Abstract
Key message
Along the stem axis phloem’s sieve elements increase in diameter basally at rates comparable to those of xylem conduits and in agreement with principles of hydraulic optimization.
Abstract
Plant physiology relies on the efficiency of the two long-distance transport systems of xylem and phloem. Xylem architecture comprises conduits of small dimensions towards the stem apex, where transpiration-induced tensions are the highest along the root-to-leaves hydraulic pathway, and widen basally to minimize the path length resistance to water flow. Instead, information on phloem anatomy and allometry is extremely scarce, although potentially relevant for the efficiency of sugar transportation. We measured the hydraulic diameter (Dh) of both xylem conduits and phloem sieve elements in parallel at different heights along the stem of a small tree of Picea abies, Fraxinus excelsior and Salix eleagnos. Dh increased from the stem apex to base in both xylem and phloem, with a higher scaling exponent (b) of sieve elements than that of tracheids in the conifer (0.19 vs. 0.14) and lower than that of vessels in the angiosperms (0.14–0.22 vs. 0.19–0.40). In addition, sieve elements were larger than tracheids in P. abies and narrower than angiosperms vessels at any height along the stem. In conclusion, axial conduit widening would seem to be a key feature of both xylem and phloem long-distance transport architectures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascular plants are living organisms relying on the efficiency of the long-distance transportation network of the xylem to supply water and nutrients absorbed from roots up to the leaves to sustain evapotranspiration and photosynthesis, and of the phloem to deliver the sugar solutions produced with photosynthesis that are necessary for cell respiration and growth to all the living tissues. Evolutionary pressures are, therefore, imposed on vascular plants to withstand the hydrodynamic constraints of distributing liquids over long distances. Water flow through xylem conduits is triggered by foliar transpiration and occurs under tension (i.e., negative pressure) (Angeles et al. 2004). Plants need to withstand this physical metastable state of water because hydraulic failure by xylem cavitation would lead to progressive crown desiccation and ultimately death. Strategies to avoid cavitation involve anatomical adjustments such as reduction in conduit size (Hacke et al. 2001), or biochemical regulation such as stomatal closure (Brodribb and Holbrook 2003; Brodribb et al. 2003). Besides this safety aspect against cavitation, plants need to compensate for the negative effect of increasing in height on the total hydraulic resistance to maintain an efficient transport network during ontogeny (Ryan et al. 2006).
Plants seem to have evolved towards a few universal criteria to cope with the need to sustain foliar transpiration and thus photosynthesis, and to preserve the efficiency of the long-distance transport system from hydraulic failure by cavitation. On the one hand, plants are characterized by widening precise axial pattern of the xylem architecture, where conduits increase in diameter from the stem apex downwards (Anfodillo et al. 2006; Lintunen and Kalliokoski 2010; Olson and Rosell 2012; Petit et al. 2009). This widened design of the whole xylem architecture seems to play a key role in the maintenance of the hydraulic efficiency during ontogeny (i.e., with height growth) because of its effect of buffering the path length effect on the total hydraulic resistance (Becker et al. 2000; Petit and Anfodillo 2009). Indeed, the theoretical power scaling of conduit diameter with tree height determining the independence of the whole tree resistance from the total tree height (West et al. 1999) received substantial support from empirical measurements (Anfodillo et al. 2006, 2013; Olson and Rosell 2012). On the other hand, plants operate physiologically at rates close to the safety limit, as recently evidenced by the convergence towards a universal safety margin against vulnerability to drought-induced cavitation in the different species in the various biomes (Choat et al. 2012).
The threat of excessive water losses with transpiration and prolonged stomatal closure during drought events has recently been considered as the starting point for a cascade of events that lead plants to consume most available carbon to sustain respiration in the different organs/tissues and ultimately die of carbon starvation (McDowell et al. 2008).In this context, the efficiency of the carbohydrates transport system may play a crucial role for plant survival (Sala et al. 2010). Nonetheless, empirical data on whole tree phloem architecture and physiology are scarce (Ryan and Asao 2014), and current knowledge on the phloem function relying on theoretical models substantially lacks this important information (Mencuccini et al. 2011).
