Abstract
The application of dendrochronology in (sub)tropical regions has been limited by the difficulty in finding trees with distinct annual rings that can be crossdated. Here, we report successful crossdating of Juniperus procera trees from North Gondar, Ethiopia. The trees form annual rings in response to a unimodal rainfall regime. The selection of mesic locations ensured that the trees did not respond to intra-seasonal weather anomalies. Crossdating was achieved by comparison of the wood anatomy directly on the surface of the core samples and purpose-adapted skeleton plotting. Wood-anatomical anomalies, such as false and indistinct rings, were regarded as potentially replicated features and used in crossdating. COFECHA yielded site-specific mean series inter-correlations between 0.52 and 0.59. AMS radiocarbon dating during the bomb era indicated that dating uncertainty is ±1 year.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crossdating Juniperus procera Hochst. ex Endlicher (African Pencil Cedar) has been complicated by irregular stem shape, sensitivity of growth ring formation to local conditions, and year-to-year variability in rainfall regime (Conway et al. 1997, 1998; Jacoby 1989; Wils et al. 2009). Successful studies identified annual rings that could be crossdated in J. procera trees from southern Ethiopia (Couralet et al. 2005, 2007; Sass-Klaassen et al. 2008a). Similar, successful studies have also been reported for Acacia species in Ethiopia (Eshete and Ståhl 1999; Gebrekirstos et al. 2008) and for other species in sub-Saharan Africa (e.g. Couralet et al. 2010; Fichtler et al. 2004; Schöngart et al. 2006; Therrell et al. 2006; Trouet et al. 2006; Worbes et al. 2003).
Juniperus is an important genus in tree-ring networks in the Mediterranean basin, the Middle East and Asia, (e.g. Bräuning 2001; Grießinger et al. 2008; Touchan et al. 1999, 2005, 2007; Touchan and Hughes 1999; Treydte et al. 2006). The development of successful crossdating strategies for J. procera is important to extend these networks into Africa. This will allow various dendroecological studies to take place, such as age determination and climate reconstruction in a region where high-resolution palaeoclimate records are underrepresented (Gasse 2000; Jansen et al. 2007; Verschuren 2004).
Crossdating aims to synchronise growth ring series by aligning patterns in ring widths (e.g. Pilcher 1990; Stokes and Smiley 1968) and/or other growth ring variables, such as isotopes (Roden 2008). It is assumed that these growth patterns are caused by growth-limiting environmental factors that affect all trees in a similar way. If the assumption that the rings are annual cannot be made a priori, such a hypothesis should be tested a posteriori by, e.g. radiocarbon dating (Wils et al. 2009; Worbes and Junk 1989).
To facilitate the crossdating process, numerous methodologies have been developed, such as skeleton plotting (Stokes and Smiley 1968), visual comparison of samples or ring width curves (Pilcher 1990), and the detection of pointer years (Schweingruber et al. 1990). In skeleton plotting, increment cores are described by bar graphs in which the height of the bar indicates the narrowness of the ring. Hence, narrow rings that tend to be better replicated in other cores than wide rings are identified and can be aligned between different cores, effectively identifying pointer years (replicated, distinctly narrow tree rings).
After the growth rings have been aligned provisionally, the association between growth ring variables can be evaluated using statistical tools, such as the Gleichläufigkeitswert (Eckstein and Bauch 1969), t values (Baillie and Pilcher 1973; Hollstein 1980; Munro 1984; Wigley et al. 1987) and the computer program COFECHA (Grissino-Mayer 2001; Holmes 1983). COFECHA standardises raw ring width series and subsequently evaluates the statistical significance of the correlation of each whole series and series segment (by default 50-ring) with a master series. The master series can be entered into the program separately or calculated by averaging all other series under consideration (Grissino-Mayer 2001). Standardisation is aimed at isolating the high-frequency variation in the ring width series, which is most strongly replicated in other trees (Stokes and Smiley 1968). Default standardisation involves fitting of a spline (rigidity 32 rings, frequency response 50% at a wavelength of 32 rings) and autoregressive modelling (Grissino-Mayer 2001). The ring width series are transformed logarithmically to reduce the skewness of the data and give more weight to narrow rings that tend to be replicated more strongly in other trees (Schweingruber et al. 1990; Stokes and Smiley 1968).
Because the actual process of crossdating is difficult to describe, only brief methods and summary statistics tend to be stated. For example, in sub-Saharan Africa, successful studies of crossdating tropical trees report mean series inter-correlations between ring width series varying from 0.24 (Trouet et al. 2006) to 0.63 (Therrell et al. 2006). More discourse on the practice of crossdating tropical trees would be beneficial, as it would create opportunities to learn from each other’s experiences and improve practices and methodologies.
