Abstract
Background
Providing extracorporeal renal support to neonates and infants involves a number of technical and clinical issues, possibly discouraging early utilization. This report aims to describe a multicenter experience of continuous kidney replacement therapy (CKRT) delivery to small infants using a device specifically designed for this age group.
Methods
A retrospective cohort analysis of all patients treated with the Carpediem™ machine (Bellco-Medtronic, Mirandola, Italy) in 6 centers between June 2013 and December 2016.
Results
Twenty-six neonates and small infants received 165 CKRT sessions in convective modality. Median age at neonatal intensive care unit admission 1 day (IQR 1–11), median body weight 2.9 kg (IQR 2.2–3.6). Median circuit duration 14 h (IQR 10–22), with delivered/prescribed time ratio of 84%. CKRT was conducted using 4 Fr (27%), 5 Fr (35%), 6.5 Fr (11%), and 7 Fr (3%) vascular access, and with umbilical and peripheral accesses (11% each) allowing overall median blood flow of 4.5 ml/kg/min (IQR 3.4–6) and median effluent flow rate 35 ml/kg/h (IQR 28–42). Circuits were primed with normal saline in 58% of treatments, colloids in 31%, and packed red blood cells in 11%. No serious adverse events directly related to machine application were reported by any center. Twenty-five (96%) patients survived their CKRT course and 13 patients (50%) survived to ICU discharge.
Conclusions
CKRT in neonates was easy to initiate and conduct when performed with small central vascular accesses coupled with this device. A dedicated technology for infant CKRT delivery enables patients to be safely treated avoiding technical complications.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) affects about 30% of critically ill neonates admitted to neonatal intensive care units (NICU) [1]. However, neonates receive renal support infrequently. According to recent data from the Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) study, kidney replacement therapy (KRT) was provided to 25 of 605 neonates with AKI (4%), and only 4 (0.7%) were treated with continuous extracorporeal KRT [1]. In this series, the lack of a specific extracorporeal KRT device available for neonates and infants might have contributed to the low number of treatments.
Continuous extracorporeal KRT (CKRT) for neonates is associated with a series of technical and clinical challenges: in fact, patients’ relatively small volume and diameters of central veins may result in inadequate extracorporeal blood flow circulation [2]. This is particularly true when adult CKRT devices, not specifically designed and tested for use in infants, are adapted for clinical application in infants. Priming the circuit with blood might prevent hypotension and hemodynamic alterations [3], but can cause acidosis, hypocalcemia, hyperkalemia, thrombocytopenia, and coagulopathy and may cause vasoplegia with relative hypovolemia and hypotension. Moreover, adult CKRT machines may lack an adequate level of fluid volume control accuracy and eventual clinical complications may occur [4, 5]. Detailed protocols and skilled staff are also required to perform CKRT in neonates and small infants, warranting its application only at tertiary pediatric hospitals. Although peritoneal dialysis (PD) is often used to treat AKI in neonates, it may be contraindicated in some patients (i.e., neonates undergoing abdominal surgical procedures); it is associated with fluid leakage and infection of the peritoneum, and efficient fluid removal may not be achieved, especially in patients who require high enteral and parenteral nutrition flows [6].
The revolution in the management of AKI in newborns has started recently with the development of new CKRT machines specifically adapted or conceived for small infants [7,8,9]. The advantages of the new devices include smaller extracorporeal volumes, ability to potentially avoid blood prime, a potentially better volume control, a more graduated flow rate adjustment, and the possibility to choose smaller catheter sizes without compromising blood flow [10, 11].
Since June 2013, the Cardio-Renal Pediatric Dialysis Emergency Machine (Carpediem™, Bellco-Medtronic, Mirandola, Italy) has been used in several pediatric centers in Italy. Here we would like to describe a 2.5-year experience with the use of this machine in treating neonates and infants using a convective modality. The aim of this analysis is to report treated patients’ characteristics, therapy data with particular focus on treatment initiation, catheters utilized, technical considerations, and overall outcomes.
