Abstract
The seasonal variation in δ13C values was measured in leaves from 17 upper canopy, five mid-canopy and in four gap tree species, as well as in five epiphyte and five vine species, in a seasonally dry lowland tropical forest at Parque Natural Metropolitano near Panama City, Republic of Panama. No seasonal variation was detected in the δ13C values of mature exposed leaves from either the upper or mid-canopy. However, canopy position did influence the δ13C value. The mean isotopic composition of leaves from the mid-canopy was more negative than that of the upper canopy throughout the year. The δ13C value was also influenced by leaf development, with juvenile leaves on average 1.5‰ less negative than mature leaves. The five epiphyte species exhibited δ13C values that were typical of crassulacean acid metabolism (CAM). Codonanthe uleana, with isotopic values of −19.9 to −22.1‰, is only the second species in the Gesneriaceae reported to express CAM, whereas values between −14.6 and −22.0‰ indicate that Peperomia macrostachya can exhibit different degrees of CAM. The isotopic composition of exposed mature leaves from the vines showed little interspecific variation and was similar to the upper-canopy leaves of the trees.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The δ13C value of leaves is widely used to identify the pathway of photosynthesis and to estimate the water-use efficiency (WUE) of plants in natural vegetation assemblages. Such analyses can be successful because the principal carboxylases of the C3, C4 and CAM pathways of photosynthesis discriminate to different extents against 13C, and because H2O loss and CO2 uptake in leaves are related to the intercellular partial pressure of CO2/ambient partial pressure of CO2, p i/p a, which is in turn related to δ13C (Farquhar et al. 1989). Because of their size, trees are hydraulically constrained, which affects their ability to acquire carbon (Meinzer 2003; Tyree 2003). Canopy leaves from trees of tropical forests often show pronounced “midday depression” in stomatal conductance and CO2 uptake when subjected to high solar radiation, as is known from desert plants, a manifestation of the fact that tight control of H2O and CO2 fluxes is of paramount importance in these exposed habitats (Zotz et al. 1995).
This study was undertaken to explore whether there is interspecific and seasonal variation in the carbon isotope signatures of canopy vegetation within a seasonally dry tropical forest composed of species with diverse life forms and leaf phenologies. To this end, and aided by the availability of a canopy crane, we measured the δ13C values of mature and juvenile leaves from trees in an upper canopy, a mid-canopy, a gap, and in associated epiphytes and vines growing on the trees.
Materials and methods
Study site
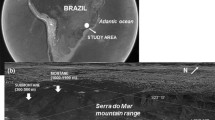
Plant material was collected in March, April, July and November 1994 from trees growing in a 75- to 150-year-old secondary growth semi-deciduous tropical lowland forest in the 265 ha Parque Natural Metropolitano (PNM; 8°58′ N, 79°23′ W), near Panama City, Republic of Panama. Access to the canopy was afforded by a 42-m canopy crane operated by the Smithsonian Tropical Research Institute (STRI; Parker et al. 1992).
The study site receives an average rainfall of ca. 1,800 mm a−1, 92% of which falls from May to December (for 9 years of climate data at the site, see the STRI Web site at http://striweb.si.edu/esp/physical_monitoring/download_pnm.htm). Further climate data for PNM are provided in Winter et al. (2001).
Species studied
Leaves were sampled from the following species:
-
Upper canopy: Acrocomia aculeata (Jacq.) Lodd. ex Mart. (Arecaceae), Albizia adinocephala (Donn. Sm.) Britton & Rose (Fabaceae), Anacardium excelsum (Bertero & Balb. ex Kunth) Skeels (Anacardiaceae), Antirrhoea trichantha Hemsl. (Rubiaceae), Astronium graveolens Jacq. (Anacardiaceae), Castilla elastica Sessé ex Cerv. (Moraceae), Cecropia longipes Pittier (Cecropiaceae), Cecropia peltata L. (Cecropiaceae), Chrysophyllum cainito L. (Sapotaceae), Cordia alliodora (Ruiz & Pav.) Oken (Boraginaceae), Didymopanax morototoni (Aubl.) Decne. & Planch. (Araliaceae), Enterolobium cyclocarpum (Jacq.) Griseb. (Fabaceae), Ficus insipida Willd. (Moraceae), Ficus maxima Mill. (Moraceae), Luehea seemannii Triana & Planch. (Tiliaceae), Pseudobombax septenatum (Jacq.) Dugand (Bombacaceae) and Spondias mombin L. (Anacardiaceae).
