Abstract
Long-term variation in tree-ring widths (1873–2006) and intra-annual dynamics of cambial activity and tree-ring formation in 2006 were studied in mature beech (Fagus sylvatica L.) trees at a typical forest site near Ljubljana (46°N, 14°40′E, 400 m a.s.l.) and related to leaf phenology and climate data. Tree-ring widths were negatively affected by minimum March and maximum August temperatures and favoured by May and July precipitation. Precipitation of the previous August and temperature of the previous November also had a positive effect. Leaf unfolding was affected by March and April temperatures, occurring later if they were low. Leaf yellowing was positively affected by minimum July temperatures and negatively by September precipitation. In 2006, leaf unfolding occurred on 16 April and was immediately followed by reactivation of cambium at breast height of the trees. One week later, the cambium obtained its maximum width (around 11 cell layers) and the rate of division increased until the end of May/beginning of June. By the end of June, 75% of the tree-ring was formed. Cambial cell divisions stopped from the end of July to mid-August. The average time of cambial activity was 100 days. Leaf yellowing occurred at the end of October, i.e. nearly 2 months after the cessation of cambial cell division. We discuss the usefulness of a combination of long-term (tree-ring width and phenology) and short-term (wood formation at a cellular level) data to understand better the environmental signals registered by a tree during growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
European beech (Fagus sylvatica L.) is among the most important forest species, with an ability to grow on a great variety of sites all over Europe (Mayer 1984). Its proportion is currently increasing in Central Europe, particularly where forests with a high percentage of conifers are being converted into more natural mixed forests (e.g. Tarp et al. 2000; Bončina et al. 2003). It has recently been reported that the competitiveness of beech might be considerably reduced due to climate change (Geßler et al. 2007). At the edge of its southern range, the changing climate may even cause a retreat of beech populations (Jump and Peñuelas 2006). Similar observations have been made in Slovenia, SE Central Europe, where beech is the basic structural element of most forest associations from the lowlands up to the high mountains (Marinček 1987) and forms more than one-third of the wood stock (Brus 2005). The climate change scenario for this region predicts a rise in temperature and more uneven distribution of precipitation associated with more frequent droughts and extreme rainfall events (Bergant and Kajfež-Bogataj 2005), which might affect the survival of beech, particularly on more extreme and marginal sites (Diaci 2007).
Because of its frequency, ability to grow on sites of wide ecological variability and its longevity, beech is well-suited for assembling spatial networks of tree-ring chronologies. The beech can be used for dendroecological studies, especially to evaluate the growth and climate relationships in different bioclimatological units, and to estimate future prospects and possible ecological risks associated with climate change (e.g. Eckstein et al. 1984; Gutierrez 1988; Biondi 1992; Rozas 2001; Dittmar et al. 2003; Piovesan et al. 2005; Lebourgeois et al. 2005; Di Filippo et al. 2007). Most dendroclimatological studies are based on analyses of tree-ring widths that integrate positive and negative environmental influences affecting the growth of a tree during the current or previous years (Eckstein 2004). The application of densitometry showed that within and between year density variations in beech provide more information at higher temporal resolution, than tree-ring widths only (Z’Graggen 1992; Bouriaud et al. 2004; Skomarkova et al. 2006). Analysis of wood structure provides even better resolution, since the morphology of the cells records the effects of different factors on a finer time scale during the time of wood formation (Eckstein 2004; Frankenstein et al. 2005). Studying the time series of vessel areas in beech wood showed that they can be used as an ecological variable indicating the impact of internal and external factors on the process of wood formation (Sass 1993; Sass and Eckstein 1995). However, if we want to understand the information on factors affecting wood formation recorded in the wood structure we need to identify which signal (and to what degree) affects a particular feature in the wood (e.g. Fonti et al. 2007).
For this purpose, studies on wood formation during the growth season are needed. Studying wood formation on a cellular level requires destructive sampling from living trees and time consuming preparation and measurement of microscopic sections (e.g. Gričar et al. 2007). This is among the main reasons that wood formation in beech has, to our knowledge, only been studied in northern Europe for one or two seasons (Schmitt et al. 2000; Werf van der et al. 2007). In northern Germany, wood formation data were combined with phenological data (Schmitt et al. 2000) and in the Netherlands with long-term tree-ring and climate data (Werf van der et al. 2007). Such combinations of data proved to be useful for explaining the environmental signals registered by a tree during different phases of wood formation.