Increasing theoretical and empirical evidence supports the hypothesis of a tight link between xylem and phloem circulations (De Schepper and Steppe 2010; Höltta et al. 2009; Knoblauch and van Bel 1998; Köckenberger et al. 1997; Lacointe and Minchin 2008; Lampinen and Noponen 2003; Minchin and Lacointe 2005; Sevanto et al. 2011). According to the Münch phloem counterflow hypothesis, transportation through the sieve cells is determined by a hydrostatic pressure gradient developing along the phloem network between sites of sugar loading (in the leaves), where the increased osmotic potential attracts xylem water inside the phloem, and sites of unloading (living cells of the different organs) (De Schepper et al. 2013). Consequently, hydration plays a key role for the phloem transport, so both the xylem and phloem flow rates correlate well with the foliar transpiration rate (De Schepper et al. 2013; De Schepper and Steppe 2010; Ho and Nichols 1975). In addition, solute unloadings from phloem to xylem seem to be of particular importance for the refilling of embolized xylem conduits as they act as nucleation agents for water absorption (Hölttä et al. 2006; Nardini et al. 2011). On the other hand, high xylem tension would remove water from phloem (Sevanto et al. 2011), thus increasing the overall phloem sap viscosity along the transportation network (Woodruff 2014) with negative effects on flow (Höltta et al. 2009). Furthermore, theoretical model simulations (Höltta et al. 2009) showed that axial variations in sieve element size can play a relevant role in phloem hydraulics and xylem/phloem interactions. In particular, a widened axial pattern of sieve tube diameters would steepen the hydrostatic pressure gradient distally in the phloem network (i.e., towards the leaves), thus speeding up sugars transportation, with a beneficial effect also on the phloem sap viscosity (Höltta et al. 2009; Mencuccini et al. 2011). Instead, a different approach not involving any axial variation in phloem cell dimension predicts a universal allometric scaling of increasing sieve element diameter with tree height for the optimization of sap velocity (Jensen et al. 2011).
Contrary to the abundant data on the xylem architecture and its universal axial conduit widening, little information is available in the literature on structural variations in phloem conduits within a plant. Phloem cross-sectional area was reported to increase with the distal leaf area in trees but not in vines (Ewers and Fisher 1991), suggesting a basal enlargement of phloem tissues where the xylem must also provide mechanical support. Mencuccini et al. (2011) reported similar results for angiosperm trees, whereas the proportion of xylem and phloem functional basal areas to the total leaf area seemed to remain unchanged with increasing diameter at breast height (dbh) in a chronosequence of Scots pine. Data on axial variations of sieve tube dimensions are also scarce. Measurements on single individuals of Eucalyptus globulus revealed no significant axial patterns in sieve tube diameter and length, although both patterns would suggest a size reduction towards the stem apex (Quilhó et al. 2000). Instead, measurements of sieve cell diameter at three points along the stem of mature Norway spruce trees seemed to indicate a reverse axial widening, with diameter increasing distally (Rosner et al. 2001). Lastly, the increase in sieve tube diameter with dbh in chronosequences of different species (Mencuccini et al. 2011) would clearly indicate a height dependence of phloem conduit size and thus a basal axial widening of both xylem and phloem conduits.
The aim of this work was to provide fundamental information on phloem anatomy at a whole tree scale by investigating the axial variation of both xylem and phloem conduits in parallel.
