Abstract
IgA nephropathy (IgAN) is the most common glomerulonephritis worldwide. It is diagnosed based on clinical and histological features including predominant IgA deposits in kidney biopsy. The multi-hit theory, based on the production of GDIgA1 and anti-GDIgA1 antibodies, and complement activation via alternative and lectin pathways and also a genetic tendency are crucial in the pathogenesis of IgAN. The aim of the present review is to summarize the utility of routine diagnostic tests in IgA nephropathy, such as IgA and C3 in serum and kidney biopsy specimens, for predicting the disease progression. The paper also contains data on new markers used in the diagnosis and prognosis of IgA nephropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
IgA nephropathy (IgAN) is the most common glomerulonephritis worldwide. It is diagnosed based on clinical and histological features including predominant IgA deposits in kidney biopsy. IgAN reduces life expectancy by more than 10 years and leads to kidney failure in 20–40% of patients by 20 years after the diagnosis [1,2,3].
In patients after kidney transplantation, the disease recurs in 50% of cases within 10 years [1]. Hypertension, elevated serum creatinine concentration, proteinuria, old age, male sex, and the absence of macroscopic hematuria have been found to be independent predictors of an unfavorable outcome [4,5,6].
Various biomarkers have been used to predict the disease progression, including pediatric Prediction Tool models, and new predictors continue to be sought [7]. According to the WHO definition, a biomarker is any substance, structure, or process, which may be measured in an organism, or its products, which affects, indicates or predicts the frequency, or the outcome of a disease [8].
In the present review, we summarize the utility of traditional biomarkers in IgAN, such as IgA and C3, for predicting the disease progression, and discuss other substances, studied in the pathogenesis of IgAN, which are or may become new biomarkers.
Epidemiology and clinical presentation
IgAN is most common in Asia, where the prevalence is 30–60% in all kidney biopsies, compared to 20–30% in Europe and below 5% in Africa [9,10,11]. The disease course and its late sequelae are more severe in Asia compared to European populations [9, 12].
Clinical manifestations may vary and include asymptomatic microscopic hematuria, proteinuria with micro- or macroscopic hematuria, overt hematuria, nephritic syndrome, or even rapidly progressive glomerulonephritis. In pediatric patients with IgAN in Poland, the most frequent symptom was microscopic hematuria with non-nephrotic proteinuria observed in 50% of patients, isolated micro or macroscopic hematuria in 29%, and nephrotic proteinuria with micro- or macroscopic hematuria in 21% of cases. Macroscopic hematuria was noticed as a first symptom of IgAN in 29% of children and kidney failure in 39% [13]. The clinical course may vary between adults and children [14, 15]. In adults it is a slow disease, leading to kidney failure in 30–40% of patients. In children, it may present with mild symptoms, but 50% of patients require kidney replacement therapy by the age of 50 [14]. Proteinuria in adults reflects the presence of chronic lesions, while in children with IgAN, it is associated with glomerular proliferative lesions [15].
A kidney biopsy showing predominant IgA deposits is the only, albeit invasive diagnostic tool for IgAN. Histopathological specimens are evaluated using the Oxford MESTC classification: 1—present, 0—absent; M—mesangial hypercellularity; E—endocapillary hypercellularity; S—segmental sclerosis/adhesion; T—tubular atrophy/interstitial fibrosis T0 0–25%, T1 26–50%, T2 > 50%; and C—crescents, C0 0%, C1 1–25%, C2 > 25% [16, 17].
Pathogenesis of IgAN
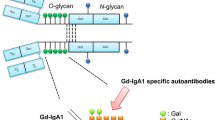
The pathogenesis of IgAN is described by the multi-hit theory. This theory assumes that the initial pathogenetic event (first hit) is a production of galactose-deficient IgA1 (GdIgA1). In the hinge region, 3–6 glycans are attached but not all of them contain a galactose moiety, which is due to impaired expression or activity of glucosyltransferases involved in post-translation modification of IgA1 [18,19,20,21].
Available data also show that abnormal IgA1 glycosylation precedes clinically overt disease and is a hereditary risk factor for the development of IgAN.
In the pathogenesis of IgAN, synthesis of GDIgA1 and IgA is also significantly affected by APRIL (a proliferation-inducing ligand), homologous with B-cell activating factor (BAFF). APRIL is coded by the TNFSF13 gene which is associated with IgA synthesis in the general population and in patients with IgAN [22, 23]. In mice, overexpression of BAFF resulted in an increased GDIgA1 level and more severe proteinuria and microscopic hematuria in IgAN [24]. An increased APRIL level leads to overexpression of its receptors on B cells and increased GDIgA1 production, which plays a role in the pathogenesis of IgAN [22].
However, an elevated GDIgA1 level is not sufficient to induce IgAN [25,26,27]. The presence of GDIgA1 leads to the formation of specific IgA or IgG antibodies (second hit). Anti-GDIgA1 IgG level correlates with the severity of disease and proteinuria [21]. Another major contributing factor is the genetic effect of MHC class II antigens, HLA-DQB1, DQA, and DRB1, of which DQB1*0602 reduces the risk of incident IgAN by 50% [21, 28].
