Abstract
A dye injection method was used to elucidate the xylem water-conducting pathways of 34 broadleaved evergreen trees growing in southern Japan: two semi-ring-porous, 26 diffuse-porous, five radial-porous and one non-vessel species. The large earlywood vessels in semi-ring-porous species have a water transport function in only the outermost annual ring, as in deciduous ring-porous species. On the other hand, the small vessels in semi-ring-porous species maintain the water transport function in many outer annual rings. For the other xylem-type species, the many vessels in many outer annual rings have a water transport function. In diffuse-porous species, we categorized the water-conducting pattern within the annual rings into two types: d1 type, where water travels through vessels in the whole region; and d2 type, where water travels mainly through the earlywood vessels. The pattern in radial-porous species is similar to that in the d1 type; the pattern in non-vessels species is similar to that in the d2 type. The vessel diameter in radial-porous species is similar to that of the earlywood vessels of semi-ring-porous species. These results suggest that the conduit diameter size is only one of many factors determining the water-conducting pathways of broadleaved evergreen species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The conduits (vessels and tracheids) in woody plants’ stems have evolved for long-distance water transport (Tyree and Zimmermann 2002). However, some conduits in the stem lose their function because of cavitation in their lumina, arising from water or freezing stress (Sperry et al. 1988; Tyree and Sperry 1989; Sperry 1993). Cavitated conduits cannot resume their water-conducting function unless refilling with sap by positive pressure, like root pressure, occurs (Scholander et al. 1955; Sperry et al. 1987, 1988; Ewers et al. 1997). Some reports suggest that tension-induced cavitation is a direct function not of the size of xylem conduits, but of the maximum pore size in their pit membranes (Sperry and Tyree 1988; Jarbeau et al. 1995; Christman et al. 2009). On the other hand, freezing-induced cavitation is strongly correlated to the diameter or volume of xylem conduits (Cochard and Tyree 1990; Sperry and Sullivan 1992; Taneda and Tateno 2005). The anatomical characteristics of the conduits can have a profound impact on the occurrence of cavitation induced by seasonal water and freezing stress.
In general, the more efficient conduits are more vulnerable to cavitation (Tyree et al. 1994; Hacke et al. 2006), and trade-offs between safety and efficiency decide the interspecific differences in water-conducting systems (Maherali et al. 2004). In the deciduous ring-porous species that have large efficient earlywood vessels, the outermost annual ring has a major role in water transport (Chaney and Kozlowski 1977; Ellmore and Ewers 1986) but the vessels in the inner annual rings do not (Utsumi et al. 1996; Umebayashi et al. 2008). On the other hand, the diffuse-porous species that have many small vessels of about the same sizes maintain their water transport function for many years (Baker and James 1933; Greenidge 1958; Chaney and Kozlowski 1977; Anfodillo et al. 1993). These structural differences in xylem correspond to the species’ distributions. The ring-porous species are distributed mainly in the northern temperate zone, while diffuse-porous species grow worldwide (Wheeler et al. 2007). Among diffuse-porous trees, the species growing in cold or dry environments tend to have narrower vessels than those growing in wet-warm environments (Tyree et al. 1994).
Leaf habit is also recognized as one of the main characteristics to explain the growth environments of trees (e.g., DeFries et al. 2000; Givnish 2002). Broadleaved evergreen species can conduct photosynthesis throughout a year (e.g., Miyazawa and Kikuzawa 2005), and dominate in regions where environmental conditions are stable (Chabot and Hicks 1982), whereas deciduous species have achieved high photosynthetic capacities through the evolution to compensate for shorter leaf life span (Chabot and Hicks 1982), and can avoid seasonal stresses (Axelrod 1966; Thomas and Sadras 2001). Seasonal changes in xylem hydraulic conductivity also differ between evergreen and deciduous species. Even in species growing sympatrically in northern temperate zones, the percentage loss of hydraulic conductivity (PLC) of broadleaved evergreen species tends to be lower than that of broadleaved deciduous species (Cavender-Bares and Holbrook 2001; Cavender-Bares et al. 2005).
The leaf habits correspond to vessel arrangement in the xylem in some cases. Most of the ring-porous woods are found in the deciduous species (Gilbert 1940; Hallé et al. 1978; Wheeler and Baas 1991). In this case, the strategy of dropping leaves and losing water transport function during the dormant season and recovering by forming new leaves and large vessels in spring is reasonable to avoid climate stress (Lechowicz 1984). However, diffuse-porous woods are present in both evergreen and deciduous species, and broadleaved evergreen trees have not only diffuse-porous wood, but also semi-ring-porous (e.g., Yamauchi 1976), radial-porous wood, where vessels are arranged radially along rays (e.g., Hirose et al. 2005; Tateishi et al. 2008), and non-vessel wood, where only tracheary elements and parenchyma are present (e.g., Wheeler et al. 1989; Feild et al. 2002). This structural variety in the xylem of evergreen species has been paid little attention from the view of water conduction.