Materials and methods
All records of patients with a CKRT prescribed with the Carpediem machine between June 2013 and December 2016 were collected into a retrospective registry. Six Italian centers (University-Hospital of Padua, Bambino Gesù Children’s Hospital, Rome, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Giovanni Paolo XXIII Children’s Hospital, Bari, San Bortolo Hospital, Vicenza, and Regina Margherita Children’s Hospital, Turin) participated to the retrospective cohort data collection. The registry was approved by each institution’s ethics committee, and informed consent for data analysis was waived due to the retrospective nature of the study. Each center followed local institutional practice with respect to timing and criteria for CKRT initiation, termination, and prescription. CKRT modality and vascular access type were also determined by the clinicians based on institutional protocols and standard of care, patient characteristics, and local availability.
The retrospective registry collected data from the time of intensive care unit (ICU) admission to 4 weeks after a patient’s hospital discharge. As used in previous neonatal studies, AKI was defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) workgroup AKI definition, modified for neonates [1, 12]. To assess illness severity, pediatric risk mortality (PRISM) 2 scores were calculated for each patient at ICU admission and CKRT initiation [13]. Percentage fluid overload (%FO) was determined at the time of CKRT initiation using the method described by Goldstein et al. [14].
Demographic data of all patients were expressed as median and interquartile range (IQR). Median blood flow was calculated utilizing the patients’ median blood flows of all treatments throughout the whole study period. Comparison between continuous variables was made using non-parametric tests, while categorical variables were analyzed using chi-square analysis. Cox regression analysis was performed to identify factors associated with patient survival. We considered p < 0.05 to be statistically significant. All statistical analyses were performed using the R software (version 3.3.3).
Results
Patient demographic data
Twenty-six children (18 males) received 165 CKRT sessions with the Carpediem machine in a convective modality. Median age at ICU admission was 1 day (interquartile range [IQR] 1–11), median body weight was 2.9 kg (IQR 2.2–3.6), and median PRISM II score was 18 (IQR 11–25). In half of cases, body weight at dialysis initiation was < 3 kg. Except for two patients aged 69 and 140 days, all patients started CKRT within 4 weeks of life. Cardiac disease was the most common primary diagnosis at ICU admission (38%), followed by sepsis (15%), inborn errors of metabolism (IEM) (15%), kidney disease (12%), primary pulmonary disease (12%), and others (8%). On average, infants were admitted to ICU 7 days (IQR 3–14) prior to CKRT initiation. All patients were critically ill, 58% received ventilatory support, 50% were treated with diuretics, and 27% were vasopressor-dependent. Median %FO at CKRT start was 14% (IQR 0–23): amount of %FO was lower than 10% in 9 patients (35%), between 10% and 20% in 6 patients (23%), and higher than 20% in the remaining 11 patients (50%). Median urinary output at CKRT initiation was 1.2 ml/kg/h (IQR 0.15–2.1), with a median estimated glomerular filtration rate (eGFR) of 28 (IQR 20–42) and 15 (IQR 8–27) ml/min/1.73m2 at ICU admission and CKRT start, respectively. Indications for CKRT were AKI with fluid overload in 22 (85%) patients, and metabolic or electrolyte imbalances in the remaining 4 cases (15%). Patient clinical characteristics are shown in Table 1.
CKRT and circuits
In 152 out of 165 CKRT sessions (92%), predilution hemofiltration was prescribed, while in 13 sessions (8%), a post-dilution continuous veno-venous hemofiltration (CVVH) was set. Generally, circuit size was selected based upon patient body surface area and weight. Operating characteristics of circuits are summarized in Table 2. Median actual blood flow was 4.5 ml/kg/min (IQR 3.4–6) and median effluent flow rate was 35 ml/kg/h (IQR 28–42). The 4 patients with IEM received 12 CKRT sessions overall with a median effluent flow rate of 60 ml/kg/h (IQR 52–69).
Three different polyethersulfone hemodialyzers are available with different surface areas (0.075, 0.15, and 0.25 m2). Significant differences were shown between prescribed settings and filter sizes, with the smaller (0.075 m2) and the larger membrane (0.25 m2) being those with the highest blood flows, net ultrafiltration, and effluent rates (Table 2).