Production of anti-GDIgA1 may be induced by exposure to infectious or dietary antigens in patients with susceptibility alleles, which are discovered in GWAS (genome-wide association study) [25, 26]. The formation of circulating immune complexes or deposits of abnormally glycosylated IgA1, which bind to anti-glycan antibodies and form in situ immune complexes in the mesangium, constitutes the third hit. However, circulating complexes may also be identified in healthy subjects or those with IgA vasculitis without nephropathy [21]. IgA-IgA complexes are considered non-nephritogenic. IgA-IgG complexes are relatively large and do not enter the Disse space and thus are not catabolized by the ASGPR receptor (asialoglicoprotein receptor), specific for IgA1, but enter the kidney circulation. They bind to the mesangium more effectively than IgA1 present in a non-immune complex form [21, 29, 30], which results in cellular and mesangial matrix proliferation, and production of cytokines including tumor necrosis factor (TNF), interleukin-6, and transforming growth factor (TGF) beta, which activate the alternative complement pathway and change glomerular permeability by modifying podocyte gene expression (fourth hit) [21, 25, 31,32,33,34].
In addition, the binding of soluble CD89 with circulating polymeric IgA1 may increase the nephritogenicity of immune complexes [25, 35, 36]. The receptor for transferrin (CD71) and transglutaminase 2 (TGase 2) on mesangial cells effectively binds immune complexes containing GDIgA1 [21, 35, 37, 38] which results in additionally increased mesangial expression and leads to the complement system activation [25]. The role of genetic factors is also important, and the existence of susceptibility alleles has been confirmed in genome-wide association studies [25,26,27]. The pathogenesis of IgAN is shown in Fig. 1.
The available literature also suggests a major role of the complement system in IgAN. Complement activation increases the inflammatory process and leads to kidney tissue damage. Complement activation in IgAN occurs via the alternative or the lectin pathway (MBL, mannose binding lectin), resulting in the presence of C3 and the absence of C1q in kidney biopsy specimens. The distribution of C3 follows that of IgA in as many as 90% of kidney biopsy specimens [3, 39]. Activation of the alternative pathway is inhibited by FH (factor H) and stimulated by FHR1-5 (factor H related protein 1–5).
Activation of the lectin pathway by MBL deposition was observed in 20–25% of cases. It also determined more severe kidney damage [40]. A marker of the activation of this pathway is the presence of C4d in kidney biopsy specimens, which was associated with worse patient outcomes during 20-year follow-up in Spanish studies [41, 42]. Complement activation pathways are shown in Fig. 2.
Complement activation pathways. MBL - mannose binding lectin; MASP - mannose associated serine proteases; GDIgA1-IC - galactose deficient IgA1 immunocomplexes; MCP(CD46) - membrane cofactor protein; FH - factor H; FI - factor I; CR1(CD35) - complement receptor 1; FHR 1–5 - factor H related proteins 1–5; FB - factor B; FD, - factor D; P - properdin; DAF(CD55) - decay-accelerating factor. Regulators are in green
In genetic GWAS studies the presence of CFHR1/CFHR3 (complement factor H-related protein 1/complement factor H-related protein 3) deletion results in a lower FHR1 level and protects from excessive complement activation, as CFHR1 and CFHR3 compete with FH for binding with C3b, which is the key activator of the terminal complement pathway [25]. The presence of homozygosity of this deletion reduces the risk of IgAN by 45% and by 26% in heterozygosity [26].
Serum biomarkers
IgA
Japanese data indicate that an elevated serum IgA level is found in 50–70% of adults and in only 16% of children with IgAN [43] while in the Polish pediatric population, IgA levels above the reference range were found in 52% of patients [44].
In the study by Tomino et al., multivariate analysis did not show the prognostic significance of elevated serum IgA level but the cutoff of > 315 mg/dL may serve as a diagnostic standard in adult patients [45].
In the present author’s study of 89 children, elevated serum IgA level at baseline was significantly more frequently associated with M1 and S1 using the Oxford classification but the Kaplan–Meier curve did not show a relation between kidney survival and baseline IgA level [43]. In another study, pre-biopsy IgA level was significantly higher in children with IgAN without proteinuria compared to those with either nephrotic or non-nephrotic proteinuria [44].
Some authors point to the importance of biomarker panel differentiating IgAN from other kidney diseases. Yanagawa et al. compared serum levels of IgA, IgG, Gd-IgA1, Gd-IgA1-specific IgG, and Gd-IgA1-specific IgA in 135 IgAN adult patients, 79 patients with chronic kidney disease (CKD), and 106 healthy controls. Forty-one percent of IgAN patients have been found to have higher serum levels of IgA, and in 91% higher serum levels of IgG anti-GDIgA1 [46].
The studies on the importance of serum IgA are now of historic interest, and current research focuses on the importance of GDIgA1 and anti-GDIgA1. While these tests are not yet readily available in routine practice in all nephrological centers, they may replace IgA assays for the diagnosis, monitoring, and prognostication of IgAN in the future.