In the SE part of Central Europe, detailed intra- and inter-annual studies of wood formation in relation to climate are still lacking in deciduous tree species. We therefore decided to study adult beech trees from a typical forest site near Ljubljana. The objectives of the study were: (1) to evaluate long-term intra-annual tree-ring characteristics and leaf phenology in relation to climate variation; and (2) to present the dynamics of cambial activity and tree-ring formation on a cellular level in the 2006 growth period. We discussed the obtained results in the context of climate change predictions.
Materials and methods
Study site characteristics
The study was carried out at a forest site Panška Reka near Ljubljana (approximately 46°N, 14°40′E, 400 m a.s.l.). The privately owned forest site belongs to the Blechno fagetum forest association. The predominant tree species in the site is beech (F. sylvatica L.).
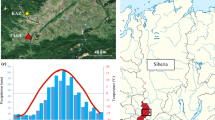
The climate at the site is humid continental. The mean annual temperature is 10.2°C (T Jan = −0.5°C, T Jul = 20.4°C) and total annual precipitation is 1,384 mm, as calculated from the 1960–2006 climate data set from the nearby Ljubljana climate station of the Environmental Agency of the Republic of Slovenia within the Ministry of the Environment and Spatial Planning. A climogram for the study site, including long-term and 2006 data, is shown in Fig. 1.
Climate diagram, meteorological station Ljubljana: mean monthly temperatures (minimum, mean, maximum) (lines) and monthly sum of precipitation (white bars) for the period 1960–2006 compared with means of 2006 (circles, triangles and grey bars). The mean annual temperature is 10.2°C (range 8.8–12.2) and total annual precipitation 1,384 mm (range 1,091–1,839). The corresponding values in 2006 are 11.4°C and 1,141 mm
Tree selection and sampling
During winter 2005–2006, 14 isolated, dominant or co-dominant, healthy beech (F. sylvatica L.) trees with diameter 40–50 cm, height 25–30 m, and ages above 100 years were selected for analysis. Six of them were visited at weekly intervals from April until September 2006 to collect intact tissue samples in order to study cambial activity and growth ring formation on a cellular level. Thereafter, during winter 2006–2007, all the 14 trees were felled and the stem discs were taken for tree-ring analysis.
Intact tissue samples
Samples (25 × 10 × 10 mm) were taken from the breast height (1.3 m) of six living trees with a chisel and knife. They contained phloem, cambium, developing and older outer xylem. They were fixed in FEA (formalin–ethanol–acetic acid solution), reduced to blocks of dimensions 2 × 2 × 3 mm, dehydrated in ethanol and embedded in paraffin (Rossi et al. 2006a). Cross sections of 12-μm thickness were stained with safranin (0.5% in 95% ethanol) and astra blue (0.5% in 95% ethanol) and observed under a Nikon Eclipse 800 light microscope (bright field and polarized light) and analysed with a Lucia G 4.8 image analysis. We examined the cambium (number of cells along three radial files and the width in μm) and the current xylem growth ring (increment width in μm along three radial lines).
In cross-sections, we distinguished among cambial cells (CC), differentiating xylem cells (vessels, fibres, axial parenchyma, ray parenchyma) in postcambial growth (PC), secondary cell wall deposition and lignification (SW), and mature cells (MT) (Fig. 2). The CC consisted of radially flattened cells with thin cell walls that stained blue with astra blue. The PC cells were larger and had thin, non-lignified, blue-stained primary cell walls. The deposition of secondary wall (SW) was observed under polarized light as the cell walls showed birefringence and their thickness started to increase. The beginning of cell wall lignification could be observed as the red staining by safranin gradually replaced the blue staining. When the process of differentiation was completed, the walls of mature cells (MT) were completely red stained and the cell lumina were empty.
