plants include most gymnosperms, which can be classified into siphonogamous gymnosperms and zoidogamous gymnosperms. The neck cells affect the development of the male gametophyte in siphonogamous gymnosperms. For example, the body cell mitosis occurs only when the pollen tube is close to the neck cells in Podocarpus totara (Wilson and Owens 1999) and, only after the tip of the pollen tube is in contact with the neck cells, the wall of the pollen tube is digested completely in Taxus baccata (Pennell and Bell 1988). Because of the importance of neck cells in the fertilization process, the developmental process and their structural characteristics have received considerable attention.
The neck cells show some similarities with synergid cells of angiosperms, e.g., both are located close to the egg cell and are important for guidance and development of pollen tubes. Studies focused on the synergids’ functions have shown that the synergids secrete small polymorphic proteins, which attract pollen tubes and induce sperm release (Márton et al. 2005; Capron et al. 2008; Okuda et al. 2009). However, to date few investigations of the function of neck cells have been reported, and particularly the role of neck cells in gymnosperm reproduction remains controversial. Owens and Bruns (2000) showed that neck cells might help to direct pollen tube growth because they have a secretory function in Pinus monticola. However, other studies have found no evidence to prove that neck cells produce chemotactic signals to guide pollen tubes in Pseudotsuga menziesii (Fernando et al. 1998; Ma et al. 1998). Therefore, it is of importance to clarify how the neck cells exert their functions in gymnosperms.
Ginkgo biloba L., which is often regarded as a ‘living fossil’, occupies an important position in seed plant evolution because of its unique morphological traits and primitive evolutionary characteristics shared with early seed plants (Hirase 1896; Zhou and Zhang 1989; Zhou and Wu 2006). Since the spermatozoid was first observed by Hirase (1896), the reproductive biology of G. biloba has attracted much attention. Some studies have noted that the primary neck cell, which originates from unequal division of the archegonial initial, undergoes two divisions to form four cells (Lee 1955; Ji et al. 1999, 2003). During archegonial development, the egg cell is tightly surrounded by jacket cells and other female gametophyte cells and only the inflated neck cells are naked in the archegonial chamber (Wang et al. 2009). Recently, Zhang et al. (2012) showed that the neck cells respond to interaction of the generative cell and central cell since the neck cells are present between the two cells. To further study the roles of the neck cells in the fertilization process in G. biloba, we applied scanning electron microscopy and semi-thin sectioning to examine the morphological and anatomical changes in neck cells during fertilization to reveal the mechanism of archegonial opening. In addition, using ultra-thin sectioning and transmission electron microscopy, we investigated the involvement of the neck cells and surrounding surface cells of the female gametophyte in secretion of the fertilization liquid to guide the spermatozoids toward the egg cell.
Materials and methods
Plant materials
Healthy female G. biloba trees were selected from the Ginkgo experimental station at Yangzhou University, Yangzhou, China (32°20′N, 119°30′E). About 30 ovules of G. biloba were collected weekly from early June to middle August in 2008 and 2009. In late August, the period for fertilization, ovules were collected daily.
Sampling and stereomicroscope observation
The female gametophyte was carefully dissected with a razor blade, the portion close to the micropylar side was observed using a Motic SMZ-168-TL stereomicroscope (Motic China Group Co. Ltd., Xiamen, China), and digital images were captured with a Nikon Coolpix 4500 camera (Jin et al. 2012). After that, the samples were fixed with different fixation solutions based on different purposes as follows and stored in a refrigerator at 4 °C until use.
Scanning electron microscopic observation
Samples were fixed in an improved FAA solution (70 % alcohol: glacial acetic acid: formaldehyde; 90:5:5) for 1 week at 4 °C, then dehydrated in a graded ethanol series (30, 50, 70, 80, 90, 95, and 100 %, 15 min at each step). After infiltration twice in 100 % acetone, the samples were moved through an acetone–isoamyl acetate series (2:1, 1:1, 1:2, and 0:1 acetone:isoamyl acetate, at 15 min intervals). After critical point drying, the specimens were coated with a layer of gold and observed under a S-4800 field-emission scanning electron microscope (Hitachi, Tokyo, Japan) at 15 kV accelerating voltage (Jin et al. 2010).