Materials and methods
Three young individuals of three different species representing different wood types were selected near the Center for Alpine Research of the University of Padova (San Vito di Cadore, BL; 1,100 m a.s.l.), in the Dolomites (Eastern Alps, Italy): Picea abies Karst. (conifer), Fraxinus excelsior L. (broadleaved, ring porous wood), Salix eleagnos Scop. (broadleaved, diffuse porous wood). Trees were healthy, without any macroscopic biotic or abiotic damage, and presented a monopodial-like branching architecture with a total height (H) of 4.3, 9.4 and 6.1 m, respectively. After felling, 10 sampling points were identified along the main stem, their distance from the stem apex (L) carefully measured and stem disks extracted. One subsample of each disk encompassing the last annual ring and the bark was cut with a sledge microtome (Reichert) to obtain thin sections of 15–25 μm. Sections were then stained with a solution of safranine (1 % in distilled water) and astrablue (0.5 % in distilled water) and permanently fixed on microscope slides with Eukitt (BiOptica, Milan, Italy). Slides were observed at 40–100× magnification under a light microscope (Leitz, Laborlux S) equipped with a digital camera. For each sample, we took one image for the xylem of the outermost ring, focused on the earlywood where the bigger conduits are found, and one image of the phloem. For each image, the number of conduits measured varied according to ring width, axial variation in conduit density and the different wood/phloem anatomy depending on species (Fig. 1). Table 1 reports the total number of xylem/phloem conduits measured at each of the 10 points along the stem. Before the analysis with WinCell (Régent Instruments Inc., Sainte-Foy, QC, Canada) for the estimate of the cell parameters, images were edited to enhance the contrast and polished from staining residuals or cell contents. For both the outermost xylem ring and the phloem, the averaged hydraulic conduit diameter (Dh) (Kolb and Sperry 1999) was assessed as
where d n is the nth conduit diameter.
Xylem and phloem anatomy at the stem apex and at around 4 m below the apex for the three analyzed trees (P. abies: a, b, c; F. excelsior: d, e, f; S. eleagnos: g, h, i). Left images were taken at the stem apex. (a L = 4 cm, d L = 2 cm, g L = 2.5 cm) and encompass xylem and phloem structures. Right images were taken at around 4 m from the apex (b, c L = 420 cm, e, f L = 400 cm, h, i L = 400 cm). b, e, h are images of the phloem. c, f, i are images of the xylem in the earlywood of the outermost ring
Statistical analyses
The scaling parameters of the power equations used to fit the data were determined from pairwise comparisons of log10-transformed data. Using reduced major axis (RMA) analysis, the scaling exponents and allometric constants were identified as the regression slopes (b-RMA) and y-intercept (a-RMA), respectively. A simple application (Bohonak 2004) was used to compute the regression coefficients and their 95 % confidence intervals by standard methods (Sokal and Rohlf 1981) using a bootstrap procedure with 100,000 replications (Davison and Hinkley 1997).
Results
Anatomical analyses of xylem conduits, tracheids in P. abies and vessels in F. excelsior and S. eleagnos, and phloem sieve elements revealed that both transportation networks are organized according to the basipetal widening of conduits.
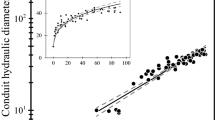
Dh increased with L continuously from the stem apex to the base (Fig. 1) following a power trajectory (Dh = aL b) in both xylem and phloem (Fig. 2; Table 2).
Variation of Dh of xylem (circles) and phloem conduits (triangles) with L for the three analyzed species. Solid and dashed lines are the regression line and the 95 % confidence intervals, respectively (details in Table 2). Linear scale in insets
The increase in Dh of xylem conduits with L varied between the analyzed trees. The degree of axial widening (i.e., the scaling exponent b) was significantly higher for the angiosperms than the conifer (b = 0.40–0.22 vs. b = 0.14), as commonly reported for young trees (Bettiati et al. 2012; Petit et al. 2011). However, it must be highlighted that Dh estimates for conifers is based on a much higher number of replicates than angiosperms, where the number of vessels per unit area strongly differ from that of conifer tracheids (in our case tens of vessels vs. hundreds of tracheids per image).
Phloem sieve elements increase in Dh from the stem apex to the base according to a power scaling with exponents (b) in the range of 0.14–0.22. Differently from the xylem, the axial widening of phloem does not significantly differ between the analyzed trees (see 95 % CI_b in Table 2). Although not significantly different, sieve elements of the conifer seemed slightly wider than those of the angiosperms (Table 2).
On the other hand, conifer and angiosperms do differ in the relative size (Dh) of phloem and xylem conduits (Figs. 1, 2). In P. abies, sieve elements and tracheids showed similar degrees of widening (see 95 % CI_b in Table 1), but the former were nearly double the size all along the stem (Fig. 2). Instead, xylem axial widening was higher in the angiosperms, with vessels being wider and increasing with L at a faster rate than phloem conduits (Figs. 1, 2, 3).