Radiocarbon dating can be applied to test the synchrony of aligned ring patterns and the annual nature of the growth rings (e.g. Dezzeo et al. 2003; Mozeto et al. 1988; Norström et al. 2005; Robertson et al. 2004, 2006; Wils et al. 2009; Worbes and Junk 1989). In the Northern Hemisphere, the radiocarbon calibration curve displays a ‘plateau’ in the period AD 1650–1955, which limits the potential to obtain accurate radiocarbon dates during this period (Stuiver et al. 1998). However, a high peak occurs after AD 1955, related to atmospheric nuclear bomb tests in the period AD 1945–1963 (Dai and Fan 1986). After AD 1963 the atmospheric radiocarbon concentration declined sharply due to the incorporation of radiocarbon in other reservoirs of the carbon cycle (Nydal et al. 1979). As the largest nuclear bomb tests were performed at Novaya Zemlya, Russia (72°N, 53°E) (Dai and Fan 1986), the peak varies with latitude. Therefore, a local or regional dataset of atmospheric radiocarbon concentration measurements is required to allow highly accurate dating after AD 1955.
In this paper we discuss crossdating of J. procera trees from northern Ethiopia. Building on experience from Wils et al. (2009), we develop a conceptual model to capture the crossdating process. We test the hypothesis that the crossdated growth rings are annual by radiocarbon dating.
Materials and methods
Study area
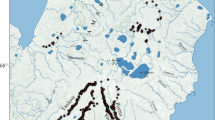
From a map displaying the climate and rainfall patterns in Ethiopia and Eritrea (after EMA 1988; Wils et al. 2009, 2010a, b), it is evident that the lowlands experience hot and dry climates, whereas the highlands experience cool and wet climates (Fig. 1). To increase the chance of obtaining annually datable tree rings, the study sites were located well inside the area experiencing a pronounced unimodal rainfall regime of summer rains, that is, in the administrative zone North Gondar, north of Lake Tana (sites B, E, K, M and W in Fig. 1).
Climate and rainfall patterns in Ethiopia and Eritrea (after EMA 1988; Wils et al. 2009, 2010a, b). Study sites are indicated: church compounds Debrebirkan Selassie (B), Kuskuam (K) and Woken-Woybila-Mariam (W), and forests Debre Kidane-Mihret (E) and Gomia-Mariam (M). Study sites of other authors are also indicated: Doba forest (A) (Wils et al. 2009) and Denkoro forest (D) (Wils 2009), Menagesha-Suba forest (G), Adaba-Dodola forest (O) and Shashemene plantation (V) (Couralet et al. 2005; Sass-Klaassen et al. 2008a), and the Abernosa woodlands (R) (Gebrekirstos et al. 2008)
Climate in Northwest Ethiopia is characterised by monsoonal rainfall from June to September. A small amount of additional rain may occur occasionally in December. Mean precipitation is highest in July (304 mm) and August (270 mm) (Fig. 2, AD 1965–1990). Mean total annual precipitation is 1,177 mm. Mean monthly temperature varies from 18.0°C in August to 22.5°C in April, but the difference in temperature between day and night can be over 20°C.
Sampling of trees took place in three enclosed church compounds and two secondary forests. In the church compounds, protection by the Ethiopian Orthodox Church had ensured the survival of J. procera trees for up to almost three centuries. Two church compounds, Debrebirkan Selassie (B: 12°37′N, 37°29′E) and Kuskuam (K: 12°37′N, 37°27′E), are located inside the urban area of the city of Gondar (Fig. 1), and are regarded as one site (BK). The third church compound, Woken-Woybila-Mariam (W: 13°1′N, 37°46′E; Fig. 3a), and the forests, Debre Kidane-Mihret (E: 13°9′N, 37°55′E) and Gomia-Mariam (M: 13°7′N, 37°58′E), are located in the vicinity of the town of Debark, 55–70 km northeast of Gondar (Fig. 1).
All sites are well-drained and vary in altitude from 2,250 to 2,975 m a.s.l. Soils are fertile and developed on Tertiary volcanic rocks. Vegetation consists of freestanding trees, mostly J. procera and some Podocarpus falcatus, and sparse grasses. The church compounds are flat to undulating spaces and are used for various purposes including cattle grazing, religious gatherings and tourism. Low tree branches have been cut off. Debrebirkan Selassie church (B) was built in AD 1681 or 1682 (1674 of the Ethiopian calendar) and Kuskuam church (K) in AD 1740 or 1741 (1733 of the Ethiopian calendar) (pers. comm. local priests and people). Debre Kidane-Mihret (E) and Gomia-Mariam (M) are secondary, monodominant forests consisting of J. procera, growing on a gentle northeast facing slope and the upper part of a south facing slope (30°), respectively, and are used for cattle grazing.
Study species
Juniperus procera (Fig. 3b) is an evergreen conifer belonging to the family of the Cupressaceae (Friis 1992). Growth rings are formed in response to the alternation of wet and dry seasons (Conway et al. 1998). The rings may be annual if there is only one cycle of wet and dry seasons per year (Couralet et al. 2005; Sass-Klaassen et al. 2008a), but intra-seasonal anomalies in weather conditions can prevent the formation of distinct rings, particularly when the trees are growing in exceptionally well-drained and windy environments (Wils et al. 2009). If seasonality in climate is less pronounced, J. procera trees tend to form indistinct ring boundaries and/or multiple rings per year that do not allow successful crossdating (Jacoby 1989; Wils et al. 2009). The formation of growth rings in juniper can be highly irregular and asymmetric. Consequently, for dendrochronological analysis either stem discs or multiple cores from relatively straight, regular stems are required.