Semi-thin sectioning
Specimens were fixed in 2.5 % glutaraldehyde in 0.2 M phosphate buffer (pH 7.2) for 1 month at 4 °C. The archegonia were dehydrated through a graded ethanol series (20, 40, 60, 80, 90, 95, and 100 %, 10 min at each step) and infiltrated with propylene oxide on a gyrator before embedding in Spurr resin at 70 °C for 12 h (Jin et al. 2011). Semi-thin sections (1 μm thick) were cut with a glass knife, stained with 1 % (w/v) toluidine blue, and observed under a Carl Zeiss microscope (Zeiss Axioskop 40, Carl Zeiss Shanghai Company Ltd, Shanghai, China).
Transmission electron microscopic observation
Dissected archegonia were prefixed in 2.5 % glutaraldehyde in 0.2 M phosphate buffer (pH 7.2) for 24 h at 4 °C, postfixed in 1 % OsO4 for 4 h at room temperature, then dehydrated in an ethanol series (50, 70, 80, 90, 95, and 100 %, 10 min at each step). After replacement with acetone (100 % twice, 10 min for each change), the specimens were infiltrated and embedded in Spurr resin (Jin et al. 2011). Sections (70 nm) were cut with a diamond knife using a Leica ultramicrotome (Leica Microsystems GmbH, Wetzlar, Germany), picked up on Formvar-coated grids, and stained for 30 min with uranyl acetate and for 15 min with lead citrate. The stained sections were examined with a Philips Tecnai 12 transmission electron microscope (JEOL Ltd, Tokyo, Japan).
Results
Morphological characteristics of neck cells before and during fertilization
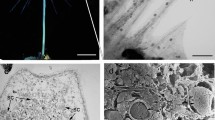
Neck cells of G. biloba originated from the archegonial primary cell, which underwent an unequal division to give rise to a large central cell and a small primary neck cell (Wang et al. 2009). The developmental process of the neck cell is summarized in Table 1. Our observations began at 55 days after pollination, when the primary neck cell divided perpendicularly into two semicircular and flattened cells. The secondary neck cells were distinctly larger than the surrounding cells (Fig. 1a). The secondary neck cells gradually expanded and enlarged during archegonium development (Fig. 1b), and progressively projected toward the archegonial chamber (Fig. 1c). In mid-August, the two elliptic-shaped secondary neck cells had almost attained their maximum volume and significantly protruded into the archegonial chamber (Fig. 1d). About 125 days after pollination, both secondary neck cells underwent oblique division to produce four cells (Fig. 1e). With further development, the four cells were arranged imbricately, consisting of two outer and two inner cells (Fig. 1f), with a layer of translucent membrane covering the surface. When approaching fertilization, the two outer cells jutted slightly upwards to induce the four cells to separate from their neighbors, and thus to produce a split among the neck cells, termed the archegonial opening (Fig. 1g). In this period, several globular particles were observed on the surface of neck cells, with cytoplasmic debris apparent at the opening. Occasionally, the neck cells were covered by clusters of material that adhered closely to the archegonial opening (Fig. 1h). At this stage, the neck cells were extremely turgid and the archegonial opening became enlarged. After fertilization, all four neck cells were distinctly shriveled (Fig. 1i), although the archegonial opening was still visible, which was irregular in shape with a maximum diameter of about 20 μm (Fig. 1i, arrowhead).
SEM micrographs of neck cells in G. biloba. a Formation of the secondary neck cells about 55 days after pollination. b, c Gradual enlargement of the secondary neck cells. d About 115 days after pollination, neck cell protrusion is obvious. e Oblique division of the secondary neck cells. f Four neck cells arranged imbricately and covered by a translucent membrane. g Separation of the four neck cells along their connecting walls. Some globular particles are on the cell surface and cytoplasmic debris is apparent at the opening. h Clustered material adhering to the neck cells. Arrowhead indicates the archegonial opening. i After fertilization, all neck cells are shriveled, but the archegonial opening (arrowhead) is still visible. j Micropylar part of female gametophyte before fertilization. k Micropylar part of the female gametophyte after fertilization. Archegonial openings (arrowhead) form opposite the tentpole. Cw cell wall, In inner neck cell, Nc neck cell, On outer neck cell, T tentpole. Bars a–i 50 μm, j, k 200 μm
In general, before the oblique division, each female gametophyte usually comprised two archegonia, which were distributed around a column of female gametophyte tissue known as the tentpole (Figs. 1j, 2a). Interestingly, the two neck cells were a similar distance from the tentpole before division (Fig. 1j), and the opening between the four neck cells that developed after the oblique division formed frequently opposite the tentpole (Fig. 1k). In addition, among the large number of female gametophytes we observed, 6.75 % of the female gametophytes were found to have three or more archegonia, and <1.125 % of the neck cells appeared to be abnormal. For example, Fig. 2c shows the micropylar end of a female gametophyte with one tentpole and two archegonia, but one of the archegonia was observed to have two pairs of secondary neck cells (Fig. 2d). Figure 2e shows four neck cells positioned side-by-side, which may be derived from two perpendicular divisions of the primary neck cell, and Fig. 2f and g, respectively, shows two and three pairs of neck cells arranged in close proximity to each other.