-
Mid-canopy: Annona spraguei Saff. (Annonaceae), Nectandra gentlei Lundell (Lauraceae), Phoebe cinnamomifolia (Kunth) Nees (Lauraceae), Xylopia frutescens Aubl. (Annonaceae) and Zuelania guidonia (Sw.) Britton & Millsp. (Flacourtiaceae).
-
Gap: Carica papaya L. (Caricaceae), Castilla elastica Sessé ex Cerv. (Moraceae), Ficus insipida Willd. (Moraceae) and Piper sp. (Piperaceae).
-
Epiphytes: Aechmea tillandsioides (Mart. ex Schult. & Schult. f.) Baker (Bromeliaceae), Codonanthe uleana Fritsch (Gesneriaceae), Epidendrum imatophyllum Lindl. (Orchidaceae), Epiphyllum phyllanthus (L.) Haw. (Cactaceae) and Peperomia macrostachya (Vahl) A. Dietr. (Piperaceae).
-
Vines: Arrabidaea patellifera (Schltdl.) Sandwith (Bignoniaceae), Bonamia trichantha Hallier f. (Convolvulaceae), Gouania lupuloides (L.) Urb. (Rhamnaceae), Mikania leiostachya Benth. (Asteraceae) and an unidentified species.
From each plant, between 3 and 12 leaves were collected and oven-dried at 65°C prior to carbon isotope analyses.
Excision of intercostal tissue from Anacardium excelsum
A mature Anacardium excelsum leaf (13.5 cm long) was harvested from the upper canopy (34 m) and oven-dried at 65°C. Intercostal tissue between the mid-rib and the leaf periphery was sampled at 3–5 mm intervals between ribs 2 and 6 and between ribs 9 and 13 of the first-order ribs numbered from the apex.
Carbon isotope ratio determinations
Carbon isotope ratios were determined for CO2 derived from the leaf samples at the Duke University Phytotron (Durham, NC) using isotope ratio mass spectrometry (Crayn et al. 2001; Pierce et al. 2002). Following the appropriate corrections for other isotopes, the abundance of 13C in each sample was calculated relative to the abundance of 13C in standard CO2 that had been calibrated against Pee Dee belemnite (Belemnitella americana). Relative abundance was determined using the relationship
Results and discussion
The influence of canopy position on leaf δ13C value
The interspecific variation in δ13C values of mature sun-exposed leaves harvested over an annual season from 17 C3 tree species in the upper canopy of a seasonally dry lowland tropical forest in Panama ranged between −29.6 and −25.4‰ (Table 1). The range in isotopic composition is small considering the pronounced seasonality and the phenotypic diversity at the site, which contains deciduous and semi-deciduous species, as well as some evergreens such as Ficus spp. and Acrocomia aculeata, a palm (Kitajima et al. 1997). The trees included pioneers with relatively short-lived leaves, such as Cecropia spp., early succession species such as Castilla elastica and Antirrhoea trichantha with leaf lifespans of about 6 months, early to late succession species such as Anacardium excelsum, Enterolobium cyclocarpum and Luehea seemannii with longer-lasting leaves, the deciduous emergent Pseudobombax septenatum, and the rainy season deciduous species Cordia alliodora.
Despite the small range of isotopic values in the upper canopy, a vertical gradient in leaf isotopic composition was detected (Fig. 1). The isotopic composition, averaged across the seasons, of the mature exposed mid-canopy leaves was significantly more negative than that of the upper canopy (P<0.01; \( \bar{x}_{{{\text{top}}}} = - 27.4\permille \pm 1.1\;{\text{SD}} \), \( \bar{x}_{{{\text{mid}}}} = - 29.9\permille \pm 1.0\,{\text{SD}} \)).