In this study, we anatomically evaluated interspecific differences in the water-conduction pathways of 34 evergreen broadleaved trees grown in Japan. These species have been categorized into four groups based on their xylem conduit arrangement: semi-ring-porous, diffuse-porous, radial-porous, and non-vessel species (Itoh 1995, 1996, 1997, 1998, 1999). We obtained an ascending dye pattern using a dye injection method that can show the pathway of dye uptake in xylem at the tissue level (Sano et al. 2005; Umebayashi et al. 2007, 2008). We compared the functional vessel area with the water distribution pattern in each conduit-arrangement category using cryo-scanning electron microscopy (cryo-SEM, Utsumi and Sano 2007). We evaluated whether the hydraulic diameter of conduits could explain the interspecific variation in the water-conducting patterns of xylem.
Materials and methods
Plant material and growth conditions
The study was carried out from 2005 to 2006 in the Kasuya Research Forest of Kyushu University (KRF; 33°38′N, 130°31′E; elevation 50–100 m; Kasuya, Fukuoka, Japan) and from 2003 to 2006 in the Shiiba Research Forest of Kyushu University (SRF; 32°22′N, 131°09′E; elevation 660–1,000 m; Shiiba, Miyazaki, Japan).
The KRF site is a warm temperate evergreen forest located in the north Kyushu mountain range. The dominant species are evergreen Fagaceae and Lauraceae species. The flora comprised on 135 deciduous and 91 evergreen broadleaved trees (Inoue et al. 2002). According to the temperature and precipitation data for 2005 and 2006 obtained from an experimental watershed (elevation 250 m; cf., Ide et al. 2007) in the KRF, the yearly mean air temperature was 16.0°C. The daily maximum temperatures for 2005 and 2006 were 37.9 and 39.5°C, respectively, and the minimum temperatures for the 2 years were −5.2 and −3.1°C, respectively. The average yearly mean precipitation for the 2 years was 1,880 mm. Mean temperature and humidity during experiment were 29.4 ± 3.1°C and 72.6 ± 12.3%, respectively. Average, maximum and minimum rainfall days per a month were 12.0 ± 1.8 mm, with 13 days in July 2005 and 9 days in August 2006, respectively.
The SRF site is cold temperate deciduous forest located in the central Kyushu mountain range. The dominant species are deciduous Fagaceae, Aceraceae and Betulaceae. The flora comprised on 188 deciduous and 30 evergreen broadleaved trees (Inoue et al. 2002). According to the temperature and precipitation data for 2003–2006 in the SRF, the yearly mean air temperature was 12.7°C. The maximum temperature reached in 2003, 2004, 2005, and 2006 was 33.0, 32.5, 33.4, and 33.5°C, respectively; the minimum temperature was −9.1, −8.5, −8.3, and −10.7°C, respectively. The average annual precipitation over the 4 years was 2,770 mm. Mean temperature and humidity during the experiment were 24.8 ± 2.3°C and 77.1 ± 9.5%, respectively. Average, maximum and minimum rainfall days per month were 18.0 ± 4.2 mm, with 24 days in July 2003 and 13 days in July 2004 and 2005, respectively.
In the 2 forests a total of 34 broadleaved evergreen species (2 semi-ring-porous species, 26 diffuse-porous species, 5 radial-porous species, and 1 non-vessel species) were used for our study. We selected 2 trees with 1–10 cm diameter at breast height for each species (Table 1).
Dye injection and collection of samples
Dye injection experiments were performed from 11:00 to 14:00 on sunny days from mid-July to August in 2003, 2004, 2005, and 2006. A watertight collar was set at height of 1 m and filled with water. The south-facing half of the stem was cut under water and the water was replaced with a 0.2% acid fuchsin aqueous solution that had been filtered through a 0.22-μm filter (GV, Millipore, Billerica, Massachusetts, USA) before the experiment. To keep water on the cut surface and avoid the adhesion of debris, the stem was wrapped with a fine cotton fabric during the replacement. After perfusion for 30 min, sample discs of thickness 2 cm were taken at 0, 10, 30, and 50 cm above the dye injection height and at 50-cm intervals from 100 cm to the maximum height of the stained xylem. Finally, a disc was taken at 10 cm below the maximum height reached by the dye solution. All discs were frozen immediately in liquid N2. The discs stored in liquid N2 were transferred to a laboratory and freeze-dried at −50°C (FZ-1, Labconco, Kansas, USA; Umebayashi et al. 2007, 2008). This process was conducted for two trees of each species.
Observation of dye distribution in xylem
The top surface of the sample discs was trimmed with a razor blade, and we observed macroscopically the xylem dye distribution in the cross-section of the stem. Subsequently, the stained portion of the disc was split into small blocks. These surfaces were planed using a sliding microtome, and we observed the dye distribution at tissue level using a stereomicroscope (SMZ800, Nikon, Tokyo, Japan). The small blocks containing at least one annual ring were trimmed and embedded in an epoxy resin (Sano et al. 2005). Finally, thin sections of thickness 4–7 μm were cut from the blocks with a semi-ultramicrotome (RM2045, Leica, Wetzlar, Germany) and the dye distribution in each xylem cell was observed using a light microscope (Optiphoto-2, Nikon, Tokyo, Japan). Images were obtained from a microscope digital camera system (DP70, Olympus, Tokyo, Japan) attached to the stereomicroscope or light microscope. Dye ascending heights of each annual ring in all species studied were measured, and the rate of decrease from the maximum height was calculated in each annual ring.