Serum complement component C3
Due to the role of complement activation in the pathogenesis of IgAN, studies in recent years also evaluated the prognostic importance of C3 level. In a Korean study of 343 adult patients with IgAN by Kim et al., serum C3 level < 90 mg/dL was found to be an independent predictor of poor outcomes [47].
In contrast to adults, the Polish study of 166 children from the Polish Pediatric IgAN Registry did not show such an association for C3 level below the reference range, although, in children with serum C3 level below the reference range, the glomerular filtration rate (GFR) at follow-up was lower compared to the group with normal baseline serum C3 level (p = 0.07). It may result from the fact that a low baseline serum C3 level was found in only 8% of patients in the study group [48].
In another pediatric study, a significantly lower C3 level was found in the T1 group compared to the T0 group (p < 0.05), with no significant differences between M1, E1, and S1 vs. M0, E0, and S0, respectively [44].
Current studies focus on activated C3 (actC3), which is present in 50% of patients. Based on the studies by Zwirner et al., elevated actC3 level is a predictor of disease progression and loss of kidney function with 75% sensitivity and 89% specificity [3, 49].
In addition, other types of biomarker activity, of alternative pathways of complement activation, have been studied in IgAN patients, such as urinary MAC, FH and P levels. Onda et al. have shown that MAC and FH in urine correlates with creatinine levels, proteinuria, presence of glomerular fibrosis, and sclerosis and may be useful as a marker of kidney failure in patients with IgAN [50].
Lower level of MBL in adult patients (< 100 ng/ml) is associated with more frequent infections with gross hematuria and independently with poor outcome (hazard ratio 5.18; 95% CI 2.50–10.72; p < 0.001). High level of MBL (> 3540 ng/ml) is associated with heavy proteinuria and higher percentage of crescents. This data points to the importance of MBL in pathogenesis of IgAN in various mechanisms [51].
A Chinese genetic study that included 1543 adult patients with IgAN confirmed an association between CFHR1 and CFHR3 deletion and CFH and C3 level. CFH positively correlated with levels of C3 and negatively with mesangial C3 deposits. CFH, CFHR1, and CFHR3 affect complement activation in IgAN [29, 52].
IgA/C3 ratio
The IgA/C3 ratio may help differentiate between IgAN and other glomerulopathies. In a Japanese study that included 195 patients with IgAN, 111 patients with other glomerulonephritides, and 418 healthy subjects, serum IgA/C3 ratio was the highest in patients with IgAN. Serum IgA/C3 in patients with IgAN was affected by both reduced C3 level and elevated IgA level [45]. In addition, studies from Asia also propose the IgA/C3 ratio as a prognostic marker. Higher values of IgA/C3 are associated with more severe histological lesions, and poor outcomes with worse kidney function, persistent proteinuria, and hematuria [3, 53,54,55].
In a Chinese study of 217 adult patients with IgAN, among whom 9.7% reached the endpoint of doubling of serum creatinine or kidney failure by 36 months, multivariate analysis showed that serum IgA/C3 ratio ≥ 3.32 was an independent adverse prognostic factor (RR = 4.31, 95% CI 1.33–13.96) [56]. In a European pediatric population of 89 children with IgAN, a prognostic value of the IgA/C3 ratio for kidney survival was not confirmed but the IgA/C3 ratio was shown to be a predictor of kidney biopsy specimen grading by the Oxford histological classification [44], as shown below (Table 1).
In another pediatric study of 44 Japanese children with IgAN, a prognostic value was shown for a combination of the IgA/C3 ratio > 2.68 and grade ≥ 2 C3 deposits in kidney biopsy [55]. In a European study of 95 adult patients with IgAN, it was confirmed that an IgA/C3 serum ratio > 2.91 may be considered an adverse prognostic factor for kidney survival with a specificity of 68% and sensitivity of 55% [57]. In a group of Japanese patients treated with steroids and tonsillectomy, a higher IgA/C3 ratio was associated with a higher number of recurrences [58].
Kidney histology
IgA deposits
Predominant IgA deposits in kidney biopsy are necessary for the diagnosis of IgAN. However, asymptomatic isolated IgA deposits were found in 6.9% of patients in the study by Varis et al. that included 753 kidney biopsies, and in 16.1% of patients in a Japanese study [59, 60]. It is unclear whether mesangial IgA deposits directly cause hematuria. Another study did not find an effect of severity of mesangial deposits and glomerular damage [61].
IgA deposits persist in some patients despite clinical remission of disease [62].
In a study by Polish researchers of 81 children with IgAN, we also found no correlation of the severity or location of IgA deposits on histopathological examination, with proteinuria, creatinine and GFR, although the percentage of children with GFR < 90 was significantly higher in the group of children with IgA + 3, + 4 versus + 1, + 2 [63].
On histopathological examination, kidney GDIgA1 is also identified using KM55 antibodies. According to US authors, immunostaining does not differentiate primary from secondary forms of IgAN, although negative or low staining may be indicative of secondary or incident IgAN without signs of nephritis, and thus may help exclude primary IgAN (especially when GD-IgA1 ≤ + 1) [64].