Xylem ring formation in beech (Fagus sylvatica): a cambium (CC) and forming growth ring in the wood with cells in postcambial growth (PC) on 24 April 2006; V vessel, F fibre, AP axial parenchyma; b wide CC and forming xylem growth ring on 20 June 2006 consisting of cells in phase of PC (stained blue), deposition of secondary wall (SW) and mature cells (MT) with empty lumina and red stained cell walls. Line 100 μm
Tree-rings and chronology computation
The discs taken from 14 trees felled in winter 2006–2007 were polished and the tree-ring widths were measured to the nearest 0.01 mm. The TSAP/X program was used for data acquisition. The tree-ring series were visually and statistically cross-dated and compared with each other by calculating the t value after Baillie and Pilcher (1973) using TSAP/X and TSAP-Win.
The tree-ring series were assembled in a local chronology using the ARSTAN programme (Holmes 1994). The individual raw tree-ring series were hereby standardized in a two-step procedure. First, the long-term trend was removed by fitting a negative exponential function or a regression line to each tree-ring series. Second, a more flexible detrending was made by a cubic smoothing spline with a 50% frequency response of 60 years, in order to reduce further non-climatic variance. Thereafter, autoregressive modelling of the residuals and bi-weight robust estimation of the mean were applied (Cook and Peters 1997). We then made three versions of the chronology, a non-detrended raw-data, a detrended standard, and a detrended residual chronology for analyses of the tree-ring and climate relations.
Phenological data
Phenological data (the date of leaf unfolding and the date of general leaf yellowing) for beech from the site in Ljubljana were obtained from the Environmental Agency of the Republic of Slovenia (Črepinšek and Zrnec 2005). The period of observations was 1960–2006.
Climate data
Climatic data (minimum, maximum and mean monthly temperatures and monthly amount of precipitation) for the period 1960–2006 were obtained from the Ljubljana climate station (299 m a.s.l., 46°04′N,14°29′E) of the Environmental Agency of the Republic of Slovenia. The station is located approximately 10 km from the forest site.
Climate–growth and climate–phenology relationships
Climate–growth relationships were calculated using the program DendroClim2002 through correlation function analysis (Biondi and Waikul 2004), where the residual version of the tree-ring chronology was the dependent variable and the regressors were the monthly minimum, maximum and mean temperatures and the monthly sums of precipitation for each year from the previous August to the current November. The program applies a bootstrap process (Guiot 1991) to assess the statistical significance of the correlation coefficients.
The influence of climate on the three phenological variables used (date of leaf unfolding, date of general leaf yellowing and the time span between these two dates, indicating the period when the trees had active leaves) was estimated using forward stepwise regression techniques with the same climate regressors.
Interannual tree-ring formation
In each intact tissue sample, we calculated the average width (X i ) of the 2006 growth ring based on measurements along three radial lines:
X i is the average width of xylem growth ring 2006, i is the date of sampling; n is the number of measurements, j is the subsequent measurement on individual sample.
The formation of the 2006 xylem growth ring was then analysed with the Gompertz function (Zeide 1993, Zeide 2004; Deslauriers et al. 2003a, b; Rossi et al. 2003) according to the equation:
where y is the weekly cumulative width, t is the day of the year (DOY), A being the upper asymptote, representing the maximum ring width, B is the placement parameter, where the lower asymptote starts, k is the rate of change parameter, B/k being the inflection point on the curve representing the maximum daily rate of growth.
Results
Inter-annual variability in tree-rings and phenology
The local tree-ring chronology of beech based on 14 trees was 134 years long and covered the period 1873–2006 (Fig. 3a, b). The average length of tree-ring series included was 105 years. The mean ring width for the period 1960–2006 was 1.89 mm (standard deviation 0.386), as calculated from the raw version of the chronology (Table 1).
After standardization, the ARSTAN standard chronology still showed a significant first order autocorrelation; the residual chronology was therefore calculated by autoregressive modelling, which showed no significant autocorrelation between tree-ring indices. The main descriptive statistics of the chronologies are presented in Table 1. The obtained chronologies and their replication are shown in Fig. 3a, b.
The time series of phenological data for the period 1960–2006 are shown in Fig. 3c. In the phenological time series there was detected no significant trend, therefore the original raw series instead of detrended ones could be used in further analysis. The average time (day of the year, DOY) of leaf unfolding was 110 ± (5.7 SD), which corresponded with the calendar date of 20 April. The long-term average DOY of general leaf yellowing was 291 ± (8.0 SD), corresponding to 18 October. The average period when the trees had leaves was thus 181 ± (10.5 SD) days (Table 2).