Cryo-SEM observation
In August 2006, two sample trees were selected for cryo-SEM observation from one semi-ring-porous species [Dendropanax trifidus (Thunb.) Makino], two diffuse-porous species [Camellia sinensis (L.) O. Kuntze and Ilex pedunculosa Miq.], two radial-porous species (Quercus glauca Thunb. ex Murray and Quercus salicina Blume) and one non-vessel species (Trochodendron aralioides Sieb. et Zucc.). A watertight collar with a plastic funnel was fitted to the stem at a height of 100 cm and filled with liquid N2 to freeze the stem before sunrise (Utsumi et al. 2003). Frozen stem blocks (5 cm long) were removed and placed in liquid N2. The top surfaces of the blocks were trimmed and planed on a sliding microtome in a cold room at −23°C. They were then lightly etched, coated with platinum and carbon in the cryo-SEM system (JSM840-a equipped with CRU-7000, JEOL, Tokyo, Japan; Fujikawa et al. 1988), and observed at 5 kV to assess the xylem water distribution in the outer three, or more, annual rings (Utsumi et al. 1996, Utsumi and Sano 2007).
Definition of vessel arrangement in an annual ring
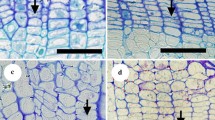
In semi-ring-porous species, we described the earlywood as the region having one row of large-diameter vessels in an annual ring (Fig. 1a, E), and latewood as the other region (Fig. 1a, L). D. trifidus had some clusters of small vessels in the earlywood. In this case, we differentiated the small-vessel earlywood region (Fig. 1b, arrows) from the large-vessel earlywood region (Fig. 1b, V). In diffuse-porous species, we described earlywood as the older half-region of annual rings (Fig. 1c, E) and latewood as the newer half-region (Fig. 1c, L; Umebayashi et al. 2008). In non-vessel species, we defined the latewood region according to Mork’s definition, that is, the region where the twofold radial thickness of the common double wall adjacent to the tracheid is larger than the radial diameter of the tracheid lumen (Fig. 1d, L; Denne 1988); earlywood is the other part (Fig. 1d, E).
Definition of earlywood and latewood in semi-ring-porous, diffuse-porous, and non-vessel species in this study. a Cross-section of Dendropanax trifidus. b Earlywood of D. trifidus. V Large earlywood vessel, arrows small earlywood vessel. c Cross-section of Myrica rubra. d Cross-section of Trochodendron aralioides. E earlywood, L latewood. Scale bars a, c, d 100 μm; b 50 μm
Xylem conduit measurements
In eight species (Table 3), the diameters of vessels and tracheids were measured on the transverse section (10 or 20 μm thickness) of the outer second annual ring at 10 cm above the dye injection height. We obtained images of transverse sections using a digital camera attached to a light microscope, and the images were imported into the Winroof software, version 5.0 (Mitani Corporation, Fukui, Japan), for analysis. We set an oblong grid to locate four vertices on the initial and terminal borders of the annual ring, enclosing 100 or more conduits. All conduits of three grids, that is, 300 or more numbers, were measured for each sample tree. The hydraulic diameter (D) of the lumen was calculated as D = √(xy), where x and y were the major and minor axis diameter of the conduit, respectively. We measured the mean hydraulic diameter, which was defined as [(ΣD 4)/N]1/4, where N was the number of conduits (Tyree and Zimmermann 2002). Finally, we calculated the mean hydraulic diameter of conduits and the mean hydraulic diameter of conduits for each region in an annual ring, as defined above.
Results
In all of the species studied, the number of annual rings stained by dye decreased with increasing stem height (Table 1). Most semi-ring-porous and radial-porous species showed the higher maximum dye injection height than diffuse-porous species. Q. glauca transported the dye solution for the longest distance over all species studied. Figure 2 shows the dye ascending pattern within outer ten annual rings. The dye ascent observed in semi-ring-porous species occurred mainly in the two outermost annual rings and had rapid decrease of ascending height on inner annual rings whereas radial-porous species decreased the ascending height gradually according to the annual ring number (Fig. 2a). Dye ascending pattern in diffuse-porous species varied among species and had no obvious trend. In some species the height of the dye was stable in ten annual rings while in other species the height decreased gradually in inner annual rings (Fig. 2b).
Dye ascending height of outer ten annual rings in two semi-ring-porous and five radial-porous species (a), 26 diffuse-porous and a non-vessel species (b). Narrow lines 20 d1 type species, heavy lines 6 d2 type species, dashed line a non-vessel species. Cs Castanopsis sieboldii, Dt Dendropanax trifidus, Ep Elaeagnus pungens, Le Lithocarpus edulis, Qg Quercus glauca, Qm Quercus myrsinaefolia, Qs Quercus salicina, Ta Trochodendron aralioides. Plots of each species were derived from the average of two samples
In two semi-ring-porous species, only the outer two annual rings were stained at the maximum dye height (Fig. 3a, b). The earlywood vessels and the adjacent latewood vessels were stained in the outermost annual ring (Fig. 3b), and only the terminal latewood vessels were stained in the second annual ring (Fig. 3b, arrow). At 10 cm above the dye injection point, the dye distribution patterns in the latewood of all stained annual rings were similar across all annual rings (Fig. 3c), and many vessels in the whole region of latewood were stained in both of the species (Fig. 3d, arrows). On the other hand, the dye distribution patterns in the earlywood differed between rings: many large earlywood vessels in the outermost annual ring were stained, but none of them in the inner annual rings were stained, in both species (Fig. 3d, e). In D. trifudus, most large earlywood vessels of the outermost annual ring maintained water (Fig. 4a, large allow); many large earlywood vessels of the inner annual rings had no water (Fig. 4a, small arrows). D. trifidus had many small earlywood vessels, and many of these vessels had dye in their lumina in all stained annual rings (Fig. 3e, arrow). Most of the small vessels and fibers had water in the outer three annual rings (Fig. 4a). In Elaeagnus pungens Thunb., at all heights, most wood fibers within the stained annual rings contained dye in their lumina (Fig. 3d; Table 2).