Roos et al. found deposits of IgA1 but not IgA2 in kidney bioassays of IgAN patients [65]. Their study also showed the presence of MBL I L-ficolin in glomeruli, which is not necessarily associated with increased serum concentrations. The biopsy finding of these lectin pathway activators was associated with increased mesangial proliferation, extracapillary proliferation, glomerular sclerosis, and interstitial infiltration, as well as increased proteinuria. These studies confirm the involvement of MBL and L-ficolin in IgAN progression. IgA polymers can activate the lectin pathway by binding to MBL through glycans present in a heavy chain of IgA [40, 65].
C3 deposits
The concomitant presence of C3 deposits in kidney biopsy are currently considered confirmatory for IgAN, in contrast to asymptomatic IgA deposits. In IgAN, C3 deposits are present together with IgA deposits in 90% of biopsies [3]. In the study by Caliskan et al. in 156 adult patients, grade ≥ 2 C3 deposits were significantly more commonly accompanied by a reduced serum C3 level, and the number of patients with GFR (glomerular filtration rate) reduction by > 50% compared to baseline was also higher in the group with grade ≥ 2 C3 deposits [6]. In the study by Kim and Koo of 343 adult patients, 21% showed grade ≥ 2 C3 deposits. An effect of grade ≥ 1 C3 deposits was also shown on the occurrence of kidney failure or doubling of serum creatinine [47].
Similarly, in the current author’s study in a pediatric population of 148 patients, reduced kidney survival was shown in the group with grade ≥ 1 C3 deposits in kidney biopsy, as well as in boys and patients with baseline GFR < 90 mL/min [48].
Proteomic studies by Paunas et al. also confirmed an association between the presence of C3 deposits and progression of IgAN [66, 67].
C4d staining may be useful in differentiating between patients with a good and unfavorable prognosis. Espinisa et al. showed that 10-year kidney survival was 43.9% in C4d-positive patients versus 90.9% in C4d-negative patients (log-rank, p = 0.0005) [41]. Deposits of other elements of the alternative complement pathway such as FH, C5, and properdin may also be found in kidney biopsy [34]. The available data on the utility of IgA and C3 as prognostic markers are summarized in Table 2.
Summary
The present review discusses the utility of widely available, routine testing for IgA and C3 in serum and kidney biopsy specimens for the diagnosis and prognostication of IgAN, but studies show that even more important are GDIgA1 and anti-GDIgA1 that have been established as more sensitive and specific markers.
Regarding the complex mechanisms of IgAN pathogenesis, it is also important to consider the possible utility of other novel biomarkers resulting from activation of the alternative and lectin pathway of complement and genetic tests. Therefore, it seems that panels of biomarkers may be the future for the diagnosis and prognosis of IgAN in children and adults.
Key summary points
-
IgA nephropathy (IgAN) is the most common glomerulonephritis worldwide. It is diagnosed based on clinical and histological features including predominant IgA deposits in kidney biopsy.
-
The pathogenesis of IgA nephropathy is described by the multi-hit theory, in which immune, genetic, and environmental factors, as well as activation of the complement system by alternative and lectin pathways, play a role.
-
Routine testing for IgA and C3 in serum and kidney biopsy specimens for the diagnosis and prognostication in IgAN are useful. Studies also show an important role for GDIgA1 and anti-GDIgA1, which have been found to be more sensitive and specific markers.
-
Regarding the complex mechanisms of IgAN pathogenesis, it is also important to consider the possible utility of other novel biomarkers resulting from activation of the alternative and lectin pathway of complement and genetic tests. Therefore, it seems that panels of biomarkers may be the future for the diagnosis and prognosis of IgAN in children and adults.
Multiple choice questions (more than 1 answer may be correct) (answers can be found after the reference list).
-
1.
IgA nephropathy:
-
a.
is the most common nephropathy in the world
-
b.
can lead to kidney failure in 20–40% of patients within 20 years
-
c.
is most prevalent in Asians, followed by Caucasians, and relatively rare in Africans
-
d.
kidney biopsy is the gold standard diagnostic method
-
e.
the diagnosis is made based on elevated serum IgA level
-
a.
-
2.
Clinical manifestations of IgA nephropathy include:
-
a.
erythrocyturia or hematuria
-
b.
proteinuria
-
c.
asymptomatic leukocyturia
-
d.
hypertension
-
e.
kidney failure
-
a.
-
3.
Factors important in the pathogenesis of IgA nephropathy include:
-
a.
formation of oligoglycosylated IgA1
-
b.
formation of anti-glycan antibodies
-
c.
genetic predisposition
-
d.
complement activation by the classical pathway
-
e.
complement activation by the alternative pathway
-
a.
-
4.
Serum IgA level:
-
a.
may be elevated in about 50% of patients with IgA nephropathy
-
b.
has no prognostic significance
-
c.
indicates GDIgA1
-
d.
is related to creatinine level
-
e.
is associated with lower proteinuria
-
a.
-
5.
C3 complement component:
-
a.
serum C3 level < 90 mg/dL in adults is an adverse prognostic factor
-
b.
serum C3 level correlates positively with the severity of C3 deposits in kidney biopsy
-
c.
elevated C3 level is present in patients with the protective CFHR1/ CFHR3deletion
-
d.
severe C3 deposits in kidney biopsy are associated with better kidney survival
-
e.
may be a component of IgA/C3 and have prognostic significance
-
a.