Effects of climate on tree-ring formation and phenology
The mean of maximum temperatures in August proved to be the most important climatic factor explaining year to year variations in the growth of beech in the study area. However, ring width was also negatively correlated with minimum temperature in March and positively with precipitation in May and July. Maximum temperature in the previous November and precipitation in the previous September also positively affected the tree-ring widths (Fig. 4).
The phenological time series showed significant correlation with some climatic variables. Year to year variations in the time of leaf unfolding (FL) can be explained to a large degree by March and April minimum temperatures (Table 3); the lower they are, the later the leaves unfold. In contrast, leaf yellowing (LY) was positively related to minimum July temperatures and negatively to September precipitation (Table 3), which means that leaf yellowing occurred later if the minimum July temperatures were higher and when September precipitation was low. The period when the trees had leaves (DwL) (time from leaf unfolding until yellowing) positively correlated with maximum March and July temperatures and September precipitation (Table 3).
Tree-ring indices, leaf unfolding, leaf yellowing and days with leaves all proved to depend on climate but there are differences in the climatic parameters and the periods affecting them. Their comparison therefore showed no significant correlation either between tree-ring indices and phenology (Fig. 5a) or between leaf unfolding and leaf yellowing (Fig. 5b).
Wood formation dynamics in 2006
Observation of cambium and newly formed xylem under a light microscope at weekly intervals during the 2006 vegetation period showed that the dormant cambium contained 3–5 cell layers. Divisional activity in the cambium started in the week between 18 and 24 April (DOY 108–114) when the number of cambial cells increased to approximately ten cell layers (Fig. 2a). The first xylem cells (fibres, vessels, axial and ray parenchyma) in postcambial growth were observed 1 or 2 weeks after reactivation of the cambium. Synthesis of the secondary wall started between 24 April and 2 May (DOY 114–122) and the first fully differentiated fibres were observed between 13 and 20 June (DOY 164–171), i.e., 5 weeks after their formation in the cambium. The period of maximum cell production was assessed between the 30 May and 6 June 2006, when more than 35 μm of wood increment was formed per day. Until 20–27 June (DOY 171–178), approximately 75% of the xylem growth ring was completed in most trees (Fig. 6). Divisions in the cambium stopped from the end of July till mid August 2006 (Figs. 6, 7, 8). Development of the latest formed xylem cells thereafter persisted for some time. The average duration of cambial activity was estimated to be 100 days.
Weekly increments (arrows) of 2006 xylem growth ring in beech no. 4: 18 April (DOY 108) onset of cambium divisions, 6 June (DOY 157) 50% of the growth ring was formed, 21 June (DOY 171) early-wood formation completed and 76% of the growth ring formed, 22 August (DOY 234) cambium divisions completed. Scale bar 100 μm
Beech (Fagus sylvatica) Ljubljana, Slovenia: phenology (FL leaf unfolding and LY leaf yellowing for 1960–2006 and 2006), dynamics of wood formation (increment width) with milestones of ring formation in 2006. Below are indicated positive (+) or negative (−) effects of climatic factors in months of the current and previous year on FL, LY and TRI (TRI tree-ring indices)
Discussion
The combined information obtained from a more than 100-year tree-ring chronology, 46-year-long leaf phenology series, the seasonal dynamics of wood formation in 2006 and its relation to climate, enabled us to present the milestones of wood formation, as well as the main climate parameters that affect year to year tree-ring variation and phenology (leaf unfolding and leaf yellowing) for beech near Ljubljana. These milestones are presented in Fig. 8 and are discussed below.
We observed a close correspondence between leaf unfolding and reactivation of the cambium. The two processes occurred at almost the same time, on 16 and 18 April (DOY 106 and108) 2006, and were slightly earlier than the average leaf unfolding date for the period 1960–2006, which is 20 April (DOY 110) (Table 2). This confirms earlier reports that leaf unfolding can be used for a rough estimate of the onset of cambial divisions and that cambium reactivation takes place immediately after bud breaking in beech as a diffuse porous species (e.g. Bosshard 1974; Panshin and de Zeeuw 1980; Suzuki et al. 1996; Schmitt et al. 2000).