Discussion
Analyses of variation of xylem conduits and phloem sieve elements along the stem revealed the coexistence of stem-to-base xylem and phloem axial widening. The assessed degree of widening (b) occurring in both xylem and phloem is consistent with principles of hydraulic optimization for long-distance transportation networks (Becker et al. 2000; Petit and Anfodillo 2009; West et al. 1999). Optimal xylem widening (b > 0.2) allows the path length resistance to water flow to not vary much with tree height, thus preventing trees from undergoing strong hydraulic and thus physiological limitations (Anfodillo et al. 2006; Petit and Anfodillo 2009). The same principle can be extended to the phloem transportation system, where the mass flow of sugar solutions through the phloem architecture also minimizes the path length effect on the hydrodynamic resistance by an optimal widening of sieve elements.
Axial xylem widening varied between the analyzed trees, but was in line with previous reports, where young angiosperms often showed steep increases in vessel diameters from the stem apex downwards, whereas in young conifers a sub-optimal widening with respect to hydraulic optimization was common (b < 0.2) (Anfodillo et al. 2006; Bettiati et al. 2012; Petit et al. 2011, 2009). Degrees of xylem widening of b > 2 were commonly found in trees exhibiting fast rates of height growth during the early stages of ontogeny (Anfodillo et al. 2006). Instead, sub-optimal widening may reflect situations of high competition for soil water and nutrients (Petit et al. 2011, 2008).
The most relevant result of this study is that we clearly found axial phloem widening in the analyzed trees. Data in the literature on the scaling of phloem cell dimensions are scarce and contradictory. Previous studies reported every kind of axial pattern and the contrary. Quilhó et al. (2000) reported no evident axial patterns in sieve tube dimensions, although from a closer view the most apical sieve elements were clearly smaller. Instead, an even base-to-apex (i.e., reverse) phloem widening was observed by Rosner et al. (2001), although they analyzed only three points along the stem (base, crown insertion and middle crown).
Other apparently contradictory results are those reporting different allometric scaling of sieve element dimension with tree size. Some studies (Jensen et al. 2012; Mencuccini et al. 2011) reported an increase in sieve element diameter with increasing tree height, whereas an opposite pattern was observed in a chronosequence of Douglas-fir trees spanning from 2 to 60 m (Woodruff 2014). In this context, our results may help put the pieces together and propose an explanation for the observed differences. Most studies in the field of phloem anatomy and physiology simply neglected the potential axial widening of phloem sieve elements, except for Mencuccini et al. (2011). We can thus hypothesize that the scaling of diameter at the stem base with the total path length (i.e., tree height) observed in Mencuccini et al. (2011) and Jensen et al. (2012) is simply the effect of the phloem basal widening. Indeed, these observed patterns are not in disagreement with Woodruff (2014), who simply reported anatomical modifications of phloem anatomy of apical branches as a response to the increased hydraulic constraints with tree height. Although our reduced sample size imposes some limitations on the consistency of the conclusions reached due to the inter-specific and intra-specific variability of the estimated scaling parameters not being assessed, the importance of this study is that we provide for the first time, through a series of measurements of phloem conduit dimensions at several points along the stem, empirical support for the existence of an axial basal conduit widening in both xylem and phloem networks.
These results need further empirical support from anatomical measurements of trees of different species, heights and environments for a better assessment of scaling variability and response mechanisms of xylem/phloem anatomy to increased physical and environmental constraints.
To conclude, we hope that this study will encourage future studies attempts at measuring or modeling phloem anatomy and physiology to consider the existence of potential axial trends. Together with further investigations on the link between xylem and phloem hydrodynamics, this type of anatomical information is of fundamental importance for physiological modeling and their implementation would help to better understand forest responses to extreme events and perhaps to forecast on a more solid basis the potential ecosystem dynamics under climate change.