Cross-sectional micrographs of the stained xylem. a Discs at 0.1, 0.5, and 1.0 m from the dye injection site in Dendropanax trifidus. b Outer two annual rings at 150 cm height in Elaeagnus pungens. Arrow stained latewood. c Outer two annual rings at 10 cm height in E. pungens. d Annual ring border area of the second and third annual rings at 10 cm height in E. pungens. Arrows dye in the latewood vessel lumen in both the second and third annual rings. e Earlywood of the second annual ring at 10 cm height in D. trifidus. Arrow dye in the small earlywood vessel lumen. f Discs at 0.1, 0.5, and 1.5 m from the dye injection site in Distylium racemosum. g Outer three annual rings at 150 cm height in D. racemosum. h–j Outer three or four annual rings at 10 cm height in D. racemosum (h), Litsea acuminata (i), and Camellia sinensis (j). k Earlywood of the second annual ring at 10 cm height in Myrica rubra. Arrow dye in the cell lumen adjacent to the vessels. l Discs of 0.1, 1.5, and 2.5 m from the dye injection site in Castanopsis sieboldii. m Outer three annual rings at 2.0 m height in C. sieboldii. n, o Outer three annual rings at 10 cm height in C. sieboldii (n) and Quercus salicina (o). p A vessel in the second annual ring at 10 cm height in Quercus glauca. Arrows dye in the cell lumen adjacent to vessel. q A vessel in the third annual ring at 10 cm height in C. sieboldii. Arrow dye in the vessel lumen. r Discs at 0.1 and 0.3 m from the dye injection site in Trochodendron aralioides. s Outer four annual rings at 0.3 m height in T. aralioides. t Outer three annual rings at 0.1 m height in T. aralioides. u The fourth annual ring at 10 cm height in T. aralioides. Arrows the dye in the earlywood tracheid lumina. g–j, m–o, s, t Arrowheads annual ring boundary. Scale bars a, f, l, r 1 cm; b, c, g, i, j, m–o, s, t 500 μm; d, e, k, p–q 20 μm; h 1 mm; u 25 μm
Cryo-scanning electron micrographs of xylem cross-sections. a The outer three annual rings of Dendropanax trifidus. Large arrow large earlywood vessel having water in the lumina. Small arrows embolic large earlywood vessel. b The second and third annual rings of Ilex pedunculosa. c The second annual ring of Camellia sinensis. d, e The second annual ring of Quercus glauca, Q. salicina, respectively. Arrows embolic vessel. f The second annual ring of Trochodendron aralioides. a–c, f Arrowheads annual ring boundary. Scale bars a 50 μm; b 200 μm; c–f 100 μm
In diffuse-porous species, the number of stained rings varied between and within species at the maximum dye height (Table 1), and eight diffuse-porous species such as Distylium racemosum Sieb. et Zucc. had discontinuous stained annual rings (Fig. 3f). The dye distribution patterns for the inner annual rings were similar at any given height for most species (Fig. 3g, h). In the outermost annual ring, whole region was stained in 15 species (Fig. 3i, j), and terminal region was not stained in 11 species (Fig. 3h). In the inner annual rings, the dye pattern was classified into two types (Table 2): d1, where most or some vessels in the whole region were stained (Fig. 3h, i), and d2, where mainly only earlywood vessels were stained (Fig. 3j). The vessels of the two diffuse-porous species (I. pedunculosa and C. sinensis) showed similar patterns of water distribution among the outer three annual rings. Most vessels of I. pedunculosa had water in both earlywood and latewood (Fig. 4b). Most earlywood vessels in C. sinensis had water (Fig. 4c) and many latewood vessels had no water (Fig. 4c). In 17 species, the wood fibers adjacent to the stained vessels had dye in their lumina (Fig. 3k, arrow; Table 2).
In all of the radial-porous species, the outermost annual ring or several outer annual rings were stained at the maximum dye height (Fig. 3l; Table 1). The percentage of stained vessels gradually decreased in the inner annual rings (Fig. 3m). At 10 cm above the dye injection point, many vessels in the outermost annual ring were stained in all of the species (Fig. 3n, o). In the inner annual rings, some vessels were not stained (Fig. 3p), but others were stained (Fig. 3q, arrow). In Q. glauca and Q. salicina, the patterns of water distribution were similar among the outer three annual rings. Many vessels had water, and some vessels lost water regardless of their diameter (Fig. 4d, e; arrows). At any height in all species, the whole region of vessels within an annual ring was stained, and the dead xylem cells adjacent to stained and non-stained vessels contained dye in their lumina (Fig. 3p, arrows; Table 2).