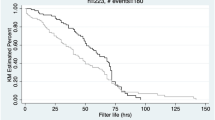
Overall, 2295 treatment hours were recorded with a mean prescribed/delivered ratio of 84%. Normal saline was used to prime the extracorporeal circuits in 58% of cases, whereas in 31% of case colloids (5% albumin), and in 11% packed red blood cells (PRBc) were added to the priming solution before CKRT start. Colloids and PRBc were mainly chosen by physicians to prevent the risk of hemodynamic instability at CKRT initiation. However, no babies required specific interventions (i.e., increase in number and dose of vasoactive drugs) to maintain blood pressure after circuit initiation. Heparin was used for systemic anticoagulation in 118 sessions (71%), whereas anticoagulation was not prescribed in the remaining 47 sessions (29%). Carpediem software requests a mandatory change of circuit every 24 h. Twenty-four-hour KRT sessions were prescribed in 86 out of 165 sessions, with a circuit patency (sessions lasting to the 24th hour) achieved in 58 (68%) of the treatments. The remaining sessions (79) were prescribed to last 12–18 h (generally due to organizational purposes). Overall, 28 times in the 24-h sessions and 20 times in the remaining ones, a premature circuit interruption was described: clotting in 22 (13%) sessions, clinical reasons in 12 (7%), vascular access malfunction in 10 (6%), unresolved software alarms, and other unspecified technical problems both in 4 (2.4%) sessions. In the whole group, median circuit duration was 14 h (IQR 10–22). However, in the 86 sessions foreseen as continuous, this duration was 18 (IQR 14–24).
Vascular access data
The most common location for vascular access was right internal jugular vein (54%), followed by femoral (31%), umbilical (11.5%), and subclavian vein (3.5%). In most patients, CKRT was conducted using a 4 Fr (27%) or a 5 Fr (35%) central vascular access; three patients were treated using a combination of 5 (venous inflow line) and 3.5 Fr (arterial outflow line), 20 cm long, umbilical catheters. Detailed vascular access data are reported in Table 3. Delivery of the prescribed CKRT was higher in patients with 5 and 6.5 Fr catheters (mean delivered/prescribed ratio of 92%) with respect to umbilical, 3 Fr, 4 Fr, and 7 Fr catheters (76%, 66%, 80%, and 81%, respectively).
Patient outcomes
Twenty-five (96%) patients survived their CKRT course and 13 patients (50%) survived both ICU and hospital discharge. Weaning from CKRT in these patients was decided upon institutional protocols, but in general, it was achieved after recovery of spontaneous diuresis. Survival of infants with weight < 3 kg (4/13, 31%) was significantly lower than that of children > 3 kg (9/13, 69%; p = 0.03). Survivors were more likely to have higher gestational age, higher body weight, lower PRISM II scores at ICU admission, shorter ICU stay prior to CKRT initiation, and lower eGFR drop from ICU admission to CKRT start (Table 1). Cox regression analysis found an association between mortality and PRISM II score (HR 1.1; 95%CI 1.01–1.15; p = 0.05), length of ICU stay (HR 1.02; 95% CI 1.01–1.04; p = 0.005), and drop in eGFR from admission to CKRT initiation (HR 1.04; 95%CI 1.01–1.09; p = 0.03).
At 28 days of follow-up, 8 out of 13 infants had a normal renal function, whereas 5 patients still showed renal dysfunction, 2 of these cases being dialysis-dependent.
Discussion
The use of devices designed for adults to deliver CKRT to younger and smaller children brings along additional risks deriving from relatively larger priming volumes, the use of blood pumps conceived for large tubes and catheters, and imprecise volume control [2]. Several groups have recently described the utilization of CKRT devices specifically designed or adapted for neonates and small infants. Askenazi et al. have modified the Aquadex™ machine set-up to provide predilution CVVH in small children requiring renal support [7]. The Newcastle infant dialysis and ultrafiltration system (NIDUS) has been designed to provide single-lumen continuous veno-venous hemodiafiltration (CVVHD) to children weighing between 800 and 8 kg [8]. Our group has recently described the development and use of a miniaturized machine, the Carpediem, to perform CVVHD and therapeutic plasma exchange [9, 10, 15,16,17]. The present study explored the utilization of this device in performing CVVH in a multicentre context for treating 26 critically ill neonates and infants weighing from 1.6 to 4.6 kg.