Relationship between δ13C value and height above the forest floor for mature sun-exposed leaves from upper-canopy (○) and mid-canopy (shaded circle) trees, from trees in a gap (▵) and from epiphytes (•) growing in a seasonally dry tropical lowland forest at Parque Natural Metropolitano, Panama. Samples were harvested in July 1994. A regression line (y=a+bx, where a=90.79053, b=2.46928 and r 2=0.339) is fitted to the upper and mid-canopy values
The principal contributors to more negative leaf isotopic values in the mid-canopy are probably the reductions in light intensity and vapour pressure deficits (VPDs) that are associated with the more sheltered canopy layers. This can result in a higher ratio of stomatal conductance to net photosynthesis, and consequently greater p i/p a ratios, i.e. a reduction in the diffusion component and a greater expression of the Rubisco component of isotopic fractionation (Farquhar et al. 1989). This effect was particularly pronounced in shade leaves of Anacardium excelsum growing at about the height of the mid-canopy trees, but sampled from deep within the crown of a 34 m upper-canopy tree (Table 1). The shade leaves had δ13C values that were 2.9–3.8‰ more negative than sun-exposed leaves. Low isotope values may also be exhibited when trees assimilate more isotopically depleted CO2, which is produced during soil or plant respiration (Medina and Minchin 1980; Sternberg et al. 1989). The contribution of the respiratory δ13C signal to the isotopic signature of mid- to upper-canopy leaves at the PNM crane site is probably minor. During daylight hours, when plants are photosynthesizing, the [CO2] measured within 10 cm of the soil surface during wet and dry seasons did not diverge extensively from the above-canopy concentration, rarely exceeding 450 ppm (Holtum and Winter 2001). At the heights and conditions at which leaves were sampled during this study, the dilution of ambient CO2 by respiratory CO2 should thus be small (Broadmeadow et al. 1992; Berry et al. 1997; Buchmann et al. 1997; Ometto et al. 2002).
Leaves from the upper surfaces of trees growing in a gap exhibited mean δ13C values that were similar to the upper canopy throughout the season, which suggests that the combined effects of VPD, light intensity and respiratory CO2 on isotopic composition were similar at the two forest sites (Table 1).
For a mature sun-exposed leaf from the upper canopy of A. excelsum sampled in March 1994, the mean ± SD δ13C value of 29 intercostal segments excised from the mid-rib to the leaf margin between the 2nd and 6th first-order ribs was −26.4±0.1, and the value for 22 segments sampled from between the 9th and 13th first-order ribs was −26.3±0.2.The similarity of isotopic composition, which was at the resolution limits of the mass spectrometer, of the 51 intercostal samples from a single mature leaf demonstrates that although stomatal aperture may often be patchy across the intercostal regions such that p i/p a may vary locally across pneumatically isolated patches (Beyschlag et al. 1992), any variation in δ13C values must be extremely local, at least in Anacardium. The observation validates the widely used procedure of taking samples for isotope determination from the centre or top-centre of the leaf blade in order to maximize the sample of the intercostal tissue and to minimize the contributions of juvenile cells, non-photosynthetic mid-rib or petiolar material that may contain an atypical δ13C composition.
δ13C value is influenced by leaf age
Juvenile leaves in the upper canopy exhibited mean δ13C values that were 1.5‰ less negative than for mature leaves (P<0.01, paired two-tailed t-test). In the nine species in which isotopic composition was measured during a temporal transition from young to old leaves (Albizia adinocephala, Antirrhoea trichantha, Castilla elastica, Cecropia longipes, Enterolobium cyclocarpum, Pseudobombax septenatum, Spondias mombin, Annona spraguei and Zuelania guidonia in Table 1), mature leaves exhibited more negative δ13C values in every instance. In Anacardium excelsum and Ficus insipida, for which juvenile and mature leaves were sampled concurrently, the mature leaves also had more negative δ13C values. Similar differences have been measured between juvenile and mature leaves in temperate and tropical species (Lowden and Dyck 1974; Sobrado and Ehleringer 1997; Terwilliger et al. 2001), though differences may sometimes be small (Ometto et al. 2002). Although it has been suggested that juvenile leaf isotopic values are less negative because the leaves are formed from carbon captured during drier periods when plants conserve water by operating at lower p i/p a, less negative δ13C values were also observed in plants that form leaves using carbon gained during the wet season (Terwilliger 1997). Terwilliger et al. (2001) speculated that the mechanistic basis for less negative δ13C values in juvenile leaves is that a significant proportion of the carbon in their structural carbohydrates is captured from the atmosphere by PEPC. It still remains to be seen to what extent fractionation processes involved in carbon storage in old leaves and stems and carbon remobilization from old leaves and/or stems contribute to less negative δ13C values in juvenile leaves.