Previous work has shown that J. procera tree-ring variables, such as ring width and isotopic signature, can be related to variation in moisture availability (Couralet et al. 2005; Krishnamurthy and Epstein 1985; Sass-Klaassen et al. 2008a). Other benefits of the species for dendrochronology include excellent preservation of the heartwood, life spans of up to several centuries and the use of its wood for construction purposes, which yields future potential for substantial chronology extension.
Sampling and sample preparation
In May 2007, 32 trees from five sites were successfully cored at three different cardinal positions, using a 5 mm diameter increment borer (Table 1). The sampled trees had relatively straight and regular trunks. It was not possible to acquire permission to collect stem discs. The cores were prepared using abrasive paper wrapped around a flexible eraser with progressively finer grit sizes (ISO 120-grit (106–125 μm) to ISO 800-grit (20.8–22.8 μm) (Orvis and Grissino-Mayer 2002)).
Ring boundary detection, crossdating and ring width measurement
The process of crossdating was developed using experience from Wils et al. (2009), who report that crossdating by comparison of ring width curves (Pilcher 1990) may be misleading, because it draws the attention of the researcher to general ring width patterns that do not necessarily contain or emphasise the information required for successful crossdating. Wils et al. (2009) suggest that comparison of the wood anatomy directly on the surface of the samples and skeleton plotting (Stokes and Smiley 1968) may provide a more robust way of crossdating, as these methodologies divert the researcher’s attention to pointer years. These methodologies also allow simultaneous alignment of wood-anatomical anomalies, such as false or indistinct rings. As these anomalies are caused by environmental factors that probably affected other trees as well, they can be regarded as potentially replicated features. The crossdating process adapted in this way is schematised in Fig. 4.
The three samples taken from each tree were crossdated by direct comparison of the wood samples under a microscope. Simultaneously, purpose-adapted skeleton plots were drawn for each increment core. Extremely narrow rings were regarded as likely false rings, and indicated by a question mark between the lines of the squared paper. Indistinct ring boundaries were judged from the degree to which they were replicated, and from the change in tracheid size, tracheid morphology and thickness of the tracheid cell walls, and marked by the abbreviations ‘db’ [density variation, ring may be part of bark-ward ring] or ‘(2,3)di’ [(2 or 3) density variation(s) inside ring]. Exclamation marks were added if doubt was above average. Other wood-anatomical anomalies, such as deformed tracheids and tracheids filled with coloured, probably phenolic substances, were recorded in a notebook.
After samples were crossdated within-tree, composite skeleton plots were drawn for each tree, and ring widths were measured with a precision of 0.01 mm using a Velmex measuring stage and the computer program TSAP-Win (Rinn 2003). For each site, trees were crossdated by aligning the composite skeleton plots. Additional to narrow rings, wood-anatomical anomalies were also regarded as a potentially replicated feature. The computer program COFECHA (Holmes 1983) was run to examine the statistical quality of the association between the aligned series. The main statistics used were the t test significance levels of the correlation coefficients between the individual series or series segments and the master series of the other aligned series. Weakly associated series were re-examined by sample inspection, comparison of skeleton plots and ring width curves, and re-runs of COFECHA.
Composite skeleton plots were drawn for each site and compared with each other. Site-specific master series calculated by COFECHA were also compared with each other. Mismatches were re-examined thoroughly. The crossdating process as described and illustrated should be regarded as its general structure.
Radiocarbon dating
AMS radiocarbon dating was performed on growth rings 14 (provisional date AD 1993) and 42 (provisional date AD 1965) from a Gomia-Mariam tree core (M8A) at the 14CHRONO Centre of Queen’s University Belfast (Wils et al. 2010a). Two atmospheric radiocarbon concentration datasets were used for calibration. For the period AD 1963–1969, at the height of the bomb peak, a dataset from Debre Zeit (40 km southeast of Addis Ababa) was available (Nydal and Lövseth 1996). Outside this period a regional dataset was used (Northern Hemisphere zone 3, Hua and Barbetti 2004).
Results
Crossdating
From the available samples, 73 ring width series from 30 trees at 5 sites could be crossdated successfully. Statistics for each site are listed in Table 2. Gomia-Mariam (M) and Debre Kidane-Mihret (E) forests are characterised by young trees with wider growth rings. Relative to the high average mean ring width, the average standard deviation is low. Average mean sensitivity is also low, indicating a relatively complacent growth behaviour. Debrebirkan Selassie (B) and Kuskuam (K) church compounds are characterised by old trees with narrower growth rings. Relative to the low average mean ring width, the average standard deviation is high. Average mean sensitivity is also high, indicating a relatively sensitive growth behaviour. Woken-Woybila-Mariam church compound (W) represents an intermediate situation. Relative to the high average mean ring width, the average standard deviation is high as well. Average mean sensitivity is intermediate.
In summary, sites with young trees (M and E) consist of trees with wider and more complacent growth rings than sites with old trees (B and K). Therefore, differences in mean ring width and mean sensitivity are at site level associated with tree age, reflecting similar observations in temperate regions (Fritts 1976). However, this association is often lacking within trees, as individual tree samples do not show a clear trend of declining ring width with age. The differences in mean ring width and mean sensitivity are therefore primarily caused by differences in site conditions.