Anatomical changes to neck cells during oblique division
About 115 days after pollination, just before oblique division of the neck cell, the archegonium was well developed and composed of one central cell, two neck cells, and a layer of jacket cells (Fig. 3a). The swollen neck cell contained a large nucleus and numerous starch grains (Fig. 3b). At this stage, the wall of the central cell was extremely thick except for some thinner areas adjacent to the jacket cells (Fig. 3c, d), which represents the site of exchange of materials between the central cell and the jacket cell, whereas the wall of the central cell adjacent to the neck cell was relatively thin (Fig. 3b). Oblique division of each neck cell gave rise to a cell wall between the outer and inner cell. The central cell also underwent an unequal division to form an egg cell and a ventral canal cell (which disappeared quickly). The nucleus of the egg cell was located in the upper part of the cell near the neck cells, and part of the cytoplasm protruded toward the neck cells (Fig. 3e). Serial sections indicated that the inner neck cell was concave in shape; in Fig. 3e the inner neck cell showed a compressed appearance with the wall adjacent to the egg cell curved toward the outer neck cell, and in Fig. 3f the inner neck cell displayed a decreased area and seemed nearly dissociated into two parts; in Fig. 3g only a small part of the inner neck cell was visible, and the outer neck cell was in contact with the egg cell. After fertilization, when the sperm entered the archegonium, the neck cells almost lost their swollen shape and all organelles were indiscernible (Fig. 3h).
Anatomical structure of neck cells in G. biloba. a A well-developed archegonium before oblique division of the neck cells. b A neck cell just before oblique division. c, d Sunken areas (arrows) in the wall of central cell. e–g Serial sections of neck cells after oblique division. h Neck cell after entry of a sperm into the archegonium. Cc central cell, Cw cell wall, Ec egg cell, In inner neck cell, Jc jacket cell, Nc neck cell, N nucleus, On outer neck cell, S starch grain, Sp sperm. Bars a 50 μm, b, c, e–h 20 μm, d 5 μm
Ultrastructure of neck cells before fertilization
About 85 days after pollination, the neck cell contained abundant mitochondria, ribosomes and some rough endoplasmic reticulum (RER) (Fig. 4a–c). Adjacent to the wall were dotted some electron-dense materials enclosed by a single membrane (Fig. 4a, b, arrows). Occasionally, parallel-arranged RER was apparent in the cytoplasm with numerous ribosomes scattered between them (Fig. 4c). In this period, lipid droplets could be observed in the cytoplasm (Fig. 4c). About 115 days after pollination, the dictyosomes and RER had increased markedly in abundance in the neck cell (Fig. 4d, e). The dictyosomes were well developed with distinct stacks and vesicles (Fig. 4d) and the dilated RER was distributed throughout the cytoplasm (Fig. 4e). Plastids containing starch grains also were increased in number and size (Fig. 4e, f). Several pockets of cytoplasm enclosed by double membranes were apparent in the neck cell (Fig. 4f, arrows). These vesicles seemed to dock, fuse and unload their contents in the space between the cell wall and plasma membrane (Fig. 4g). After unloading, the vesicle was recycled back to the cytoplasm and seemed to vanish progressively (Fig. 4g). Just before oblique division, the number of small vesicles increased in the neck cells (Fig. 4h), and abundant organelles accumulated in the central cell along the thin wall near the neck cell (Fig. 4h). In addition, gray-colored material accumulated outside the neck cells (Fig. 4h, inset image, arrow). It was notable in this period that the starch grains in plastids were gradually degraded (Fig. 4i). Plasmodesmata were completely absent in the wall of the neck cells, but the dictyosomes were still distinct (Fig. 4j). After the oblique division, the cytoplasm of the neck cells contained some mitochondria, dictyosomes and scattered plastids (Fig. 4k). With formation of the archegonial opening, the four neck cells contained many small vacuoles, and some small vacuoles fused to form larger vacuoles occupying most of the cell volume, and consequently the cytoplasm was confined to a narrow peripheral layer surrounding the vacuoles (Fig. 4l).