δ13C values of vines
The δ13C signatures of sun-exposed leaves from five vine species sampled in March 1994 from the upper canopy were remarkably uniform, varying by less than 1‰ (Table 2), and did not significantly differ from the adjacent sun-exposed leaves of the upper-canopy trees that were sampled concurrently. It is not known whether this relative constancy of carbon isotope ratio is maintained throughout the year. Compared to trees, vines tend to allocate more biomass to photosynthetic tissues than to supporting structures (Castellanos 1991) and, in general, leaf production and turnover rates are greater in vines than in trees (Hegarty 1990). As a consequence, one might expect the δ13C of vine leaves to respond more rapidly to changing environmental conditions. The relative area of the canopy that is covered by vine and tree leaves certainly differs seasonally at the PNM site; during the dry season vine and tree leaves occupy 14% and 51% of the canopy area respectively whilst 35% is bare, whereas during the wet season vine and tree leaves cover 31% and 44% respectively whilst 25% is bare (Avalos and Mulkey 1999).
δ13C values of epiphytes
The δ13C values between −12.3 and −22.0‰ for canopy epiphytes were consistent with the operation of crassulacean acid metabolism (CAM) in all five species examined (Table 1; Silvera et al. 2005). The δ13C ratios of Aechmea tillandsioides, Epidendrum imatophyllum and Epiphyllum phyllanthus indicate that these species from three different families, the Bromeliaceae, Orchidaceae and Cactaceae, are strong CAM plants that gain about 74–89% of their carbon via dark CO2 fixation (Winter and Holtum 2002). A. tillandsioides exhibited strong CAM yet grew under extreme shade conditions, an ability also exhibited by some other bromeliads such as the terrestrial species Aechmea magdalenae (Skillman and Winter 1997; Skillman et al. 1999; Crayn et al. 2004). E. phyllanthus sampled in this study, growing well-shaded or in patchy shade, did not exhibit seasonal variation in δ13C values. In contrast, shadehouse-grown plants exposed to 9.2 mol m−2 day−1 exhibited little CAM when well-watered but predominately dark fixation when water-stressed (Andrade and Nobel 1997). Analogy between the shade-house and canopy-grown plants suggests that the plants in the canopy either experienced similar levels of water stress the year round, which is unlikely considering the wet-dry seasonality of the site, or that the extent of the expression of CAM in this species is also affected by other factors, such as light intensity.
Codonanthe uleana is only the second species within the Gesneriaceae known to express CAM. Guralnick et al. (1986) reported that under well-watered conditions C. crassifolia (Focke) can refix respiratory CO2 and accumulate titratable acidity, but does not exhibit net CO2 uptake in the dark (CAM cycling; Holtum and Winter 1999). When water-stressed, C. crassifolia closes its stomata in the dark, but still exhibits diurnal acid fluctuations (CAM idling). The δ13C value of −24.6‰ published for C. crassifolia is consistent with dark CO2 fixation contributing about 14% to the leaf carbon gain (Winter and Holtum 2002, 2005). In contrast, it can be calculated that C. uleana, with δ13C values of between −19.9 and −22.1‰, gains between 29% and 43% of its carbon from dark CO2 uptake. It is possible that more CAM species will be detected in the genus Codonanthe, which lies within the Episcieae, a tribe that contains the majority of epiphytic species within the subfamily Gesnerioideae (Smith 2000).
Peperomia macrostachya has been reported as one of many species in the genus that exhibits CAM cycling (Ting et al. 1985; Holthe et al. 1992). However, the isotopic range of −14.6 to −22.0‰ observed by us is equivalent to a contribution of dark CO2 fixation to net carbon gain of between 30% and 75%, suggesting that dark CO2 uptake makes a substantial contribution to growth, rather than merely reducing carbon loss by refixing respiratory CO2. The range of δ13C values measured for P. monostachya indicates that the species can respond to environmental changes by expressing CAM to variable degrees in the field, as is known for members of the neotropical genus Clusia (Holtum et al. 2004).