Climatic conditions in March and April proved to affect the time of leaf unfolding. In the study area, minimum April temperature is on average 5.1°C although in particular years it can be lower than 3°C or higher than 8°C; in 2006 it was 6.3°C. Such inter-annual variations in temperature cause a variation in the time of leaf unfolding. It is delayed if the temperatures are lower or anticipated if the temperatures are higher. The effect of temperatures on leaf unfolding in beech has also been shown in other studies (e.g. Črepinšek et al. 2006; Dittmar and Elling 2006). Variability in March–April minimum temperatures therefore seems to play an important role in defining the beginning of the growing season and it could indirectly affect the final tree-ring width.
The rate of cell divisions was low in the first weeks after cambial reactivation and maximal at the end of May–beginning of June, i.e., more than 1 month after reactivation of the cambium. Correlations with climate indicate that May precipitation affects year to year tree-ring variation and that the tree-rings were wider when May was wet. It is likely that precipitation in May has both direct and indirect effect on growth, the latter due to affecting the amount of available water in summer, which also depends on the water storage capacity of the soil (e.g. Bouriaud et al. 2004).
June can be considered the most important month for tree-ring formation. In June 2006, we observed the highest monthly amount of wood production, when approximately 35% of the entire tree-ring was formed. The average mean June temperature in the study area is 18.4°C, with an average maximum of 24.2°C and average minimum of 12.9°C. June is also the month with the highest average sum of precipitation in the study area (143 mm). However, June 2006 was characterized by below average precipitation and slightly higher temperatures than the long-term average.
The maximum weekly production of wood occurred between 30 May and 6 June 2006, which is more than 2 weeks before the summer solstice (21 June). The importance of June and the summer solstice with the longest photoperiod, has been described for conifers from various sites in North America and Europe (Rossi et al. 2006b; Gričar 2007), whereas maximum cell production in deciduous species proved to take place earlier, i.e., at the end of May (Eschrich 1995; Marion et al. 2007).
During July, cambial activity in investigated beech trees gradually decreased and the cessation of cell division took place from late July to mid–August 2006. Numerous similar studies on various conifers from the temperate climatic zone have shown that cambial activity stopped at the latest by the end of August (e.g. Antonova and Stasova 1993; Horacek et al. 1999; Schmitt et al. 2004; Rossi et al. 2006b; Gričar 2007). On the other hand, the wood formation in beech in the Netherlands ended in October (Werf van der et al. 2007) and in northern Germany in September (Schmitt et al. 2000). Similarly, Marion et al. (2007) found that cambial activity in Norway maple in Ljubljana continued until the first part of September. Bouriaud et al. (2004) reported that the radial increment in beech trees stopped quickly, or was greatly limited when a soil water deficit occurred.
We assume that maturation of the latest formed latewood cells continued after the cessation of cambial activity, as also observed in several studies on different tree species (Whitmore and Zahner 1966; Gindl et al. 2000; Gričar et al. 2005; De Luis et al. 2007).
After the cessation of cell division in the cambium, the trees retained active leaves capable of photosynthesis for two more months, until 21 October 2006. General leaf yellowing in 2006 occurred 3 days later than the long-term average. The products of photosynthesis in the period after the cessation of cambial activity were probably accumulated as reserves in storage tissues. This may explain the effect of the climate of the previous year on current tree-ring width. We namely observed a positive effect of previous August precipitation and previous November maximum temperatures on tree ring width.
Our study, showed no relation between the time when the trees had leaves and ring widths. We also demonstrated that leaf unfolding, leaf yellowing, time with leaves and ring formation, are linked to different climatic effects.
Climatic conditions on the study site are apparently optimal for the growth of beech. This explains the relatively low mean sensitivity, which was 0.14 for raw and standard chronologies, and 0.17 for residual. These values are lower than, at several sites in Italy, Austria, and Slovenia (Di Filippo et al. 2007) or in other European sites (Dittmar et al. 2003) where the climate is possibly more limiting for growth and thus explains the higher amount of year to year variation in tree-ring widths.