Ultrastructure of neck cells in G. biloba. a–c Ultrastructure of neck cells at about 85 days after pollination. a Mitochondria were numerous with various shapes and distinct cristae. Arrows indicate small vesicles. b Small vesicles (arrows) distributed adjacent to the cell wall. Position indicated by small black box in inset image. c Parallel-arranged rough endoplasmic reticulum and some lipid droplets in the cytoplasm. d–g Ultrastructure of neck cells at about 115 days after pollination. d Many mitochondria and dictyosomes (arrows) scattered near the nucleus. e Increased abundance of rough endoplasmic reticulum and starch grains. f Cystic structures (arrows) in the cytoplasm. g Inclusions of cystic structures were unloaded into the space between the cell wall and plasmalemma. h–j Ultrastructure of neck cells just before oblique division. h Abundant vesicles in the cytoplasm. Gray-colored material (arrow) outside the neck cell wall is shown in the inset image. i Degradation of starch grains in plastids. j Degenerated mitochondria. k–l Ultrastructure of neck cells after oblique division. k Cytoplasm of neck cells. l Organelles restricted to the cell periphery. Cc central cell, Cw cell wall, D dictyosome, Ld lipid droplet, M mitochondrion, Nc neck cell, N nucleus, Pm plasmalemma, Rer rough endoplasmic reticulum, S starch grain, V vesicle, Va vacuole. Bars a, b, j, l 1 μm, c, g 0.5 μm, d–f, i, k 2 μm, h 5 μm
The micropylar part of the female gametophyte consisted of a tentpole, 2–5 archegonia and numerous small cells (Fig. 5a). During female gametophyte development and until fertilization, the surface cells of the tentpole and the female gametophyte around the neck cells were filled with lipid droplets (Fig. 5b, c). After fertilization, the density of lipid droplets in these cells decreased considerably (Fig. 5d, e). Moreover, in the surface cells of the tentpole, dictyosomes were distributed around the cell periphery (Fig. 5d), and a large number of vesicles were evident in the surface cells of the female gametophyte (Fig. 5e).
Ultrastructure of surrounding surface cells of the tentpole and female gametophyte during fertilization. a Micropylar part of female gametophyte. b Surface cell of tentpole filled with lipid droplets before fertilization. c Surface cells of female gametophyte containing numerous lipid droplets before fertilization. d Surface cells of tentpole showing decreased lipid droplets and increased dictyosomes (arrows) after fertilization. e Lipid droplets (arrows) were less frequent in surface cells of female gametophyte after fertilization. Ar archegonium, Cw cell wall, Fgs surface cells of female gametophyte, M mitochondrion, Nc neck cell, N nucleus, S starch grain, T tentpole, Ts surface cells of tentpole. Bars a 200 μm, b, d 2 μm, c, e 5 μm
Discussion
Neck cells facilitate opening of archegonia in G. biloba during fertilization
Neck cells are essential to enable the male gametophyte to enter the archegonium and achieve fertilization in gymnosperms. The pollen tube grows toward the archegonium and enters the archegonium between the neck cells in siphonogamous gymnosperms (Wang 1948; Pennell and Bell 1988; Owens et al. 1995). During the process of male gamete entry, some pollen tubes destroy the neck cells (Owens and Morris 1998), some grow between the neck cells because of the comparatively large intercellular space (Burlingame 1915), and some grow through the previously degenerated neck cells (Owens and Morris 1990). In addition, Owens et al. (1995) reported that pollen tubes may enter the archegonium through female gametophyte tissue other than the neck cells. Nevertheless, such entry points damage the egg cells and consequently fail to accomplish fertilization. In contrast to siphonogamous plants, opening of the neck cells in zoidogamous gymnosperms, such as cycads, during fertilization is initiated by osmotic changes; increasing turgor pressure in each cell may lead to cell expansion and consequent separation of the cell walls (Norstog 1972; Takaso et al. 2013). After fertilization, neck cells can also block the archegonial opening to avoid multiple sperm entering the egg cells (Steyn et al. 1996). In the present investigation, we observed that the two turgid outer neck cells, which imbricately covered the two inner cells, jutted upwards to create the archegonial opening during fertilization, and after fertilization all four neck cells degenerated. Although the neck cells in the above-mentioned gymnosperms are inconsistent in their arrangement and behavior during fertilization, they have important functions in the sexual reproduction process in all of the species.