In the non-vessel T. aralioides, several outer annual rings were stained discontinuously at all heights in all sample trees (Fig. 3r, s, t), and most tracheids in the earlywood region were stained (Fig. 3u, arrows).
The most earlywood tracheids had water and most latewood tracheids had no water in the outer three annual rings (Fig. 4f).
In all of sample trees studied, colored heartwood was not found and no ray parenchyma cells were stained (e.g., Fig. 3c, o). One non-vessel species had 47% conducting sapwood area per total sapwood area, and the value of other species was more than 80%. Only five radial-porous species exhibited tyloses in some non-stained vessels.
Discussion
We investigated the water-conducting pathways in 34 broadleaved evergreen species from two different temperate forests in Japan. Semi-ring-porous species had a different water transport system from species with other vessel arrangements. In semi-ring-porous species, the outermost annual ring was major water transport region (Figs. 2a, 3a, b), and most of the large earlywood vessels carried dye solution and held water in the outermost annual ring, whereas most large earlywood vessels in the inner annual rings did not take up dye solution (Fig. 3d, e) and had no water in their lumina (Fig. 4a, small arrows). These results indicate that the water transport function of large earlywood vessels in semi-ring-porous species is confined to the first growth season. This limited, 1-year water-conducting function of earlywood is the same as for large earlywood vessels of deciduous ring-porous species (Utsumi et al. 1996; Umebayashi et al. 2008). One definition of semi-ring-porous wood is the transitional structure between ring-porous and diffuse-porous wood (Wheeler et al. 1989). This study is the first report to show that semi-ring-porous evergreen species are not aberrant diffuse-porous species with larger vessels, but are very similar to ring-porous species from the standpoint of water conduction.
It is well known that the water-conducting system of ring-porous species is more vulnerable to freezing stress (Sperry and Sullivan 1992; Sperry et al. 1994) than that of diffuse-porous species. The large earlywood vessels of ring-porous species lose their water-conducting function at the first freeze–thaw cycle (Utsumi et al. 1999), and the xylem conductivity decreases (Sperry et al. 1994). Larger conduits are more vulnerable than smaller conduits to freeze–thaw cycle induced cavitation (Ewers 1985; Sperry and Sullivan 1992; Tyree et al. 1994; Tyree and Cochard 1996). In this study, the hydraulic diameter of large earlywood vessels in semi-ring-porous species was considerably larger than that of small earlywood vessels and latewood vessels (Table 3), and larger than the diameter to be vulnerable to cavitation by the freeze–thaw cycle (Davis et al. 1999; Pittermann and Sperry 2003; Taneda and Tateno 2005). Therefore, these large earlywood vessels would lose their function because of freezing stress like those of ring-porous species.
On the other hand, many small earlywood and latewood vessels in semi-ring-porous species had a water-conducting function lasting several years (Figs. 3d, e, 4a). In general, small conduits have a higher resistance to freezing stress (Sperry and Sullivan 1992; Sperry et al. 1994) and water stress (Hacke et al. 2006) than larger ones. Utsumi et al. (1996) reported on a ring-porous deciduous species where small vessels maintained water in their lumina, while large earlywood vessels in the outermost annual ring cavitated during winter. The small earlywood and latewood vessels of semi-ring-porous species would be less vulnerable to cavitation. The small vessels would have a role as the main alternative pathway when the water-conduction function of the large earlywood vessels was lost.
What causes the difference between the deciduous ring-porous and evergreen semi-ring-porous species in the vessel arrangement? Many ring-porous and semi-ring-porous species generally do not grow in tropical rainforests that have a great deal of precipitation through the year, but are more common in the northern temperate zones where environmental seasonal stress occurs (Wheeler and Baas 1993). In regions where climate conditions cause freezing or water stress, both ring-porous species and semi-ring-porous species would have evolved to transport water efficiently using large earlywood vessels from the beginning of the short growing season. In deciduous ring-porous species, the cambial activity precedes leaf emergence (Lechowicz 1984), and old latewood does not need to play major role of water transport except for the short period of the leaf out before the large earlywood vessels mature (Suzuki et al. 1996). However, evergreen semi-ring-porous species transpirate throughout the year and may have a weaker relationship between cambial activity and leaf emergence than ring-porous species. The latewood vessels of semi-ring-porous species have to be active longer than for deciduous ring-porous species after the large earlywood vessels are cavitated. Evergreen semi-ring-porous species only distribute in the Kasuya Research Forest but not in the Shiiba Research Forest (Inoue et al. 2002; Itoh 1995, 1996, 1997, 1998, 1999). Kasuya Research Forest is a warm tempered evergreen broadleaved forest and has less freeze stress during winter. As a result, evergreen semi-ring-porous species may have a greater vulnerability to cavitation but may also have more efficient latewood vessel near the earlywood vessel and more ambiguous porosity than ring-porous species.