It should be noted that June 2006 was extremely dry, with only 46 mm of precipitation (compared to 143 mm long-term average) and July was extremely hot, with absolute maximum values of monthly mean, minimum and maximum temperatures in the period 1960–2006. Hot and dry June and July conditions are in agreement with climatic change predictions for Slovenia (Bergant and Kajfež-Bogataj 2005), so climate change might affect the productivity of beech. Reduced productivity, competitiveness, and survival chances of beech on more extreme sites have already been observed in the Mediterranean (Peñuelas and Boada 2003; Jump and Peñuelas 2006) and in central European sites NW of our study area (Geßler et al. 2007).
References
Antonova GF, Stasova VV (1993) Effects of environmental factors on wood formation in Scots pine stems. Trees 7(4):214–219
Baillie MGL, Pilcher JR (1973) A simple cross-dating program for tree-ring research. Tree Ring Bull 33:7–14
Bergant K, Kajfež-Bogataj L (2005) N-PLS regression as empirical downscaling tool in climate change studies. Theor Appl Climatol 81:11–23
Biondi F (1992) Development of a tree-ring network for the Italian Peninsula. Tree Ring Bull 52:15–29
Biondi F, Waikul K (2004) DENDROCLIM2002: a C++ program for statistical calibration of climate signals in tree-ring chronologies. Comput Geosci 30:303–311
Bončina A, Diaci J, Gašperšič F (2003) Long-term changes in tree species composition in the Dinaric mountain forests of Slovenia. For Chron 79:227–232
Bosshard HH (1974) Holzkunde, vol 2. Birkhäuser, Basel, pp 34–71
Bouriaud O, Breda N, Le Moguedec G, Nepveu G (2004) Modelling variability of wood density in beech as affected by ring age, radial growth and climate. Trees 18:264–276. doi:10.1007/s00468-003-0303-x
Brus R (2005) Dendrologija za gozdarje (Dendrology for foresters). Biotechnical Faculty, Department of Forestry and Renewable Resources, Ljubljana
Cook ER, Peters K (1997) Calculating unbiased tree-ring indices for the study of climatic and environmental change. Holocene 7:361–370
Črepinšek Z, Zrnec C (2005) Petinpetdeset let fenoloških opazovanj v Sloveniji, 1951–2005 (Fifty-five years of phenological observations in Slovenia, 1951–2005). Acta Agric Slov 85:283–297
Črepinšek Z, Kajfež-Bogataj L, Bergant K (2006) Modelling of weather variability effect on phytophenology. Ecol Modell 194:256–265
Deslauriers A, Morin H, Urbinati C, Carrer M (2003a) Daily weather response of balsam fir (Abies balsamea (L.) Mill.) stem radius increment from denrometer analysis in the boreal forest of Quebec (Canada). Trees 17:477–484. doi:10.1007/s00468-003-0260-4
Deslauriers A, Morin H, Begin Y (2003b) Cellular phenology of annual ring formation of Abies balsamea in the Quebec boreal forest (Canada). Can J For Res 33:190–200
De Luis M, Gričar J, Čufar K, Raventós J (2007) Seasonal dynamics of wood formation in Pinus halepensis from dry and semi-arid ecosystems in Spain. IAWA J 28:389–404
Diaci J (2007) Prilagajanje gojenja gozdov podnebnim spremembam (Adapting silviculture to climate change). In: Jurc M (ed) Podnebne spremembe: vpliv na gozd in gozdarstvo (Climate change: impact on forest and forestry). Studia Forestalia Slovenica 130, Ljubljana, pp 117–132
Di Filippo A, Biondi F, Čufar K, De Luis M, Grabner M, Maugeri M, Presutti Saba E, Schirone B, Piovesan G (2007) Bioclimatology of beech (Fagus sylvatica L.) in the Eastern Alps: spatial and altitudinal climatic signals identified through a tree-ring network. J Biogeogr 34:1873–1892. doi:10.1111/j.1365-2699.2007.01747.x
Dittmar C, Elling W (2006) Phenological phases of common beech (Fagus sylvatica L.) and their dependence on region and altitude in Southern Germany. Eur J Forest Res 125:181–188. doi:10.1007/s10342-005-0099-x