In G. biloba, the spermatozoids move toward the archegonia via their flagella after their release from the suspended pollen tube. Simultaneously, to facilitate entry of the spermatozoid, some cytoplasm of the egg cell protrudes through the neck of the archegonium (Lee 1955; Zhang et al. 2012). Although earlier studies revealed that neck cells are essential for the spermatozoids to enter the archegonium, little information is available on the archegonial opening process. Moreover, the two inner neck cells cannot be directly observed because they are invariably covered by the two elliptic-shaped outer neck cells. Here, using serial sectioning, we observed that the two outer cells were extremely turgid, whereas the two inner cells were concave in shape. On the basis of these findings we hypothesized that, after division, the two inner cells are curved opposite to each other, with the visible portions in contact and the concealed portions separate, and all of the neck cells are in contact with the egg cell (Fig. 6a, b). During fertilization, the four neck cells separated from each other in the region of contact, which ultimately led to opening of the archegonium (Fig. 6c, d), indicated that the neck cells formed intercellular space to enable the spermatozoids entering the archegonium. Importantly, formation of the archegonial opening involved morphological changes in the neck cells: the outer cells were extruded toward the archegonial chamber and the inner cells were pushed to two sides (Fig. 6c, d). Both changes might be effected by the protrusion of the egg cell cytoplasm. Because the neck cells do not separate to a large extent, the archegonial opening would correspondingly be oblique rather than perpendicular to the archegonium. Taken together, we proposed the mechanism for archegonial opening in G. biloba that the neck cells have uniquely morphological characteristic, and they would undergo distinctively structural changes which contribute to the archegonial opening during fertilization.
Schematic illustration of neck cell morphology in G. biloba after oblique division. a Four neck cells of an archegonium after oblique division before archegonial opening. b Perspective drawing of a, showing part of inner neck cells (pink) concealed by outer neck cells (green). c Neck cells during archegonial opening (arrowhead). d Perspective drawing of c, showing the two inner neck cells forced to two sides. Arrowhead indicates the archegonial opening. In inner neck cell, On outer neck cell (color figure online)
Neck cells of G. biloba have a secretory function to facilitate fertilization
It has long been considered that the neck cells in archegoniate plants are equivalent to the synergid cells in angiosperms, as both cell types are located close to the egg cell and may act as a canal for growth of the male gametophyte. Most synergid cells contain a unique structure, the filiform apparatus, which greatly increases the surface area of the plasma membrane and assists the synergid cells to absorb and transport metabolites (Kasahara et al. 2005; Punwani et al. 2007; Okuda et al. 2009) and direct pollen tube growth (Higashiyama et al. 2001; Higashiyama 2002). In addition, one of the two synergid cells may initiate programmed cell death for pollen tube entry and sperm release (Huang and Russell 1992). Studies of the fertilization process in some archegoniate species indicate that neck cells show similar characteristics with synergids, such as attraction of pollen tubes and degeneration during fertilization (Ottley 1909; Owens and Molder 1979; Owens and Morris 1991). In the present study, we observed that the archegonia were frequently located around the tentpole, with the neck cells close to the egg cell protruding toward the archegonial chamber, which implied that the neck cells of G. biloba probably exert functions in the guidance of the male gametophytes.
In contrast to most gymnosperms, in which pollen tubes show a more or less similar behavior of growth toward the neck cells, pollen tubes of G. biloba do not show such behavior and instead branch many times in the intercellular space of the pollen chamber, by which they may absorb nutrients and help to anchor themselves (Gifford and Lin 1975). Before fertilization in G. biloba, the unbranched end of the pollen tube swells and bursts to release the spermatozoids into the archegonial chamber (Friedman 1987). In the present study, abundant mitochondria, dictyosomes, vesicles and RER were observed in the neck cells just before fertilization. Although we did not observe plasmodesmata in the neck cell walls, we found many vesicles and wrinkled plasmalemma, which indicated that the neck cells in G. biloba were biochemically active and dynamic during fertilization, and had a secretory function. Considering that the archegonial chamber is filled with liquid during spermatozoid release, we propose that the neck cells play a role in production of the liquid. The secretory characteristics and function of neck cells have been reported also in P. menziesii (Owens and Morris 1991), interior spruce (Picea) (Runions and Owens 1999), and Larix decidua (Rafińska and Bednarska 2011). However, the size of neck cells is not in proportion to the volume of fertilization liquid, therefore, we speculate that an additional origin(s) is involved in the secretion. Our transmission electron microscopic observations on the surface cells of the tentpole and female gametophyte clearly showed a marked decrease in number of lipid droplets during fertilization, which implied that these cells were probably the greatest source of the liquid. Taken together, we suggest that the neck cells only contribute a small portion of the total liquid volume, and the secretions may be involved in signal transduction that function as an attractant to guide the spermatozoids toward the archegonia.