The forests (M and E) are located on a slope where down-slope groundwater flow may provide an additional supply of moisture. Furthermore, they suffer less anthropogenic disturbance than the church compounds (B, K and W), particularly regarding the cutting-off of branches. Due to the urban location of sites B and K, wood harvesting may have been more prevalent than at the rural site W (also reflected by a higher height of the first living branches (Table 1, at sites B and K). Trees produce wider rings at site E than at site M because of differences in aspect. Site E is northeast facing and therefore moister than the south facing site M. Besides, site E exhibits a more open forest, indicating less interaction with other trees for access to resources.
The major distinction between the forests (M and E) and the church compounds in Gondar (B and K), and the intermediate position of site W, is also evident in the challenges that occurred during sampling, sample preparation, ring boundary detection and crossdating. The old samples from sites B and K were more decayed, and therefore brittle, causing many samples to break during sampling, packaging, transport or sample preparation. During crossdating the major challenge was the identification of missing rings. Samples from sites M and E did not exhibit missing rings, but displayed a large amount of false rings and (partially) indistinct ring boundaries. Both problems decreased towards the bark of the samples. The distinction between true (wide) and false (extremely narrow) rings became stronger, and false rings less abundant. Indistinct ring boundaries were also less frequent and less indistinct near the bark. Near the pith part of the sample had to be discarded in many cases.
Crossdating within-tree is illustrated for tree M9 in Figs. 5, 6 and 7. Figure 5 illustrates the aligning of pointer rings by direct comparison of the wood samples. Figure 6 illustrates the major challenges associated with within-tree crossdating, that is, indistinct ring boundaries (left) and identification of false rings (right). In some cases these features are replicated in multiple samples, facilitating aligning within-tree, but complicating the identification of false rings as such, and hence crossdating between trees. Figure 7 illustrates the recording of a match achieved by direct comparison of wood samples in matching skeleton plots. The multitude and partial synchrony of question marks and comments, particularly near the pith, illustrates the abundance of false rings and indistinct ring boundaries, and the potential they provide for crossdating as a potentially replicated feature.
Illustration of within-tree crossdating by direct comparison of the wood in tree M9. Dark grey lines link pointer rings (ring 9, 17, 24, 35, 40, 50 and 63) and dashed squares indicate the location of the photographs in Fig. 6. Scale bar 10 mm
Partially indistinct ring boundary (left, ring 38–39, marked by dashed lines) and false rings (right, ring 50–51, marked by arrows) in all three cores from tree M9 (for locations see Fig. 5). The boundary between ring 38 and 39 is indistinct in core A and B, but not in core C. False rings occur in all three cores both before and after ring 51. In three pictures a pinprick is visible. Growth is from left to right. Magnification is ×4 and scale bar is 500 μm
Illustration of within-tree crossdating by skeleton plotting in tree M9. Growth is from left to right. Lines indicate narrow rings and the length of the lines indicates the narrowness. A question mark between two rings denotes a false ring; an arrow denotes a false ring that is potentially a true ring; the comment db denotes that the bark-ward ring boundary is indistinct; an exclamation mark expresses above-average doubt; and the comment piece denotes that the sample is broken, and that the marked ring(s) are located in one piece. Shaded bands indicate pointer rings
Crossdating between trees proceeded by aligning tree composite skeleton plots, in which the occurrence of false and indistinct rings appeared fairly consistent between trees, too. Two trees from Kuskuam (K1 and K2) could not be crossdated. In these trees, rings were extremely narrow (average mean ring width, 0.94 mm; average standard deviation, 0.88 mm), suggesting an abundance of missing rings. Results from COFECHA, run after crossdating between trees per site, are summarised in Table 2. Mean series inter-correlations are reasonable and similar for all sites (0.54–0.59). The percentage of 50-ring segments that is insignificantly correlated with the master series of the other crossdated series is low and remarkably similar for all sites (7%). Periodically weak matches may be caused by between-tree variation in response to factors that do not affect all trees, e.g. damage.
Comparisons of site composite skeleton plots and master series with each other showed clear similarities in growth patterns, but correlation coefficients are not always statistically significant (Table 3). The correlation between the master series of sites M and BK (r = 0.45, p < 0.01), 70 km apart, is however a strong indication that those sites are (almost) correctly synchronised, particularly regarding the high number of samples at site M.
Radiocarbon dating
Details on the AMS radiocarbon dating of growth rings 14 and 42 from core M8A are given in Table 4 (Wils et al. 2010a). Ring 42, provisionally dated to 1965, was most probably formed in 1964 or 1966, and ring 14, provisionally dated to 1993, was most probably formed in 1993 (Wils et al. 2010a). It is clear from the radiocarbon dates that the crossdated growth rings of the J. procera trees from North Gondar are mostly annual in nature. Growth rings formed in the main wet season can be identified and crossdated with an accuracy of ±1 year.