Dittmar C, Zech W, Elling W (2003) Growth variations of Common beech (Fagus sylvatica L.) under different climatic and environmental conditions in Europe-a dendroecological study. For Ecol Manage 173:63–78
Eckstein D (2004) Change in past environments—secrets of the tree hydrosystem. New Phytol 163:1–4. doi:10.1111/j.1469-8137.2004.01117.x
Eckstein D, Richter K, Aniol RW, Quiehl G (1984) Dendroklimatologische Untersuchungen zum Buchensterben im südwestlichen Vogelsberg. Forstwiss Cent 103:274–289
Eschrich W (1995) Funktionelle Pflanzenanatomie. Springer, Berlin
Fonti P, Solomonoff N, García-González I (2007) Earlywood vessels of Castanea sativa record temperature before their formation. New Phytol 173:562–570. doi:10.1111/j.1469-8137.2006.01945.x
Frankenstein C, Eckstein D, Schmitt U (2005) The onset of cambium activity—a matter of agreement? Dendrochronologia 23:57–68. doi:10.1016/j.dendro.2005.07.007
Geßler A, Keitel C, Kreuzwieser J, Matyssek R, Seiler W, Rennenberg H (2007) Potential risks for European beech (Fagus sylvatica L.) in a changing climate. Trees 21:1–11. doi:10.1007/s00468-006-0107-x
Gindl W, Grabner M, Wimmer R (2000) The influence of temperature on latewood lignin content in treeline Norway spruce compared with maximum density and ring width. Trees 14:409–414
Gričar J (2007) Xylo- and phloemogenesis in silver fir (Abies alba Mill.) and Norway spruce (Picea abies (L.) Karst.). Slovenian Forestry Institute, Ljubljana
Gričar J, Čufar K, Oven P, Schmitt U (2005) Differentiation of terminal latewood tracheids in silver fir during autumn. Ann Bot 95:959–965. doi:10.1093/aob/mci112
Gričar J, Zupančič M, Čufar K, Oven P (2007) Wood formation in Norway spruce studied by pinning technique and intact tissue sampling method. Wood Res Slov 52:1–9
Guiot J (1991) The bootstrapped response function. Tree Ring Bull 51:39–41
Gutierrez E (1988) Dendroecological study of Fagus silvatica L. in the Montseny Mountains (Spain). Acta Oecol Oecol Plant 9:301–309
Holmes RL (1994) Dendrochronology program library user’s manual. Laboratory of Tree-Ring Research. University of Arizona, Tucson
Horacek P, Slezingerova J, Gandelova L (1999) Effects of environment on the xylogenesis of Norway spruce (Picea abies [L.] Karst.). In: Wimmer R, Vetter RE (eds) Tree-ring analysis. Biological, methodological and environmental aspects. CAB International, Oxford, pp 33–54
Jump AS, Peñuelas J (2006) Running to stand still: adaptation and the response of plants to rapid climate change. Ecol Lett 8:1010–1020. doi:10.1111/j.1461-0248.2005.00796.x
Lebourgeois F, Breda N, Ulrich E, Granier A (2005) Climate-tree-growth relationship of European beech (Fagus sylvatica L.) in the French Permanent Plot Network (RENECOFOR). Trees 19:385–401. doi:10.1007/s00468-004-0397-9
Marinček L (1987) Bukovi gozdovi na Slovenskem (Beech forests in Slovenia). Delavska enotnost, Ljubljana
Marion L, Gričar J, Oven P (2007) Wood formation in urban Norway maple trees studied by the micro-coring method. Dendrochronologia 25:97–102. doi:10.1016/j.dendro.2007.05.001
Mayer H (1984) Waldbau auf soziologisch-ökologischer Grundlage, 3 Aufl., Fischer, Stuttgart-New York
Panshin AJ, De Zeeuw C (1980) Textbook of wood technology, Fourth edn. McGraw-Hill, New York
Peñuelas J, Boada M (2003) A global change-induced biome shift in the Montseny mountains (NE Spain). Glob Change Biol 9:131–140
Piovesan G, Biondi F, Bernabei M, Di Filippo A, Schirone B (2005) Spatial and altitudinal bioclimatic zones of the Italian peninsula identified from a beech (Fagus sylvatica L.) tree-ring network. Acta Oecol 27:197–210. doi:10.1016/j.actao.2005.01.001