In the diffuse-porous species studied, both water-conducting pathway between annual rings and within annual rings were found to vary between species. The water-conducting patterns within annual rings were categorized into two types: d1, conducting water through vessels in the whole region, and d2, conducting water mainly in earlywood vessels. The water-conducting pathway of non-vessel species was similar to that of the d2 type (Figs. 3t, 4f). Umebayashi et al. (2008) reported that deciduous diffuse-porous species had three water-conducting types, which were d1, d2 and the species conducting water mainly in latewood. However, no evergreen diffuse-porous species in this study transported water mainly in latewood. In the d2 type and non-vessel species, the conduit diameter in earlywood and latewood was almost the same or less than that of the d1 type (Table 3). As shown in Table 3, for example, the latewood vessel diameter of Pieris japonica (Thunb.) D. Don (d2 type) was smaller than that of I. pedunculosa (d1 type), which had the smallest vessel diameter among the d1 type species. In addition, the latewood vessel diameter of the d2 type and non-vessel species was smaller than the earlywood vessel diameter (Table 3). In this case the conduit diameter cannot regulate the conducting pattern. Utsumi et al. (2003) indicated that in coniferous species most tracheids in the transition zone from earlywood to latewood lost water and that structural changes in the pit membranes within one annual ring may cause this difference in vulnerability to cavitation. If the pit membranes of latewood conduits in d2 type and non-vessel species have a larger pore size than earlywood conduits, the membranes would have higher vulnerability to tension-induced cavitation. To explain why the latewood vessels are more vulnerable than earlywood vessel, further micro-structural analysis of the pit membranes within the annual rings is needed.
The water-conducting pattern in all of the radial-porous species was similar to that of the d1 type of diffuse-porous species (Fig. 3n, o). However, the vessel diameter of the radial-porous species was considerably larger than that of diffuse-porous and non-vessel trees, and slightly larger or smaller than that of earlywood in semi-ring-porous trees (Table 3). Even in Q. salicina, which grows in the Shiiba Research Forest where several freeze–thaw events occur during winter, the vessel diameter was larger than the earlywood of semi-ring-porous species and showed the same water-conducting pattern as other radial-porous species. This result appears to be in conflict with previous reports that species with larger vessel diameters tend to be more vulnerable to freezing stress (Cavender-Bares et al. 2005). It is known that some evergreen Quercus spp. grow at about 3,000 m above sea level in the Himalayan Mountains (Sakai 1980). The majority of vessels in Quercus spp. might be refilled in every spring by root pressure after the vessels had been embolized by freeze stress during winter. However, some researchers indicated the negative data on positive pressure after freezing in some Quercus spp. (Cavender-Bares and Holbrook 2001; Taneda and Tateno 2005). An alternative explanation, the large ray tissue that is common in Quercus spp. (Fig. 3o) may secrete sugars to the vessel lumina and achieve supercooling of the sap which may avoid freezing. Some Quercus spp. increase the soluble sugars in the stem during winter (Cavender-Bares et al. 2005). Radial-porous species might have low stomatal conductance and consequently high water potential during winter and avoid the embolism by freezing stress. Pratt et al. (2005) showed a high freeze-tolerant evergreen species had low stomatal conductance in the leaves. Further studies are needed to understand the water-conducting mechanism of radial-porous species.
In this study we showed that water-conducting pattern in the stem of evergreen broadleaved species was associated with the structural variety of xylem. Water conduction of evergreen semi-ring-porous species had similar water-conducting system as deciduous ring-porous species. The diffuse-porous species, radial-porous species and non-vessel species had different water-conducting patterns within annual rings. The vessel diameter could not explain the water-conducting pattern within annual rings of diffuse-porous species and radial-porous species, although the diameter is an important parameter to quantify the water conduction. Lechowicz (1984) reported the early leaf emerging type of deciduous diffuse-porous species tends to have narrow-diameter vessels. Ecological and physiological studies should be linked to understand the water-conducting mechanisms in evergreen species in conjunction with precise anatomical study of xylem.