Regarding this dating error, the crossdated samples, skeleton plots and ring width series were re-examined to improve the dating during the late twentieth century. This period is crucial in dendroclimatology, as most instrumental data is only then available. The abnormally frequent occurrence of a missing or potentially false ring in or close to ring 20 in trees from the church compounds (B, K and W) appeared to be the most likely dating error. Consequently, 1966 was assumed to be the most likely date for growth ring 42 from core M8A. Applying this assumption, the samples were re-crossdated following the process in Fig. 4. Samples that could not be re-dated by removal of a missing or potentially false ring were assumed to contain a double ring, formed in response to severe stress during the main growing season. In some cases the strongest association was still obtained without adjustments. New statistics for each site are listed in Table 2 and site master series are compared in Fig. 8 and Table 3, showing results similar to those originally obtained. Correlation coefficients are statistically significant (p < 0.1) between the site with the highest replication (M) and all other sites. Insignificant correlation coefficients may be caused by low replication, particularly at site W. In general, most correlation coefficients are fairly low due to the substantial environmental differences between the sites.
Master series spanning the common interval for the sites Gomia-Mariam (M, red, AD 1901–2006), Debre Kidane-Mihret (E, blue, AD 1946–2006) and Woken-Woybila-Mariam (W, thick grey, AD 1852–2006), and for the site cluster Debrebirkan Selassie & Kuskuam (BK, thick black, AD 1717–2006). Pointer years are indicated by arrows (colour figure online)
Discussion
The results are in stark contrast to findings by Wils et al. (2009) and Wils (2009) for J. procera trees from South Wollo (sites A and D in Fig. 1). In these studies, the formation of multiple rings per year in Doba forest (A) and the formation of indistinct rings in Denkoro forest (D) inhibited successful crossdating. This growth behaviour was attributed to the prevailing rainfall regime with strong year-to-year variability in the number of wet and dry seasons, and to extreme sensitivity of growth ring formation to local conditions. Trees in Doba forest, growing on a relatively dry mountain ridge, responded excessively to short spells of drought (Wils et al. 2009), whereas trees in Denkoro forest, growing on a relatively wet slope fed by groundwater flow, maintained cambial activity throughout all dry seasons (Wils 2009). In North Gondar, the intermediate conditions in site water status and the stronger unimodal character of the rainfall regime appear to have provided the conditions for a successful application of dendrochronology.
Other studies in Ethiopia reporting successful crossdating, of J. procera (Couralet et al. 2005; Sass-Klaassen et al. 2008a) and of Acacia species (Gebrekirstos et al. 2008), took place in environments that are to some degree similar (sites G, O, R and V in Fig. 1). Although these study sites experience a minor wet season, this does not seem to inhibit the recognition of annual growth rings. This may be the result of a relatively short and wet minor dry season in most years, preventing a substantial intra-annual slowing-down of tree growth. Furthermore, the study sites are also not located at the ecological limit of the studied species, but at locations with intermediate conditions in water status. This suggests that the principle of site selection near the ecological limit of the studied species, as postulated by Fritts (1976), is not valid in (sub)tropical regions.
The most important challenges encountered during crossdating were false, indistinct and missing rings. These features could be related to ring width in the sense that false and indistinct rings were more frequent in series of wide rings, particularly near the pith, while missing rings were more frequent in series of narrow rings. This association has also been noted by other authors. De Luis et al. (2007) report that false rings are more frequent in wide rings in Pinus halepensis Mill. from Spain. Jacoby (1989) found indistinct rings in vigorous J. procera trees growing in a wet environment in Kenya. As trees tend to grow faster in the early stages of growth, the more frequent occurrence of false and indistinct rings near the pith can be expected. Finally, Sass-Klaassen et al. (2008b) report multiple missing rings in drought-stressed, slow-growing Juniperus excelsa M. Bieb. subsp. polycarpos (K.Koch) Takht from Oman. The observation that false, indistinct and missing rings are potentially replicated was also reported by Cherubini et al. (2003).
The crossdated samples show a strong between-site variation in average mean ring width (1.51–3.71 mm, Table 2). This implies that age estimates are difficult to obtain from diameter measurements only. Similar conclusions were drawn by Therrell et al. (2007) for Pterocarpus angolensis D.C. from southern Africa (Botswana, Mozambique, South Africa, Zambia and Zimbabwe) and by Worbes et al. (2003) for various tree species from Cameroon. Stand-level conditions tend to be important in determining mean ring width.
The crossdated samples also show a strong between-site variation in average mean sensitivity (0.25–0.50, Table 2). To some degree this is inversely related to variations in average mean ring width, except for site M, where relatively narrow rings are associated with the lowest average mean sensitivity. Trouet et al. (2006) report average mean sensitivity values for Brachystegia spiciformis Benth. from Zambia varying from 0.39 to 0.49, which are not related to average mean ring width. Couralet et al. (2010) report average mean sensitivity values for three understory tree species from the Democratic Republic of Congo varying from 0.42 to 0.50, which are also not related to average mean ring width. Fichtler et al. (2004) report average mean sensitivity values for Burkea africana Hook and Pterocarpus angolensis D.C. from Namibia varying from 0.27 to 0.29 and 0.31 to 0.41, respectively. The values reported here are thus in the same range as those published elsewhere.