δ13C and WUE
The use of δ13C to estimate intrinsic WUE, integrated over the lives of leaves, is insensitive to variations in VPD because the relationship between WUE and δ13C is not causal; rather, both are independently linked with p i/p a (Farquhar et al. 1988). Therefore, the importance given to WUE of factors that independently influence leaf-to-air VPD may be unrecognized in studies of WUE based solely on δ13C. Although we observed little variation in δ13C values amongst exposed canopy leaves from a variety of tropical tree species, there could be substantial differences in the actual amount of H2O transpired per CO2 fixed due to differences in leaf morphology and orientation (Winter et al. 2005). The nature of the canopy itself can also influence VPD. Depending upon the extent of canopy closure and mixing of the air, evapotranspiration can increase the local humidity and reduce leaf temperatures, thereby lowering H2O loss without overly affecting p i/p a.
The most obvious changes in δ13C observed in the upper- and mid-canopy leaves were those associated with leaf development (Table 1). However, the sampling design of the isotopic analyses reported here was of insufficient frequency to enable us to comment upon the intrinsic WUE of seasonal leaf phenotypes (Kitajima et al. 1997). An isotope-based analysis of WUE of leaves of such phenotypes requires more frequent monitoring of leaf cohorts of known time of leaf initiation. It would be of particular interest to examine the water-use strategies of leaves of species that produce a second flush around the end of rainy season, which supports the production of reproductive structures.
References
Andrade JL, Nobel PS (1997) Microhabitats and water relations of epiphytic cacti and ferns in a lowland neotropical forest. Biotropica 29:261–270
Avalos G, Mulkey SS (1999) Seasonal changes in liana cover in the upper canopy of a neotropical dry forest. Biotropica 31:186–192
Berry SC, Varney GT, Flanagan LB (1997) Leaf δ13C in Pinus resinosa trees and understory plants: variation associated with light and CO2 gradients. Oecologia 109:499–506
Beyschlag W, Pfanz H, Ryel RJ (1992) Stomatal patchiness in mediterranean evergreen sclerophylls – phenomenology and consequences for the interpretation of the midday depression in photosynthesis and transpiration. Planta 187:546–553
Broadmeadow MSJ, Griffiths H, Maxwell C, Borland AM (1992) The carbon isotope ratio of plant organic material reflects temporal and spatial variations in CO2 within tropical forest formations in Trinidad. Oecologia 89:435–441
Buchmann N, Guehl J-M, Barigah TS, Ehleringer JR (1997) Interseasonal comparisons of CO2 concentrations, isotopic composition and carbon dynamics in an Amazonian rainforest (French Guiana). Oecologia 110:120–131
Castellanos AE (1991) Photosynthesis and gas exchange of vines. In: Putz FE, Mooney HA (eds) The biology of vines. Cambridge University Press, Cambridge, pp 181–204
Crayn DM, Smith JAC, Winter K (2001) Carbon-isotope ratios and photosynthetic pathways in the neotropical family Rapateaceae. Plant Biol 3:569–576
Crayn DM, Winter K, Smith JAC (2004) Multiple origins of crassulacean acid metabolism and the epiphytic habit in the Neotropical family Bromeliaceae. Proc Natl Acad Sci USA 101:3703–3708
Farquhar GD, Hubick KT, Condon AG, Richards RA (1988) Carbon isotope fractionation and plant water-use efficiency. In: Rundel PW, Ehleringer JR, Nagy KA (eds) Stable isotopes in ecological research. Springer, Berlin Heidelberg New York, pp 21–40
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537
Guralnick LJ, Ting IP, Lord EM (1986) Crassulacean acid metabolism in the Gesneriaceae. Am J Bot 73:336–345
Hegarty EE (1990) Leaf life-span and leafing phenology of lianas and associated trees during a rainforest succession. J Ecol 78:300–312
Holthe PA, Patel A, Ting IP (1992) The occurrence of CAM in Peperomia. Selbyana 13:77–87
Holtum JAM, Winter K (1999) Degrees of crassulacean acid metabolism in tropical epiphytic and lithophytic ferns. Aust J Plant Physiol 26:749–757
Holtum JAM, Winter K (2001) Are plants growing close to the floors of tropical forests exposed to markedly elevated concentrations of carbon dioxide? Aust J Bot 49:629–636