Rossi S, Deslauriers A, Morin H (2003) Application of the Gompertz equation for the study of xylem cell development. Dendrochronologia 21:33–39
Rossi S, Anfodillo T, Menardi R (2006a) Trephor: a new tool for sampling microcores from tree stems. IAWA J 27:89–97
Rossi S, Deslauriers A, Anfodillo T, Morin H, Saracino A, Motta R, Borghetti M (2006b) Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytol 170:301–310. doi:10.1111/j.1469-8137.2006.01660.x
Rozas V (2001) Detecting the impact of climate and disturbances on tree-rings of Fagus sylvatica L. and Quercus robur L. in a lowland forest in Cantabria, Northern Spain. Ann For Sci 58:237–251
Sass U (1993) Die Gefäße der Buche als ökologische Variable—Bildanalytische Erfassung, Dendroklimatologische Prüfung, ökologische Bewertung. Dissertation, Universität Hamburg
Sass U, Eckstein D (1995) The variability of vessel size in beech (Fagus sylvatica L.) and its ecophysiological interpretation. Trees 9:247–252
Schmitt U, Möller R, Eckstein D (2000) Seasonal wood formation dynamics of beech (Fagus sylvatica L.) and black locust (Robinia pseudoacacia L.) as determined by the “pinning” technique. J Appl Bot 74:10–16
Schmitt U, Jalkanen R, Eckstein D (2004) Cambium dynamics of Pinus sylvestris and Betula spp. in the northern boreal forest in Finland. Silva Fenn 38:167–178
Skomarkova MV, Vaganov EA, Mund M, Knohl A, Linke P, Boerner A, Schulze ED (2006) Inter-annual and seasonal variability of radial growth, wood density and carbon isotope ratios in tree rings of beech (Fagus sylvatica) growing in Germany and Italy. Trees 20:571–586. doi:10.1007/s00468-006-0072-4
Suzuki M, Kiyotsugu Y, Suzuki H (1996) Phenological comparison of the onset of vessel formation between ring-porous and diffuse-porous deciduoud trees in a Japanese temperate forest. IAWA J 17:431–444
Tarp P, Helles F, Holten-Andersen P, Larsen JB, Strange N (2000) Modelling near-natural silvicultural regimes for beech—an economic sensitivity analysis. For Ecol Manage 130:187–198
Werf van der GW, Sass-Klaassen U, Mohren GMJ (2007) The impact of the 2003 summer drought on the intra-annual growth pattern of beech (Fagus sylvatica L.) and oak (Quercus robur L.) on a dry site in the Netherlands. Dendrochronologia 25:103–112
Whitmore FW, Zahner R (1966) Development of the xylem ring in stems of young red pine trees. For Sci 12:198–210
Z’Graggen S (1992) Dendrohistometrisch- klimatologische Untersuchung an Buchen (Fagus sylvatica L.). Dissertation, University of Basel
Zeide B (1993) Analysis of growth equations. For Sci 39:591–616
Zeide B (2004) Intrinstic units in growth modelling. Ecol Modell 175:249–259
Acknowledgments
Climatic and phenological data originated from the Environmental Agency of the Republic of Slovenia within the Ministry of the Environment and Spatial Planning. We thank Prof. Dr. Lučka Kajfež-Bogataj for enabling us to use them. We thank Marko Beber, Martin Zupančič and Luka Krže for their work in the field and laboratory. The work was funded by the Ministry of Higher Education, Science and Technology of the Republic of Slovenia, Research Program “Lesarstvo”, and by the Spanish Ministry of Education and Science, project CGL2005-04270.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.G. Jones.
Rights and permissions
About this article
Cite this article
Čufar, K., Prislan, P., de Luis, M. et al. Tree-ring variation, wood formation and phenology of beech (Fagus sylvatica) from a representative site in Slovenia, SE Central Europe. Trees 22, 749–758 (2008). https://doi.org/10.1007/s00468-008-0235-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-008-0235-6