In theory, high mean sensitivity values may improve the potential for crossdating as strong variation in ring width supports the identification of pointer years and indicates that the trees are sensitive to strongly variable, mostly large-scale meteorological factors. This was recognised by Fritts (1976) and made him suggest to sample trees at the ecological limit of the species, where tree growth is limited most strongly by the factor that determines the particular ecological limit, causing trees to respond sensitively to this factor. However, in the present study this conclusion cannot be reached. Trees with a high mean sensitivity, such as at site BK, exhibit multiple missing rings and, as shown by Wils et al. (2009) for site A, may indicate that trees are too responsive to allow the development of identifiable annual rings. On the other hand, trees with a low mean sensitivity may be associated with the formation of indistinct growth bands, such as at site D (Wils 2009), but also with the occasional formation of false rings that can be identified relatively easy, such as at site M. It appears that intermediate values of mean sensitivity, hence trees growing at mesic locations, provide the most promising potential for crossdating J. procera from Ethiopia.
A relationship between average mean ring width and crossdating potential is not apparent in Table 2. All sites were crossdated with a similar mean series inter-correlation (0.52–0.59) at the upper edge of those published elsewhere [0.24 (Trouet et al. 2006) to 0.63 (Therrell et al. 2006)] and with a similar percentage of insignificant segment correlations (7–10%). Combined with the lack of a clear relationship between average mean ring width and average mean sensitivity and between average mean sensitivity and crossdating potential, this suggests that average mean ring width is not an indicator for crossdating potential. Nevertheless, trees with narrow rings are favourable for the development of long chronologies as increment borers have a limited length.
Conclusion
Seventy-tree cores from 30 J. procera trees from North Gondar, Ethiopia, were crossdated successfully by comparison of the wood anatomy directly on the surface of the samples and purpose-adapted skeleton plotting. Wood-anatomical anomalies, such as false and indistinct rings, were regarded as potentially replicated features, and explicitly and successfully used in crossdating. Statistical evaluation by COFECHA yielded site-specific mean series inter-correlations of 0.52–0.59. AMS radiocarbon dating indicated that dating uncertainty was ±1 year. This success can be attributed to site selection in mesic locations under a pronounced unimodal rainfall regime. Dating could be improved during the late twentieth century, yielding a preliminary tree-ring record that can be used towards, e.g. the development of dendroclimatological reconstructions.
References
Baillie MGL, Pilcher JR (1973) A simple cross-dating program for tree-ring research. Tree-ring Bull 33:7–14
Bräuning A (2001) Climate history of the Tibetan Plateau during the last 1000 years derived from a network of juniper chronologies. Dendrochronologia 19:127–137
Cherubini P, Gartner BL, Tognetti R, Bräker OU, Schoch W, Innes JL (2003) Identification, measurement and interpretation of tree rings in woody species from mediterranean climates. Biol Rev 78:119–148
Conway D, Brooks N, Briffa KR, Desta S, Merrin PD, Jones PD (1997) Exploring the potential for dendroclimatic analysis in northern Ethiopia. University of East Anglia, Norwich, p 38
Conway D, Brooks N, Briffa KR, Merrin PD (1998) Historical climatology and dendroclimatology in the Blue Nile River basin, northern Ethiopia. In: Servat E, Hughes D, Fritsch JM, Hulme M (eds) Water resources variability in Africa during the XXth century. IAHS Publications 252, pp 243–251
Couralet C, Sass-Klaassen U, Sterck F, Bekele T, Zuidema PA (2005) Combining dendrochronology and matrix modelling in demographic studies: an evaluation for Juniperus procera in Ethiopia. For Ecol Manag 216:317–330
Couralet C, Sass-Klaassen U, Sahle Y, Sterck F, Bekele T, Bongers F (2007) Dendrochronological investigations on Juniperus procera from Ethiopian Dry Afromontane Forests. TRACE Dendrosymposium 2006. Forschungszentrum Jülich, pp 73–79
Couralet C, Sterck FJ, Sass-Klaassen U, van Acker J, Beeckman H (2010) Species-specific growth responses to climate variations in understory trees of a Central African rainforest. Biotropica 42:503–511
Dai K-M, Fan CY (1986) Bomb produced 14C content in tree rings grown at different latitudes. Radiocarbon 28:346–349
De Luis M, Gričar J, Čufar K, Raventós J (2007) Seasonal dynamics of wood formation in Pinus halepensis from dry and semi-arid ecosystems in Spain. IAWA J 28:389–404
Dezzeo N, Worbes M, Ishii I, Herrera R (2003) Annual tree rings revealed by radiocarbon dating in seasonally flooded forest of the Mapire River, a tributary of the lower Orinoco River, Venezuela. Plant Ecol 168:165–175