References
Burlingame LL (1915) The morphology of Araucaria brasiliensis. III. Fertilization, the embryo, and the seed. Bot Gaz 59:1–39
Capron A, Gourgues M, Neiva LS, Faure JE, Berger F, Pagnussat G, Krishnan A, Alvarez-Mejia C, Vielle-Calzada JP, Lee YR, Liu B, Sundaresan V (2008) Maternal control of male-gamete delivery in Arabidopsis involves a putative GPI–anchored protein encoded by the LORELEI gene. Plant Cell 20:3038–3049
Fernando D, Owens JN, von Aderkas P (1998) In vitro fertilization from co-cultured pollen tubes and female gametophytes of Douglas fir (Pseudotsuga menziesii). Theor Appl Genet 96:1057–1063
Friedman WE (1987) Growth and development of the male gametophyte of Ginkgo biloba within the ovule (in vivo). Am J Bot 74:1797–1815
Gifford EM, Lin J (1975) Light microscope and ultrastructural studies of the male gametophyte in Ginkgo biloba: the spermatogenous cell. Am J Bot 62:974–981
Higashiyama T (2002) The synergid cell: attractor and acceptor of the pollen tube for double fertilization. J Plant Res 115:149–160
Higashiyama T, Yabe S, Sasaki N, Nishimura Y, Miyagishima S, Kuroiwa H, Kuroiwa T (2001) Pollen tube attraction by the synergid cell. Science 293:1480–1483
Hirase S (1896) On the spermatozoid of Ginkgo biloba. Bot Mag Tokyo 10:325–328
Huang B, Russell S (1992) Female germ unit: organization, isolation, and function. Int Rev Cytol 140:233–292
Ji CJ, Yang X, Li ZL (1999) Morphological studies on megaspore formation in Ginkgo biloba. Acta Bot Sin 41:219–221
Ji CJ, Aniwar M, Fang JY (2003) Current status on female gametophyte and fertilization in Ginkgo biloba. Acta Bot Boreal Occident Sin 23:158–163
Jin B, Wang L, Wang J, Teng NJ, He XD, Mu XJ, Wang YL (2010) The structure and roles of sterile flowers in Viburnum macrocephalum f. keteleeri (Adoxaceae). Plant Biol 12:853–862
Jin B, Wang L, Wang J, Jiang KZ, Wang Y, Jiang XX, Ni CY, Wang YL, Teng NJ (2011) The effect of experimental warming on leaf functional traits, leaf structure and leaf biochemistry in Arabidopsis thaliana. BMC Plant Biol 11:35
Jin B, Zhang L, Lu Y, Wang D, Jiang XX, Zhang M, Wang L (2012) The mechanism of pollination drop withdrawal in Ginkgo biloba L. BMC Plant Biol 12:59
Kasahara RD, Portereiko MF, Sandaklie-Nikolova L, Rabiger DS, Drews GN (2005) MYB98 is required for pollen tube guidance and synergid cell differentiation in Arabidopsis. Plant Cell 17:2981–2992
Lee CL (1955) Fertilization in Ginkgo biloba. Bot Gaz 117:79–100
Ma Y, Weber M, Dumont-BeBoux N, Webber J, von Aderkas P (1998) Megagametophytes of Douglas fir (Pseudotsuga menziesii) and hybrid larch (Larix eurolepis) in culture: multiplication of neck cells and the formation of binucleate cells. Protoplasma 204:219–225
Márton ML, Cordts S, Broadhvest J, Dresselhaus T (2005) Micropylar pollen tube guidance by egg apparatus 1 of maize. Science 307:573–576
Norstog K (1972) Role of archegonial neck cells of Zamia and other cycads. Phytomorphology 22:125–130
Okuda S, Tsutsui H, Shiina K, Sprunck S, Takeuchi H, Yui R, Kasahara RD, Hamamura Y, Mizukami A, Susaki D, Kawano N, Sakakibara T, Namiki S, Itoh K, Otsuka K, Matsuzaki M, Nozaki H, Kuroiwa T, Nakano A, Kanaoka MM, Dresselhaus T, Sasaki N, Higashiyama T (2009) Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature 458:357–361
Ottley AM (1909) The development of the gametophytes and fertilization in Juniperus communis and Juniperus virginiana. Bot Gaz 48:31–46