Holtum JAM, Aranda J, Virgo A, Gehrig HH, Winter K (2004) δ13C values and crassulacean acid metabolism in Clusia species from Panama. Trees 18:658–668
Kitajima K, Mulkey SS, Wright SJ (1997) Seasonal leaf phenotypes in the canopy of a tropical dry forest: photosynthetic characteristics and associated traits. Oecologia 109:490–498
Lowden J, Dyck W (1974) Seasonal variation in the isotope ratios and carbon in maple leaves and other plants. Can J Earth Sci 11:79–88
Medina E, Minchin P (1980) Stratification of δ13C values of leaves in an Amazonian rain forest. Oecologia 45:377–378
Meinzer FC (2003) Functional convergence in plant responses to the environment. Oecologia 134:1–11
Ometto JPHB, Flanagan LB, Martinelli LA, Moroera MZ, Higuchi N, Ehleringer JR (2002) Carbon isotope discrimination in forest and pasture ecosystems of the Amazon basin, Brasil. Global Biogeochem Cycles16:1109. DOI:10.1029/2001GB001462
Parker GG, Smith AP, Hogan KP (1992) Access to the upper forest canopy with a large tower crane. Bioscience 42:664–670
Pierce S, Winter K, Griffiths H (2002) Carbon isotope ratio and the extent of daily CAM use by Bromeliaceae. New Phytol 156:75–84
Silvera K, Santiago LS, Winter K (2005) Distribution of crassulacean acid metabolism in orchids of Panama: evidence of selection for weak and strong modes. Funct Plant Biol 32:397-407
Skillman JB, Winter K (1997) High photosynthetic capacity in a shade-tolerant crassulacean acid metabolism plant. Plant Physiol 113:441–450
Skillman JB, Garcia M, Winter K (1999) Whole-plant consequences of crassulacean acid metabolism for a tropical forest understory plant. Ecology 80:1584–1593
Smith JF (2000) Phylogenetic resolution within the tribe Episcieae (Gesneriaceae): congruence of ITS and NDHF sequences from parsimony and maximum-likelihood analyses. Am J Bot 87:883–897
Sobrado MA, Ehleringer JR (1997) Leaf carbon isotope ratios from a tropical dry forest in Venezuela. Flora 192:121–124
Sternberg LSL, Mulkey SS, Wright SJ (1989) Ecological interpretation of leaf carbon isotope ratios: influence of respired carbon dioxide. Ecology 70:1317–1324
Terwilliger VJ (1997) Changes in the δ13C values of trees during a tropical rainy season: some effects in addition to diffusion and carboxylation by rubisco? Am J Bot 84:1693–1700
Terwilliger VJ, Kitajima K, Le Roux-Swarthout DJ, Mulkey S, Wright SJ (2001) Intrinsic water-use efficiency and heterotrophic investment in tropical leaf growth of two neotropical pioneer tree species as estimated from δ13C values. New Phytol 152:267–281
Ting IP, Bates L, Sternberg LO, DeNiro MJ (1985) Physiological and isotopic aspects of photosynthesis in Peperomia. Plant Physiol 78:246–247
Tyree MT (2003) Hydraulic limits on tree performance, transpiration, carbon gain and growth of trees. Trees 17:85–100
Winter K, Holtum JAM (2002) How closely do the δ13C values of crassulacean acid metabolism plants reflect the proportion of CO2 fixed during day and night? Plant Physiol 129:1843–1851
Winter K, Holtum JAM (2005) The effects of salinity, crassulacean acid metabolism, and plant age on the carbon isotope composition of Mesembryanthemum crystallinum L., a halophytic C3-CAM species. Planta (in press)
Winter K, Aranda J, Garcia M, Virgo A, Paton SR (2001) Effect of elevated CO2 and soil fertilization on whole-plant growth and water use in seedlings of a tropical pioneer tree, Ficus insipida Willd. Flora 196:458–464
Winter K, Aranda J, Holtum JAM (2005) Carbon isotope composition and water-use efficiency in plants with crassulacean acid metabolism. Funct Plant Biol 32:381-388
Zotz G, Harris G, Königer M, Winter K (1995) High rates of photosynthesis in the tropical pioneer tree, Ficus insipida Willd. Flora 190:265–272
Acknowledgements
This study was supported by the Andrew W. Mellon Foundation and the Smithsonian Tropical Research Institute. J.A.M.H. received additional assistance from the JCU Special Studies Programme and from Dr R.G. Dunn
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holtum, J.A.M., Winter, K. Carbon isotope composition of canopy leaves in a tropical forest in Panama throughout a seasonal cycle. Trees 19, 545–551 (2005). https://doi.org/10.1007/s00468-005-0413-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-005-0413-8