Eckstein D, Bauch J (1969) Beitrag zur Rationalisierung eines dendrochronologischen Verfahrens und zur Analyse seiner Aussagesicherheit. Forstwiss Centralblatt 88:230–250
EMA (1988) National atlas of Ethiopia. Ethiopian Mapping Authority, Addis Ababa
Eshete G, Ståhl G (1999) Tree rings as indicators of growth periodicity of acacias in the Rift Valley of Ethiopia. For Ecol Manag 116:107–117
Fichtler E, Trouet V, Beeckman H, Coppin P, Worbes M (2004) Climatic signals in tree rings of Burkea africana and Pterocarpus angolensis from semiarid forests in Namibia. Trees Struct Funct 18:442–451
Friis I (1992) Forests and forest trees of northeast tropical Africa. HMSO, London
Fritts HC (1976) Tree rings and climate. Academic Press, London
Gasse F (2000) Hydrological changes in the African tropics since the Last Glacial Maximum. Quat Sci Rev 19:189–211
Gebrekirstos A, Mitlöhner R, Teketay D, Worbes M (2008) Climate–growth relationships of the dominant tree species from semi-arid savanna woodland in Ethiopia. Trees Struct Funct 22:631–641
Grießinger J, Bräuning A, Thomas A, Schleser GH (2008) Stable oxygen isotopes in juniper trees from the Tibetan plateau as a proxy for monsoonal activity. TRACE Dendrosymposium 2007. Forschungszentrum Jülich, pp 110–114
Grissino-Mayer HD (2001) Evaluating crossdating accuracy: a manual and tutorial for the computer program COFECHA. Tree-Ring Res 57:205–221
Hollstein E (1980) Mitteleuropäische Eichenchronologie. Verlag Philipp von Zabern, Mainz am Rhein, Germany
Holmes RL (1983) Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull 43:69–78
Hua Q, Barbetti M (2004) Review of tropospheric bomb 14C data for carbon cycle modeling and age calibration purposes. Radiocarbon 46:1273–1298
Jansen E, Overpeck J, Briffa KR, Duplessy J-C, Joos F, Masson-Delmotte V, Olago D, Otto-Bliesner B, Peltier WR, Rahmstorf S, Ramesh R, Raynaud D, Rind D, Solomina O, Villalba R, Zhang D (2007) Palaeoclimate. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK
Jacoby GC (1989) Overview of tree-ring analysis is tropical regions. IAWA Bull 10:99–108
Krishnamurthy RV, Epstein S (1985) Tree ring D/H ratio from Kenya, East Africa, and its palaeoclimatic significance. Nature 317:160–162
Mozeto AA, Fritz P, Moreira MZ, Vetter E, Aravena R, Salati E, Drimmie RJ (1988) Growth rates of natural Amazonian forest trees based on radiocarbon measurements. Radiocarbon 30:1–6
Munro MAR (1984) An improved algorithm for crossdating tree-ring series. Tree-ring Bull 44:17–27
Norström E, Holmgren K, Mörth C-M (2005) Rainfall-driven variations in δ13C composition and wood anatomy of Breonadia salicina trees from South Africa between AD 1375 and 1995. S Afr J Sci 101:162–168
Nydal R, Lövseth K (1996) Carbon-14 measurements in atmospheric CO2 from Northern and Southern Hemisphere sites, 1962–1993. Oak Ridge National Laboratory, Oak Ridge, TE
Nydal R, Lövseth K, Gullicksen S (1979) A survey of radiocarbon variation in nature since the test ban treaty. In: Berger R, Suess HE (eds) Radiocarbon dating. University of California Press, Berkeley, CA, pp 313–323
Orvis KH, Grissino-Mayer HD (2002) Standardizing the reporting of abrasive papers used to surface tree-ring samples. Tree-Ring Res 58:47–50
Pilcher JR (1990) Sample preparation, cross-dating and measurement. In: Cook ER, Kairiukstis LA (eds) Methods of dendrochronology applications in the environmental sciences. Kluwer, Dordrecht, pp 40–51
Rinn F (2003) TSAP-Win, Software for tree-ring measurement analysis and presentation. Rinntech, Heidelberg
Robertson I, Froyd CA, Walsh RPD, Newbery DM, Woodborne S, Ong RC (2004) The dating of dipterocarp tree rings: establishing a record of carbon cycling and climatic change in the tropics. J Quat Sci 19:657–664
Robertson I, Loader NJ, Froyd CA, Zambatis N, Whyte I, Woodborne S (2006) The potential of the baobab (Adansonia digitata L.) as a proxy climate archive. Appl Geochem 21:1674–1680
Roden J (2008) Cross-dating of tree ring δ18O and δ13C time series. Chem Geol 252:72–79
Sass-Klaassen U, Couralet C, Sahle Y, Sterck FJ (2008a) Juniper from Ethiopia contains a large-scale precipitation signal. Int J Plant Sci 169:1057–1065
Sass-Klaassen U, Leuschner HH, Buerkert A, Helle G (2008b) Tree-ring analysis of Juniperus excelsa from the northern Oman mountains. TRACE Dendrosymposium 2007. Forschungszentrum Jülich, pp 99–108
Schöngart J, Orthmann B, Hennenberg KJ, Porembski S, Worbes M (2006) Climate-growth relationships of tropical tree species in West Africa and their potential for climate reconstruction. Glob Change Biol 12:1139–1150
Schweingruber FH, Eckstein D, Serre-Bachet F, Bräker OU (1990) Identification, presentation and interpretation of event years and pointer years in dendrochronology. Dendrochronologia 8:15–38
Stokes MA, Smiley TL (1968) An introduction to tree-ring dating. University of Chicago Press, Chicago
Stuiver M, Reimer PJ, Bard E, Beck JW, Burr GS, Hughen KA, Kromer B, McCormac G, Plicht Jvd, Spurk M (1998) INTCAL98 radiocarbon age calibration, 24,000–0 cal BP. Radiocarbon 40:1041–1083