Owens JN, Bruns D (2000) Western white pine (Pinus monticola Dougl.) reproduction: I. Gametophyte development. Sex Plant Reprod 13:61–74
Owens JN, Molder M (1979) Sexual reproduction of white spruce (Picea glauca). Can J Bot 57:152–169
Owens JN, Morris SJ (1990) Cytological basis for cytoplasmic inheritance in Pseudotsuga menziesii. I. Pollen tube and archegonial development. Am J Bot 77:433–445
Owens JN, Morris SJ (1991) Cytological basis for cytoplasmic inheritance in Pseudotsuga menziesii. II. Fertilization and proembryo development. Am J Bot 78:1515–1527
Owens JN, Morris SJ (1998) Factors affecting seed and cone development in Pacific silver fir (Abies amabilis). Can J For Res 28:1146–1163
Owens JN, Catalano GL, Morris SJ, Aitken-Christie J (1995) The reproductive biology of kauri (Agathis australis). II. Male gametes, fertilization, and cytoplasmic inheritance. Int J Plant Sci 156:404–416
Pennell R, Bell P (1988) Insemination of the archegonium and fertilization in Taxus baccata L. J Cell Sci 89:551–559
Punwani JA, Rabiger DS, Drews GN (2007) MYB98 positively regulates a battery of synergid-expressed genes encoding filiform apparatus-localized proteins. Plant Cell 19:2557–2568
Rafińska K, Bednarska E (2011) Localisation pattern of homogalacturonan and arabinogalactan proteins in developing ovules of the gymnosperm plant Larix decidua Mill. Sex Plant Reprod 24:75–87
Runions CJ, Owens JN (1999) Sexual reproduction of interior spruce (Pinaceae). II. Fertilization to early embryo formation. Int J Plant Sci 160:641–652
Steyn EM, Strydom WF, Botha A (1996) Fertilization and rejection of spermatozoids by egg cells in artificially pollinated ovules of Encephalartos (Zamiaceae). Sex Plant Reprod 9:175–185
Takaso T, Kimoto Y, Owens JN, Kono M, Mimura T (2013) Secretions from the female gametophyte and their role in spermatozoid induction in Cycas revoluta. Plant Reprod 26:17–23
Wang FX (1948) Life history of Keteleeria. I. Strobili, development of the gametophytes and fertilization in Keteleeria evelyniana. Am J Bot 35:21–27
Wang L, Jin B, Lin MM, Lu Y, Teng NJ, Chen P (2009) Studies of the development of female reproductive organs in Ginkgo biloba. Chin Bull Bot 44:673–681
Wilson VR, Owens JN (1999) The reproductive biology of totara (Podocarpus totara) (Podocarpaceae). Ann Bot 83:401–411
Zhang ZM, Clayton SC, Cui KM, Lee CL (2012) Developmental synchronization of male and female gametophytes in Ginkgo biloba and its neck mother cell division prior to fertilization. Physiol Plantarum 147:541–552
Zhou ZY, Wu X (2006) The rise of ginkgoalean plants in the early Mesozoic: a data analysis. Geol J 41:363–375
Zhou ZY, Zhang BL (1989) A middle Jurassic Ginkgo with ovule-bearing organs from Henan, China. Palaeontogr Abt B 211:113–133
Acknowledgments
We gratefully acknowledge the technical advice of Prof. Zhong Wang and Prof. Peng Chen (Yangzhou University, China). This work was financially supported by the National Natural Science Foundation of China (No. 31200145) and the Natural Science Foundation of Jiangsu Province (No. BK2011444).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Lin.
Rights and permissions
About this article
Cite this article
Wang, D., Lu, Y., Zhang, M. et al. Structure and function of the neck cell during fertilization in Ginkgo biloba L.. Trees 28, 995–1005 (2014). https://doi.org/10.1007/s00468-014-1013-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-014-1013-2