One of the main advantages in the application of the Carpediem machine is the possibility of utilizing an integrated last generation machine with priming volumes ranging from 27 to 41 ml. In the Prospective Pediatric CRRT (ppCRRT) Registry’s experience, 96.5% of the circuits used for treating 48 infants weighing < 5 kg was initiated with a blood prime, and only 5 circuits (3%) with saline [2]. In the more recent experience with the Aquadex™ system adapted to children, the extracorporeal volume of 33 ml required blood to prime all circuits in infants with a body weight under 4 kg [7]. In our cohort, despite the small size and the young median age (excluding the two cases who started CKRT out of the neonatal period), only 11% of circuits required blood prime, whereas normal saline (58%) or albumin was used in the majority of circuits without hemodynamic instability during the patient connection. This is a requirement for a wide diffusion of the therapy to NICUs, especially when not equipped with a permanent and skilled CKRT team.
Another important concern regarding CKRT in neonates and infants is the choice of vascular access. It should be adapted to the small size of the patient and his/her vessels in order to match the highest blood flow rate with the lowest risk for vein occlusion [11]. Data from the ppCRRT Registry clearly demonstrated that CKRT circuit patency in children was reduced when catheters smaller than 7 Fr were utilized [18]. In particular, the authors found that none of the 5 Fr catheters lasted longer than 20 h. More recently, Westrope and coworkers remarked the importance of catheter site rather than size in order to warrant circuit survival [19]. In our series, 16 out of 26 patients (61%) were treated using a central line of 4 and 5 Fr; Qb in these patients was in the range of 10 to 25 ml/min (up to 4.4 ml/kg/min). Patients with 5 and 6.5 Fr catheters showed about 90% of circuits reaching the 24th hour confirming the feasibility of small catheters coupled with the small blood pump of this machine [11, 20]. Still good performances were obtained in terms of flow rate for 3 Fr (5.4 ml/kg/min) and umbilical (6.4 ml/kg/min) accesses, but with a smaller prescribed to delivered ratio in comparison. Not surprisingly, the performance of 4 Fr catheters appeared to be in between, in terms of prescribed to delivered ratio.
Even if there is no universally accepted effluent dose target in children [21], the median effluent flows were adequate with close to the “classic” prescription of 35 ml/kg/h [21], with a tendency to deliver higher intensity with smaller and larger circuits. It has previously been shown that, generally, this prescription is able to provide adequate solute control in terms of creatinine and urea levels [17]. Net ultrafiltration rate was also high, likely due to the need to remove excess fluids and to achieve a neutral fluid balance in severely fluid overloaded patients [22]: interestingly, in this small cohort, no significant relationship appeared between FO amount and mortality. In this regard, only a minority of patients was treated with a FO above 20%, whereas in general, the level of FO at CKRT start was below this threshold. This aspect could be possibly due to timely intervention in all patients. However, this small sample was not powered to explore relevant mortality predictors. As a matter of fact, overall ICU mortality of these patients remains high.
Data from case series of infants treated with CKRT have demonstrated a trend towards an improvement in survival over time [2, 7, 23]. In a rough analysis from published data, survival has increased from 25% in 2003 to almost 50% in the recent Aquadex™ case series [2, 7]. In our cohort, 50% of infants also survived at ICU and hospital discharge, but it is important to note that the overall survival was influenced by the higher mortality (69%) experienced in infants weighing < 3 kg. Smaller, younger patients generally display the worst outcomes, especially when the most severe diseases are treated. Interestingly, however, the feasibility and efficacy of this device showed that most of the patients were successfully weaned from the treatment, in all cases due to recovery of spontaneous diuresis. It has to be highlighted that 38% of survivors showed signs of incomplete recovery of renal function. The survival of more neonates with AKI and eventual chronic kidney disease could lead to a change in the topography of pediatric population with kidney failure, with emerging long-term comorbidities and changing demands on resources [24]. At the same time, easy application of the technique can expand the indications and the role of neonatal CKRT.