References
Anfodillo T, Carraro V, Carrer M, Fior C, Rossi S (2006) Convergent tapering of xylem conduits in different woody species. New Phytol 169:279–290
Anfodillo T, Petit G, Crivellaro A (2013) Axial conduit widening in woody species: a still neglected anatomical pattern. IAWA 34:352–364
Angeles G, Bond B, Boyer JS, Brodribb T, Brooks JR, Burns MJ, Cavender-Bares J, Clearwater M, Cochard H, Comstock J, Davis SD, Domec JC, Donovan L, Ewers F, Gartner B, Hacke U, Hinckley T, Holbrook NM, Jones HG, Kavanagh K, Law B, Lopez-Portillo J, Lovisolo C, Martin T, Martinez-Vilalta J, Mayr S, Meinzer FC, Melcher P, Mencuccini M, Mulkey S, Nardini A, Neufeld HS, Passioura J, Pockman WT, Pratt RB, Rambal S, Richter H, Sack L, Salleo S, Schubert A, Schulte P, Sparks JP, Sperry J, Teskey R, Tyree M (2004) The cohesion-tension theory. New Phytol 163:451–452
Becker P, Gribben RJ, Lim CM (2000) Tapered conduits can buffer hydraulic conductance from path-length effects. Tree Physiol 20:965–967
Bettiati D, Petit G, Anfodillo T (2012) Testing the equi-resistance principle of the xylem transport system in a small ash tree: empirical support from anatomical analyses. Tree Physiol 32:171–177
Bohonak AJ (2004) RMA: software for reduced major axis regression v.1.17. University of San Diego
Brodribb TJ, Holbrook NM (2003) Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiol 132:2166–2173
Brodribb TJ, Holbrook NM, Edwards EJ, Gutiérrez MV (2003) Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant Cell Environ 26:443–450
Choat B, Jansen S, Brodribb TJ, Cochard H, Delzon S, Bhaskar R, Bucci SJ, Feild TS, Gleason SM, Hacke UG, Jacobsen AL, Lens F, Maherali H, Martínez-Vilalta J, Mayr S, Mencuccini M, Mitchell PJ, Nardini A, Pittermann J, Pratt RB, Sperry JS, Westboy M, Wright IJ, Zanne AE (2012) Global convergence in the vulnerability of forests to drought. Nature 491:752–755
Davison AC, Hinkley DV (1997) Bootstrap methods and their applications. Cambridge University Press, New York
De Schepper V, Steppe K (2010) Development and verification of a water and sugar transport model using measured stem diameter variations. J Exp Bot 61:2083–2099
De Schepper V, De Swaef T, Bauweraerts I, Steppe K (2013) Phloem transport: a review of mechanisms and controls. J Exp Bot 64:4839–4850
Ewers F, Fisher J (1991) Why vines have narrow stems: histological trends in Bauhinia (Fabaceae). Oecologia 88:233–237
Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloch KA (2001) Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126:457–461
Ho LC, Nichols R (1975) The role of phloem transport in the translocation of sucrose along the stem of carnation cut flowers. Ann Bot 39:439–446
Höltta T, Mencuccini M, Nikinmaa E (2009) Linking phloem function to structure: analysis with a coupled xylem-phloem transport model. J Theor Biol 259:325–337
Hölttä T, Vesala T, Perämäki M, Nikinmaa E (2006) Refilling of embolised conduits as a consequence of ‘Münch water’ circulation. Funct Plant Biol 33:949–959
Jensen KH, Lee J, Bohr T, Bruus H, Holbrook NM, Zwieniecki MA (2011) Optimality of the Münch mechanism for translocation of sugars in plants. J R Soc Interface 8:1155–1165
Jensen KH, Liesche J, Bohr T, Schulz A (2012) Universality of phloem transport in seed plants. Plant Cell Environ 35:1065–1076
Knoblauch M, van Bel AJE (1998) Sieve tubes in action. Plant Cell 10:35–50
Köckenberger W, Pope JM, Xia Y, Jeffrey KR, Komor E, Callaghan PT (1997) A non-invasive measurement of phloem and xylem water flow in castor bean seedlings by nuclear magnetic resonance microimaging. Planta 201:53–63
Kolb KJ, Sperry JS (1999) Differences in drought adaptation between subspecies of sagebrush (Artemisia tridentata). Ecology 80:2373–2384