References
Berthoux FC, Mohey H, Afiani A (2008) Natural history of primary IgA nephropathy. Semin Nephrol 28:4–9. https://doi.org/10.1016/j.semnephrol.2007.10.001
Hastings MC, Bursac Z, Julian BA, Villa Baca E, Featherston J, Woodford SY, Bailey L, Wyatt RJ (2018) Life expectancy for patients from the Southeastern United States with IgA nephropathy. Kidney Int Rep 3:99–104. https://doi.org/10.1016/j.ekir.2017.08.008
Rizk DV, Maillard N, Julian BA, Knoppova B, Green TJ, Novak J, Wyatt RJ (2019) The emerging role of complement proteins as a target for therapy of IgA nephropathy. Front Immunol 10:504. https://doi.org/10.3389/fimmu.2019.00504
Caliskan Y, Kiryluk K (2014) Novel biomarkers in glomerular disease. Adv Chronic Kidney Dis 21:205–216
Tan M, Li W, Zou G, Zhang C, Fang J (2015) Clinicopathological features and outcomes of IgA nephropathy with hematuria and/or minimal proteinuria. Kidney Blood Press Res 40:200–206
Caliskan Y, Ozluk Y, Celik D, Oztop N, Aksoy A, Ucar AS, Yazici H, Kilicaslan I, Sever MS (2016) The clinical significance of uric acid and complement activation in the progression of IgA nephropathy. Kidney Blood Press Res 41:148–157. https://doi.org/10.1159/000443415
Barbour SJ, Coppo R, Er L, Russo ML, Liu ZH, Ding J, Katafuchi R, Yoshikawa N, Xu H, Kagami S, Yuzawa Y, Emma F, Cambier A, Peruzzi L, Wyatt RJ, Cattran DC, International IgA Nephropathy Network (2021) Updating the International IgA Nephropathy Prediction Tool for use in children. Kidney Int 99:1439–1450. https://doi.org/10.1016/j.kint.2020.10.033
World Health Organization (2001) Environmental Health Criteria 222. Biomarkers in Risk Assessment: Validity and Validation. http://www.inchem.org/documents/ehc/ehc/ehc222.htm. Accessed 2001
Zhang H, Barratt J (2021) Is IgA nephropathy the same disease in different parts of the world? Semin Immunopathol 43:707–715. https://doi.org/10.1007/s00281-021-00884-7
Schena FP, Nistor I (2018) Epidemiology of IgA nephropathy: a global perspective. Semin Nephrol 38:435–442
Lai KN, Tang SC, Schena FP, Novak J, Tomino Y, Fogo AB, Glassock RJ (2016) IgA nephropathy. Nat Rev Dis Primers 2:16001
Barbour SJ, Cattran DC, Kim SJ, Levin A, Wald R, Hladunewich MA, Reich HN (2013) Individuals of Pacific Asian origin with IgA nephropathy have an increased risk of progression to end-stage renal disease. Kidney Int 84:1017–1024
Mizerska-Wasiak M, Turczyn A, Such A, Cichoń-Kawa K, Małdyk J, Miklaszewska M, Pietrzyk J, Rybi-Szumińska A, Wasilewska A, Firszt-Adamczyk A, Stankiewicz R, Szczepańska M, Bieniaś B, Zajączkowska M, Pukajło-Marczyk A, Zwolińska D, Siniewicz-Luzeńczyk K, Tkaczyk M, Gadomska-Prokop K, Grenda R, Demkow U, Pańczyk-Tomaszewska M (2016) IgA nephropathy in children: a multicenter study in Poland. Adv Exp Med Biol 952:75–84. https://doi.org/10.1007/5584_2016_65
Coppo R, Robert T (2020) IgA nephropathy in children and in adults: two separate entities or the same disease? J Nephrol 33:1219–1229. https://doi.org/10.1007/s40620-020-00725-0
Cambier A, Rabant M, El Karoui K, Peuchmaur M, Servais A, Hertig A, Deschenes G, Salomon R, Hogan J, Robert T (2020) Clinical and histological differences between adults and children in new onset IgA nephropathy. Pediatr Nephrol 35:1897–1905. https://doi.org/10.1007/s00467-020-04614-3
Coppo R, Feehally J, Glassock RJ (2010) IgA nephropathy at two score and one. Kidney Int 77:181–186
Trimarchi H, Barratt J, Cattran DC (2017) Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int 91:1014–1021
Mestecky J, Moro I, Kerr MA, Woof JM (2005) Mucosal immunoglobulins. In: Mestecky J, Bienenstock J, Lamm ME, Mayer L, McGhee JR, Strober W (eds) Mucosal Immunology, 3rd edn. Elsevier Academic Press, Amsterdam, pp 153–181
Moldoveanu Z, Wyatt RJ, Lee J, Tomana M, Julian BA, Mestecky J, Huang WQ, Anreddy S, Hall S, Hastings MC, Lau KK, Cook WJ, Novak J (2007) Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int 71:1148–1154
Suzuki H, Fun R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J (2009) Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 119:1668–1677
Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG, Julian BA (2011) The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22:1795–1803. https://doi.org/10.1681/ASN.2011050464
Zhai YL, Zhu L, Shi SF, Liu LJ, Lv JC, Zhang H (2016) Increased APRIL Expression Induces IgA1 Aberrant glycosylation in IgA nephropathy. Medicine (Baltimore) 95:e3099. https://doi.org/10.1097/MD.0000000000003099
Yu XQ, Li M, Zhang H et al (2012) A genome-wide association study in Han Chinese identifies multiple susceptibility loci for IgA nephropathy. Nat Genet 44:178–182
McCarthy DD, Kujawa J, Wilson C, Papandile A, Poreci U, Porfilio EA, Ward L, Lawson MA, Macpherson AJ, McCoy KD, Pei Y, Novak L, Lee JY, Julian BA, Novak J, Ranger A, Gommerman JL, Browning JL (2011) Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest 121:3991–4002. https://doi.org/10.1172/JCI45563.Erratum.In:JClinInvest(2012)122:778
Kiryluk K, Novak J (2014) The genetics and immunobiology of IgA nephropathy. J Clin Invest 124:2325–2332. https://doi.org/10.1172/JCI74475
Kiryluk K, Li Y, Sanna-Cherchi S, Rohanizadegan M et al (2012) Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet 8:e1002765
Gharavi AG, Moldoveanu Z, Wyatt RJ, Barker CV, Woodford SY, Lifton RP, Mestecky J, Novak J, Julian BA (2008) Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol 19:1008–1014
Solberg OD, Mack SJ, Lancaster AK, Single RM, Tsai Y, Sanchez-Mazas A, Thomson G (2008) Balancing selection and heterogeneity across the classical human leukocyte antigen loci: A meta-analytic review of 497 population studies. Hum Immunol 69:443–464
Knoppova B, Reily C, Maillard N, Rizk DV, Moldoveanu Z, Mestecky J, Raska M, Renfrow MB, Julian BA, Novak J (2016) The origin and activities of IgA1-containing immune complexes in IgA nephropathy. Front Immunol 7:117. https://doi.org/10.3389/fimmu.2016.00117
Wisse E, Jacobs F, Topal B, Frederik P, De Geest B (2008) The size of endothelial fenestrae in human liver sinusoids: implications for hepatocyte-directed gene transfer. Gene Ther 15:1193–1199. https://doi.org/10.1038/gt.2008.60
Lai KN, Leung JC, Chan LY, Saleem MA, Mathieson PW, Lai FM, Tang SC (2008) Activation of podocytes by mesangial-derived TNF-alpha: glomerulo-podocytic communication in IgA nephropathy. Am J Physiol Renal Physiol 294:F945–F955
Lai KN, Leung JC, Chan LY, Saleem MA, Mathieson PW, Tam KY, Xiao J, Lai FM, Tang SC (2009) Podocyte injury induced by mesangial-derived cytokines in IgA nephropathy. Nephrol Dial Transplant 24:62–72
Zhang J, Li Y, Shan K, Wang L, Qiu W, Lu Y, Zhao D, Zhu G, He F, Wang Y (2014) Sublytic C5b–9 induces IL-6 and TGF-β1 production by glomerular mesangial cells in rat Thy-1 nephritis through p300-mediated C/EBPβ acetylation. FASEB J 28:1511–1525
Medjeral-Thomas NR, Cook HT, Pickering MC (2021) Complement activation in IgA nephropathy. Semin Immunopathol 43:679–690. https://doi.org/10.1007/s00281-021-00882-9
Berthelot L, Papista C, Maciel TT, Biarnes-Pelicot M, Tissandie E, Wang PH, Tamouza H, Jamin A, Bex-Coudrat J, Gestin A, Boumediene A, Arcos-Fajardo M, England P, Pillebout E, Walker F, Daugas E, Vrtosvnik F, Flamant M, Benhamou M, Cogné M, Moura IC, Monteiro RC (2012) Transglutaminase is essential for IgA nephropathy development acting through IgA receptors. J Exp Med 209:793–806. https://doi.org/10.1084/jem.20112005
Launay P, Grossetête B, Arcos-Fajardo M, Gaudin E, Torres SP, Beaudoin L, Patey-Mariaud de Serre N, Lehuen A, Monteiro RC (2000) Fcalpha receptor (CD89) mediates the development of immunoglobulin A (IgA) nephropathy (Berger’s disease). Evidence for pathogenic soluble receptor-Iga complexes in patients and CD89 transgenic mice. J Exp Med 191:1999–2009
Moura IC, Arcos-Fajardo M, Sadaka C, Leroy V, Benhamou M, Novak J, Vrtovsnik F, Haddad E, Chintalacharuvu KR, Monteiro RC (2004) Glycosylation and size of IgA1 are essential for interaction with mesangial transferrin receptor in IgA nephropathy. J Am Soc Nephrol 15:622–634