References
Anfodillo T, Sigalotti GB, Tomasi M, Semenzato P, Valentini R (1993) Applications of a thermal imaging technique in the study of the ascent of sap in woody species. Plant Cell Environ 16:997–1001
Axelrod DI (1966) Origin of deciduous and evergreen habits in temperate forests. Evolution 20:1–15
Baker H, James WO (1933) The behaviour of dyes in the transpiration stream of sycamores (Acer pseudoplatanus L.). New Phytol 32:245–260
Cavender-Bares J, Holbrook NM (2001) Hydraulic properties and freezing-induced cavitation in sympatric evergreen and deciduous oaks with contrasting habitats. Plant Cell Environ 24:1243–1256
Cavender-Bares J, Cortes P, Rambal S, Joffre R, Miles B, Rocheteau A (2005) Summer and winter sensitivity of leaves and xylem to minimum freezing temperatures: a comparison of co-occurring Mediterranean oaks that differ in leaf lifespan. New Phytol 168:597–612
Chabot BF, Hicks DJ (1982) The ecology of leaf life spans. Ann Rev Ecol Syst 13:229–259
Chaney WR, Kozlowski TT (1977) Patterns of water movement in intact and excised stems of Fraxinus americana and Acer saccharum seedlings. Ann Bot 41:1093–1100
Christman MA, Sperry JS, Adler FR (2009) Testing the ‘rare pit’ hypothesis for xylem cavitation resistance in three species of Acer. New Phytol 182:664–674
Cochard H, Tyree MT (1990) Xylem dysfunction in Quercus: vessel sizes, tyloses, cavitation and seasonal changes in embolism. Tree Physiol 6:393–407
Davis SD, Sperry JS, Hacke UG (1999) The relationship between xylem conduit diameter and cavitation caused by freezing. Am J Bot 86:1367–1372
DeFries RS, Hansen MC, Townshend JRG, Janetos AC, Loveland TR (2000) A new global 1-km dataset of percentage tree cover derived from remote sensing. Glob Change Biol 6:247–254
Denne MP (1988) Definition of latewood according to Mork (1928). IAWA Bull New Ser 10:59–62
Ellmore GS, Ewers FW (1986) Fluid flow in the outermost xylem increment of a ring-porous tree, Ulmus americana. Am J Bot 73:1771–1774
Ewers FW (1985) Xylem structure and water conduction in conifer trees, dicot trees, and lianas. IAWA Bull New Ser 6:309–317
Ewers FW, Cochard H, Tyree MT (1997) A survey of root pressures in vines of a tropical lowland forest. Oecologia 110:191–196
Feild TS, Brodribb T, Holbrook NM (2002) Hardly a relict: freezing and the evolution of vesselless wood in Winteraceae. Evolution 56:464–478
Fujikawa S, Suzuki T, Ishikawa T, Sakurai S, Hasegawa Y (1988) Continuous observation of frozen biological-materials with cryo-scanning electron microscope and freeze-replica by a new cryo-system. J Electron Microsc 37:315–322
Gilbert SG (1940) Evolutionary significance of ring porosity in woody angiosperms. Bot Gaz 102:105–120
Givnish TJ (2002) Adaptive significance of evergreen vs. deciduous leaves: solving the triple paradox. Silva Fenn 36:703–743
Greenidge KNH (1958) Rates and patterns of moisture movement in trees. In: Thimann KV (ed) The physiology of forest trees. Ronald, New York, pp 19–41
Hacke UG, Sperry JS, Wheeler JK, Castro L (2006) Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiol 26:689–701
Hallé F, Oldeman RAA, Tomlinson PB (1978) Tropical trees and forests: an architectural analysis. Springer, Berlin
Hirose S, Kume A, Takeuchi S, Utsumi Y, Otsuki K, Ogawa S (2005) Stem water transport of Lithocarpus edulis, an evergreen oak with radial-porous wood. Tree Physiol 25:221–228
Ide J, Nagafuchi O, Chiwa M, Kume A, Otsuki K, Ogawa S (2007) Effects of discharge level on the load of dissolved and particulate components of stream nitrogen and phosphorus from a small afforested watershed of Japanese cypress (Chamaecyparis obtusa). J For Res 12:45–56
Inoue S, Utsumi Y, Otsuki K, Okano T, Koga S, Tashiro N, Nakai T (2002) Forest and tree of Kyushu University. Kyushu University Forest, Fukuoka
Itoh T (1995) Anatomical description of Japanese hardwoods I. Wood Res Tech Notes 31:81–181
Itoh T (1996) Anatomical description of Japanese hardwoods II. Wood Res Tech Notes 32:66–176
Itoh T (1997) Anatomical description of Japanese hardwoods III. Wood Res Tech Notes 33:83–201
Itoh T (1998) Anatomical description of Japanese hardwoods IV. Wood Res Tech Notes 34:30–166
Itoh T (1999) Anatomical description of Japanese hardwoods V. Wood Res Tech Notes 35:47–175
Jarbeau JA, Ewers FW, Davis SD (1995) The mechanism of water-stress-induced embolism in two species of chaparral shrubs. Plant Cell Environ 18:189–196
Lechowicz MJ (1984) Why do temperate deciduous trees leaf out at different times? Adaptation and ecology of forest communities. Am Nat 124:821–842
Maherali H, Pockman WT, Jackson RB (2004) Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology 85:2184–2199
Miyazawa Y, Kikuzawa K (2005) Winter photosynthesis by saplings of evergreen broad-leaved trees in a deciduous temperate forest. New Phytol 165:857–866
Pittermann J, Sperry J (2003) Tracheid diameter is the key trait determining the extent of freezing-induced embolism in conifers. Tree Physiol 23:907–914
Pratt RB, Ewers FW, Lawson MC, Jacobsen AL, Brediger MM, Davis SD (2005) Mechanisms for tolerating freeze-thaw stress of two evergreen chaparral species: Rhus ovata and Malosma laurina (Anacardiaceae). Am J Bot 92:1102–1113