Therrell MD, Stahle DW, Ries LP, Shugart HH (2006) Tree-ring reconstructed rainfall variability in Zimbabwe. Clim Dyn 26:677–685
Therrell MD, Stahle DW, Mukelabai MM, Shugart HH (2007) Age, and radial growth dynamics of Pterocarpus angolensis in southern Africa. For Ecol Manag 244:24–31
Touchan R, Hughes MK (1999) Dendrochronology in Jordan. J Arid Environ 42:291–303
Touchan R, Meko DM, Hughes MK (1999) A 396-year reconstruction of precipitation in southern Jordan. J Am Water Resour 35:45–55
Touchan R, Xoplaki E, Funkhouser G, Luterbacher J, Hughes MK, Erkan N, Akkemik Ü, Stephan J (2005) Reconstructions of spring/summer precipitation for the Eastern Mediterranean from tree-ring widths and its connection to large-scale atmospheric circulation. Clim Dyn 25:75–98
Touchan R, Akkemik Ü, Hughes MK, Erkan N (2007) May-June precipitation reconstruction of southwestern Anatolia, Turkey during the last 900 years from tree rings. Quat Res 68:196–202
Treydte KS, Schleser GH, Helle G, Frank DC, Winiger M, Haug GH, Esper J (2006) The twentieth century was the wettest period in northern Pakistan over the past millennium. Nature 440:1179–1182
Trouet V, Coppin P, Beeckman H (2006) Annual growth ring patterns in Brachystegia spiciformis reveal influence of precipitation on tree growth. Biotropica 38:375–382
Verschuren D (2004) Decadal and century-scale climate variability in tropical Africa during the past 2000 years. In: Battarbee RW, Gasse F, Stickley CE (eds) Past climate variability through Europe and Africa. Springer, Dordrecht, pp 139–158
Wigley TML, Jones PD, Briffa KR (1987) Cross-dating methods in dendrochronology. J Archaeol Sci 14:51–64
Wils THG (2009) Isotope dendroclimatological studies on Juniperus procera from Ethiopia: towards a reconstruction of Blue Nile baseflow. School of the Environment and Society, PhD thesis. Swansea University, Swansea, pp 364
Wils THG, Robertson I, Eshetu Z, Sass-Klaassen UGW, Koprowski M (2009) Periodicity of growth rings in Juniperus procera from Ethiopia inferred from crossdating and radiocarbon dating. Dendrochronologia 27:45–58
Wils THG, Robertson I, Eshetu Z, Koprowski M, Sass-Klaassen UGW, Touchan R, Loader NJ (2010a) Towards a reconstruction of Blue Nile baseflow from Ethiopian tree rings. The Holocene (in press). doi:10.1177/0959683610365940
Wils THG, Sass-Klaassen UGW, Eshetu Z, Bräuning A, Gebrekirstos A, Couralet C, Robertson I, Touchan R, Koprowski M, Conway D, Briffa K, Beeckman H (2010b) Dendrochronology in Ethiopia: opportunities and challenges. Trees Struct Funct (submitted)
Worbes M, Junk WJ (1989) Dating tropical trees by means of 14C from bomb tests. Ecology 70:503–507
Worbes M, Staschel R, Roloff A, Junk WJ (2003) Tree ring analysis reveals age structure, dynamics and wood production of a natural forest stand in Cameroon. For Ecol Manag 173:105–123
Acknowledgments
The work was funded by a Swansea University studentship and co-funded by the Royal Geographical Society, the Dudley Stamp Memorial Trust (Royal Society) and the Quaternary Research Association. Additional support was obtained from the Association for Tree-ring Research, the University of Arizona, the European Union funded Millennium project (017008-2), the UK Natural Environment Research Council (NE/B501504), Nicolaus Copernicus University and the International Incoming Short Visits programme of the Royal Society. Iain Robertson was supported by the Climate Change Consortium of Wales (C3W). Ute Sass-Klaassen was supported by the Netherlands Organisation for Scientific Research (MEERVOUD grant, 83605030). The Forestry Research Centre (FRC) of the Ethiopian Institute of Agricultural Research (EIAR) in Addis Ababa provided vehicle and other logistic support. The Ethiopian Orthodox Church, Gondar Tourist Office, and Amhara, North Gondar, Debark, Dabat and Gondar governmental offices for agriculture and rural development are acknowledged for support and kind permission to work at the field sites. The National Meteorological Agency (NMA) of Ethiopia (Addis Ababa) is acknowledged for provision of meteorological data. We would like to thank Dr Alemu Gezahegn and Mr Negash Mammo (both FRC directors) for dedicated support, Mr Tesfaye Ayalew for excellent driving and help at the field sites, and Mr Kibruyesfa Sisay and Mr Yeshiber Dagnew for help in the field. We also would like to thank Mrs Nicola Jones and Ms Anna Ratcliffe for drawing maps, Dr Declan Conway and Prof. Keith Briffa (both University of East Anglia) for advice, help and support, and Mgr Hurkmans (Bishop of ‘s-Hertogenbosch, The Netherlands) for writing a letter of recommendation to the authorities of the Ethiopian Orthodox Church.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Braeuning.
Contribution to the special issue “Tropical Dendroecology”.
Rights and permissions
About this article
Cite this article
Wils, T.H.G., Robertson, I., Eshetu, Z. et al. Crossdating Juniperus procera from North Gondar, Ethiopia. Trees 25, 71–82 (2011). https://doi.org/10.1007/s00468-010-0475-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-010-0475-0