Moura IC, Arcos-Fajardo M, Gdoura A, Leroy V, Sadaka C, Mahlaoui N, Lepelletier Y, Vrtovsnik F, Haddad E, Benhamou M, Monteiro RC (2005) Engagement of transferrin receptor by polymeric IgA1: evidence for a positive feedback loop involving increased receptor expression and mesangial cell proliferation in IgA nephropathy. J Am Soc Nephrol 16:2667–2676
Jennette J (1988) The immunohistology of IgA nephropathy. Am J Kidney Dis 12:348–352. https://doi.org/10.1016/S0272-6386(88)80022-2
Roos A, Rastaldi MP, Calvaresi N, Oortwijn BD, Schlagwein N, van Gijlswijk-Janssen DJ, Stahl GL, Matsushita M, Fujita T, van Kooten C, Daha MR (2006) Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol 17:1724–1734
Espinosa M, Ortega R, Sanchez M, Segarra A, Salcedo MT, Gonzalez F, Camacho R, Valdivia MA, Cabrera R, Lopez K, Pinedo F, Gutierrez E, Valera A, Leon M, Cobo MA, Rodriguez R, Ballarin J, Arce Y, Garcia B, Munoz MD, Praga M (2014) Association of C4d deposition with clinical outcomes in IgA nephropathy. Clin J Am Soc Nephrol 9:897–904
Daha MR, van Kooten C (2016) Role of complement in IgA nephropathy. J Nephrol 29:1–4. https://doi.org/10.1007/s40620-015-0245-6
Mizerska-Wasiak M, Małdyk J, Pańczyk-Tomaszewska M, Turczyn A, Cichoń-Kawa K, Rybi-Szumińska A, Wasilewska A, Firszt-Adamczyk A, Stankiewicz R, Bieniaś B, Zajączkowska M, Gadomska-Prokop K, Grenda R, Miklaszewska M, Pietrzyk J, Pukajło-Marczyk ZD, Szczepańska M, Demkow U, Roszkowska-Blaim M (2015) Increased serum IgA in children with IgA nephropathy, severity of kidney biopsy findings and long-term outcomes. Adv Exp Med Biol 873:79–86. https://doi.org/10.1007/5584_2015_160
Mizerska-Wasiak M, Małdyk J, Rybi-Szumińska A, Wasilewska A, Miklaszewska M, Pietrzyk J, Firszt-Adamczyk A, Stankiewicz R, Bieniaś B, Zajączkowska M, Gadomska-Prokop K, Grenda R, Pukajło-Marczyk A, Zwolińska D, Szczepańska M, Turczyn A, Roszkowska-Blaim M (2015) Relationship between serum IgA/C3 ratio and severity of histological lesions using the Oxford classification in children with IgA nephropathy. Pediatr Nephrol 30:1113–1120. https://doi.org/10.1007/s00467-014-3024-z
Tomino Y, Suzuki S, Imai H, Saito T, Kawamura T, Yorioka N et al (2000) Measurement of serum IgA and C3 may predict the diagnosis of patients with IgA nephropathy prior to renal biopsy. J Clin Lab Anal 14:220–223. https://doi.org/10.1002/1098-2825(2000)14:5%3C220::AID-JCLA4%3E3.0.CO;2-2
Yanagawa H, Suzuki H, Suzuki Y, Kiryluk K, Gharavi AG, Matsuoka K, Makita Y, Julian BA, Novak J, Tomino Y (2014) A panel of serum biomarkers differentiates IgA nephropathy from other renal diseases. PLoS One 9:e98081. https://doi.org/10.1371/journal.pone.0098081
Kim SJ, Koo HM, Lim BJ, Oh HJ, Yoo DE, Shin DH, Lee MJ, Doh FM, Park JT, Yoo TH, Kang SW, Choi KH, Jeong HJ, Han SH (2012) Decreased circulating C3 levels and mesangial C3 deposition predict renal outcome in patients with IgA nephropathy. PLoS One 7:e40495. https://doi.org/10.1371/journal.pone.0040495
Mizerska-Wasiak M, Such-Gruchot A, Cichon-Kawa K, Turczyn A, Małdyk J, Miklaszewska M, Drożdż D, Firszt-Adamczyk A, Stankiewicz R, Rybi-Szumińska A, Wasilewska A, Szczepanska M, Bieniaś B, Sikora P, Pukajło-Marczyk A, Zwolińska D, Pawlak-Bratkowska M, Tkaczyk M, Zachwieja J, Drożyńska-Duklas M, Żurowska A, Gadomska-Prokop K, Grenda R, Pańczyk-Tomaszewska M (2021) The role of complement component C3 activation in the clinical presentation and prognosis of IgA nephropathy – a national study in children. J Clin Med 10:4405. https://doi.org/10.3390/jcm10194405
Zwirner J, Burg M, Schulze M, Brunkhorst R, Gotze O et al (1997) Activated complement C3: a potentially novel predictor of progressive IgA nephropathy. Kidney Int 51:1257–1264
Onda K, Ohsawa I, Ohi H, Tamano M, Mano S, Wakabayashi M, Toki A, Horikoshi S, Fujita T, Tomino Y (2011) Excretion of complement proteins and its activation marker C5b–9 in IgA nephropathy in relation to renal function. BMC Nephrol 12:64. https://doi.org/10.1186/1471-2369-12-64
Guo WY, Zhu L, Meng SJ, Shi SF, Liu LJ, Lv JC, Zhang H (2017) Mannose-binding lectin levels could predict prognosis in IgA nephropathy. J Am Soc Nephrol 28:3175–3181. https://doi.org/10.1681/ASN.2017010076