Lacointe A, Minchin PEH (2008) Modelling phloem and xylem transport within a complex architecture. Funct Plant Biol 35:772–780
Lampinen MJ, Noponen T (2003) Thermodynamic analysis of the interaction of the xylem water and phloem sugar solution and its significance for the cohesion theory. J Theor Biol 224:285–298
Lintunen A, Kalliokoski T (2010) The effect of tree architecture on conduit diameter and frequency from small distal roots to branch tips in Betula pendula, Picea abies and Pinus sylvestris. Tree Physiol 30:1433–1447
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739
Mencuccini M, Hölttä T, Martinez-Vilalta J (2011) Comparative criteria for models of the vascular transport systems of tall trees. In: Meinzer FC, Lachenbruch B, Dawson TE (eds) Size- and age-related changes in tree structure and function, vol 4. Springer, Netherlands, pp 309–339
Minchin PEH, Lacointe A (2005) New understanding on phloem physiology and possible consequences for modelling long-distance carbon transport. New Phytol 166:771–779
Nardini A, Lo Gullo MA, Salleo S (2011) Refilling embolized xylem conduits: is it a matter of phloem unloading? Plant Sci 180:604–611
Nepstad DC, Tohver IM, Ray D, Moutinho P, Cardinot G (2007) Mortality of large trees and lianas following experimental drought in an Amazon forest. Ecology 88:2259–2269
Olson ME, Rosell JA (2012) Vessel diameter-stem diameter scaling across woody angiosperms and the ecological causes of xylem vessel diameter variation. New Phytol 197:1204–1213
Petit G, Anfodillo T (2009) Plant physiology in theory and practice: an analysis of the WBE model for vascular plants. J Theor Biol 259:1–4
Petit G, Anfodillo T, Mencuccini M (2008) Tapering of xylem conduits and hydraulic limitations in sycamore (Acer pseudoplatanus) trees. New Phytol 177:653–664
Petit G, Anfodillo T, De Zan C (2009) Degree of tapering of xylem conduits in stems and roots of small Pinus cembra and Larix decidua trees. Botany 87:501–508
Petit G, Anfodillo T, Carraro V, Grani F, Carrer M (2011) Hydraulic constraints limit height growth in trees at high altitude. New Phytol 189:241–252
Quilhó T, Pereira H, Richter HG (2000) Within-tree variation in phloem cell dimensions and proportions in Eucalyptus globulus. IAWA 21:31–40
Rosner S, Baier P, Kikuta S (2001) Osmotic potential of Norway spruce [Picea abies (L.) Karst.] secondary phloem in relation to anatomy. Trees 15:472–482
Ryan MG, Asao S (2014) Phloem transport in trees. Tree Physiol 34:1–4
Ryan MG, Phillips N, Bond BJ (2006) The hydraulic limitation hypothesis revisited. Plant Cell Environ 29:367–381
Sala A, Piper F, Hoch G (2010) Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol 186:274–281
Sevanto S, Höltta T, Holbrook NM (2011) Effects of the hydraulic coupling between xylem and phloem on diurnal phloem diameter variation. Plant Cell Environ 34:690–703
Sokal RR, Rohlf FJ (1981) Biometry. WH Freeman, New York
West GB, Brown JH, Enquist BJ (1999) A general model for the structure and allometry of plant vascular systems. Nature 400:664–667
Woodruff DR (2014) The impacts of water stress on phloem transport in Douglas-fir trees. Tree Physiol 34:5–14
Acknowledgments
The manuscript was inspired and supported by the EU COST Action FP1106 (STReESS). Alan Crivellaro received financial support from the University of Padova (“Assegno di Ricerca Junior” CPDr124554/12).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Adams.
Rights and permissions
About this article
Cite this article
Petit, G., Crivellaro, A. Comparative axial widening of phloem and xylem conduits in small woody plants. Trees 28, 915–921 (2014). https://doi.org/10.1007/s00468-014-1006-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-014-1006-1