Sakai A (1980) Freezing resistance of broad-leaved evergreen trees in the warm-temperate zone. Low Temp Sci Ser B 38:1–14
Sano Y, Okamura Y, Utsumi Y (2005) Visualizing water-conduction pathways of living trees: selection of dyes and tissue preparation methods. Tree Physiol 25:269–275
Scholander PF, Love WE, Kanwisher JW (1955) The rise of sap in tall grapevines. Plant Physiol 30:93–104
Sperry JS (1993) Winter xylem embolism and spring recovery in Betula cordifolia, Fagus grandifolia, Abies balsamea and Picea rubens. In: Borghetti M, Grace J, Raschi A (eds) Water transport in plants under climatic stress. Cambridge University, Cambridge, pp 86–98
Sperry JS, Sullivan JEM (1992) Xylem embolism in response to freeze-thaw cycles and water stress in ring-porous, diffuse-porous, and conifer species. Plant Physiol 100:605–613
Sperry JS, Tyree MT (1988) Mechanism of water stress-induced xylem embolism. Plant Physiol 88:581–587
Sperry JS, Holbrook NM, Zimmermann MH, Tyree MT (1987) Spring filling of xylem vessels in wild grapevine. Plant Physiol 83:414–417
Sperry JS, Donnelly JR, Tyree MT (1988) Seasonal occurrence of xylem embolism in sugar maple (Acer saccharum). Am J Bot 75:1212–1218
Sperry JS, Nichols KL, Sullivan JEM, Eastlack SE (1994) Xylem embolism in ring-porous, diffuse-porous, and coniferous trees of northern Utah and interior Alaska. Ecology 75:1736–1752
Suzuki M, Yoda K, Suzuki H (1996) Phenological comparison of the onset of vessel formation between ring-porous and diffuse-porous deciduous trees in a Japanese temperate forest. IAWA J 17:431–444
Taneda H, Tateno M (2005) Hydraulic conductivity, photosynthesis and leaf water balance in six evergreen woody species from fall to winter. Tree Physiol 25:299–306
Tateishi M, Kumagai T, Utsumi Y, Umebayashi T, Shiiba Y, Inoue K, Kaji K, Cho K, Otsuki K (2008) Spatial variations in xylem sap flux density in evergreen oak trees with radial-porous wood: comparisons with anatomical observations. Trees 22:23–30
Thomas H, Sadras VO (2001) The chapter and gratuitous disposal of resources by plants. Funct Ecol 15:3–12
Tyree MT, Cochard H (1996) Summer and winter embolism in oak: impact on water relations. Ann Sci For 53:173–180
Tyree MT, Sperry JS (1989) Vulnerability of xylem to cavitation and embolism. Annu Rev Plant Physiol Mol Biol 40:19–38
Tyree MT, Zimmermann MH (2002) Xylem structure and the ascent of sap, 2nd edn. Springer, Berlin
Tyree MT, Davis SD, Cochard H (1994) Biophysical perspectives of xylem evolution: is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction? IAWA J 15:335–360
Umebayashi T, Utsumi Y, Koga S, Inoue S, Shiiba Y, Arakawa K, Matsumura J, Oda K (2007) Optimal conditions for visualizing water-conducting pathways in a living tree by the dye injection method. Tree Physiol 27:993–999
Umebayashi T, Utsumi Y, Koga S, Inoue S, Fujikawa S, Arakawa K, Matsumura J, Oda K (2008) Conducting pathways in north temperate deciduous broadleaved trees. IAWA J 29:247–263
Utsumi Y, Sano Y (2007) Cryoplaning technique for visualizing the distribution of water in woody tissues by cryoscanning electron microscopy. In: Kuo J (ed) Electron microscopy: methods and protocols, 2nd edn. Humana, New Jersey, pp 497–506
Utsumi Y, Sano Y, Ohtani J, Fujikawa S (1996) Seasonal changes in the distribution of water in the outer growth rings of Fraxinus mandshurica var. japonica: a study by cryo-scanning electron microscopy. IAWA J 17:113–124
Utsumi Y, Sano Y, Funada R, Fujikawa S, Ohtani J (1999) The progression of cavitation in earlywood vessels of Fraxinus mandshurica var japonica during freezing and thawing. Plant Physiol 121:897–904
Utsumi Y, Sano Y, Funada R, Ohtani J, Fujikawa S (2003) Seasonal and perennial changes in the distribution of water in the sapwood of conifers in a sub-frigid zone. Plant Physiol 131:1826–1833
Wheeler EA, Baas P (1991) A survey of the fossil record for dicotyledonous wood and its significance for evolutionary and ecological wood anatomy. IAWA Bull New Ser 12:275–332
Wheeler EA, Baas P (1993) The potentials and limitations of dicotyledonous wood anatomy for climatic reconstructions. Paleobiology 19:487–498
Wheeler EA, Baas P, Gasson PE (1989) IAWA list of microscopic features for hardwood identification: with an appendix on non-anatomical information. IAWA Bull New Ser 10:219–332
Wheeler EA, Baas P, Rodgers S (2007) Variations in dicot wood anatomy: a global analysis based on the insidewood database. IAWA J 28:229–258
Yamauchi F (1976) Anatomical studies of wood in Japanese Elaeagnus. Bull Natl Sci Mus Seri B (Bot) 2:107–118
Acknowledgments
The authors thank I. Murata, T. Inoue, H. Nagasawa, K. Kubota, S. Inoue, S. Osaki, T. Mabuchi, Y. Shiiba, and T. Itoh for their technical support. This work was supported in part by a Grant-in-Aid for Scientific Research (Nos. 17380096 and 19780121) from the Ministry of Education, Science and Culture, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Lovelock.
Rights and permissions
About this article
Cite this article
Umebayashi, T., Utsumi, Y., Koga, S. et al. Xylem water-conducting patterns of 34 broadleaved evergreen trees in southern Japan. Trees 24, 571–583 (2010). https://doi.org/10.1007/s00468-010-0428-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-010-0428-7