Zhu L, Zhai YL, Wang FM, Hou P, Lv JC, Xu DM et al (2015) Variants in complement factor H and complement factor H-related protein genes, CFHR3 and CFHR1, affect complement activation in IgA nephropathy. J Am Soc Nephrol 26:1195–1204. https://doi.org/10.1681/ASN.2014010096200
Komatsu H, Fujimoto S, Hara S, Sato Y, Yamada K, Eto T (2004) Relationship between serum IgA/C3 ratio and progression of IgA nephropathy. Intern Med 43:1023–1028. https://doi.org/10.2169/internalmedicine.43.1023
Ishiguro C, Yaguchi Y, Funabiki K, Horikoshi S, Shirato I, Tomino Y (2002) Serum IgA/C3 ratio may predict diagnosis and prognostic grading in patients with IgA nephropathy. Nephron 91:755–758. https://doi.org/10.1159/000065043
Kawasaki Y, Maeda R, Ohara S, Suyama K, Hosoya M (2018) Serum IgA/C3 and glomerular C3 staining predict severity of IgA nephropathy. Pediatr Int 60:162–167. https://doi.org/10.1111/ped.13461
Zhang J, Wang C, Tang Y, Peng H, Ye ZC, Li CC, Lou TQ (2013) Serum immunoglobulin A/C3 ratio predicts progression of immunoglobulin A nephropathy. Nephrology (Carlton) 8:125–131. https://doi.org/10.1111/nep.12010
Stefan G, Stancu S, Boitan B, Zugravu A, Petre N, Mircescu G (2020) Is there a role for IgA/C3 ratio in IgA nephropathy prognosis? An outcome analysis on a European population. Iran J Kidney Dis 14:470–477
Hirano K, Amano H, Kawamura T, Watanabe K, Koike K, Shimizu A et al (2016) Tonsillectomy reduces recurrence of IgA nephropathy in mesangial hypercellularity type categorized by the Oxford classification. Clin Exp Nephrol 20:425–432. https://doi.org/10.1007/s10157-015-1170-7
Varis J, Rantala I, Pasternack A, Oksa H, Jantti M, Paunu ES et al (1993) Immunoglobulin and complement deposition in glomeruli of 756 subjects who had committed suicide or met with a violent death. J Clin Pathol 46:607–610
Suzuki K, Honda K, Tanabe K, Toma H, Nihei H, Yamaguchi Y (2003) Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int 63:2286–2294. https://doi.org/10.1046/j.1523-1755.63.6s.2.x
Yamaji K, Suzuki Y, Suzuki H et al (2014) The kinetics of glomerular deposition of nephritogenic IgA. PLoS One 9:e113005
Wyatt RJ, Julian BA (2013) IgA nephropathy. N Engl J Med 368:2402–2414
Cichoń-Kawa K, Mizerska-Wasiak M, Małdyk J, Turczyn A, Rybi-Szumińska A, Wasilewska A, Firszt-Adamczyk A, Stankiewicz R, Bieniaś B, Sikora P, Gadomska-Prokop K, Grenda R, Pańczyk-Tomaszewska M (2018) Influence of intensity, localization and type of deposits in renal biopsy for disease symptoms and follow up in children with IgA nephropathy. Pol Merkur Lekarski 44:177–182
Cassol CA, Bott C, Nadasdy GM, Alberton V, Malvar A, Nagaraja HN, Nadasdy T, Rovin BH, Satoskar AA (2020) Immunostaining for galactose-deficient immunoglobulin A is not specific for primary immunoglobulin A nephropathy. Nephrol Dial Transplant 35:2123–2129. https://doi.org/10.1093/ndt/gfz152
Roos A, Bouwman LH, van Gijlswijk-Janssen DJ, Faber-Krol MC, Stahl GL, Daha MR (2001) Human IgA activates the complement system via the mannan-binding lectin pathway. J Immunol 167:2861–2868. https://doi.org/10.4049/jimmunol.167.5.2861
Paunas TIF, Finne K, Leh S, Marti HP, Mollnes TE, Berven F et al (2017) Glomerular abundance of complement proteins characterized by proteomic analysis of laser-captured microdissected glomeruli associates with progressive disease in IgA nephropathy. Clin Proteom 14:30. https://doi.org/10.1186/s12014-017-9165-x
Tortajada A, Gutierrez E, Pickering MC, Praga Terente M, Medjeral-Thomas N (2019) The role of complement in IgA nephropathy. Mol Immunol 114:123–132. https://doi.org/10.1016/j.molimm.2019.07.017
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Answers
1. a, b, c, d; 2. a, b, d, e; 3. a, b, c, e; 4. a, b; 5. a, c, e
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mizerska-Wasiak, M. How to take advantage of easily available biomarkers in patients with IgA nephropathy: IgA and C3 in serum and kidney biopsies. Pediatr Nephrol 38, 1439–1448 (2023). https://doi.org/10.1007/s00467-022-05644-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05644-9