The expected outcome benefit when devices specifically designed for infants are applied should be compared with those delivered with non-specific devices in order to confirm that a lower complication rate may occur during patient connection and treatment conduction. In addition, widespread use of easy-to-use technology enables patients to be treated at an early stage of the critical illness, potentially ameliorating the course of the disease. In our registry, technical issues were reported in only 2% of sessions and in no case appeared to be related to adverse events.
This registry presents limitations mainly deriving from its retrospective nature and small sample size. However, we described the first use of a new machine in a challenging group of patients using non-standardized protocols both for treatment initiation and discontinuation. This might have resulted in a tendency towards a sub-optimal application of this new technology, and perhaps, more accurate data will derive from a future prospective registry. Furthermore, the technical requirement of this machine to interrupt the treatments at the 24th hour may imply that relatively shorter circuit patency is possible with the Carpediem compared with other experiences [19]. This technical requirement is the other side of the coin when a miniaturized circuit is applied: durability of small circuit components is not warranted longer than this threshold. The clinical impact of a planned circuit change every 24 h in small infants has to be evaluated and strategies to optimize this issue (e.g., cross-priming with a second machine or technical evolution leading to prolonged circuit durability) will be tested in the future.
In conclusion, we report here the largest case series of neonates and infants treated with a new machine specifically designed for performing CKRT in low body weight patients. Our results confirm that CKRT is feasible even with relatively small venous catheters. In our experience, the new blood pump coupled with a 5 Fr catheter represents the best compromise between low vascular impact and adequate extracorporeal treatment.
References
Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, Chishti AS, Woroniecki R, Mammen C, Swanson JR, Sridhar S, Wong CS, Kupferman JC, Griffin RL, Askenazi DJ, Neonatal Kidney Collaborative (NKC) (2017) Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 1:184–194
Askenazi DJ, Goldstein SL, Koralkar R, Fortenberry J, Baum M, Hackbarth R, Blowey D, Bunchman TE, Brophy PD, Symons J, Chua A, Flores F, Somers MJ (2013) Continuous renal replacement therapy for children ≤10 kg: a report from the prospective pediatric continuous renal replacement therapy registry. J Pediatr 162:587–592.e3
Lopez-Herce J, Ruperez M, Sanchez C, Garcia C, Garcia E (2006) Effects of initiation of continuous renal replacement therapy on hemodynamics in a pediatric animal model. Ren Fail 28:171–176
Santiago MJ, López-Herce J, Urbano J, Solana MJ, del Castillo J, Ballestero Y, Botrán M, Bellón JM (2009) Complications of continuous renal replacement therapy in critically ill children: a prospective observational evaluation study. Crit Care 13:R184
Ricci Z, Morelli S, Vitale V, Di Chiara L, Cruz D, Picardo S (2007) Management of fluid balance in continuous renal replacement therapy: technical evaluation in the pediatric setting. Int J Artif Organs 30:896–901
Ricci Z, Goldstein SL (2016) Pediatric continuous renal replacement therapy. Contrib Nephrol 187:121–130
Askenazi D, Ingram D, White S, Cramer M, Borasino S, Coghill C, Dill L, Tenney F, Feig D, Fathallah-Shaykh S (2016) Smaller circuits for smaller patients: improving renal support therapy with Aquadex™. Pediatr Nephrol 31:853–860
Coulthard MG, Crosier J, Griffiths C, Smith J, Drinnan M, Whitaker M, Beckwith R, Matthews JN, Flecknell P, Lambert HJ (2014) Haemodialysing babies weighing <8 kg with the Newcastle infant dialysis and ultrafiltration system (Nidus): comparison with peritoneal and conventional haemodialysis. Pediatr Nephrol 29:1873–1881
Ronco C, Garzotto F, Brendolan A, Zanella M, Bellettato M, Vedovato S, Chiarenza F, Ricci Z, Goldstein SL (2014) Continuous renal replacement therapy in neonates and small infants: development and first-in-human use of a miniaturised machine (CARPEDIEM). Lancet 383:1807–1813
Vidal E, Cocchi E, Paglialonga F, Ricci Z, Garzotto F, Peruzzi L, Murer L, Ronco C (2019) Continuous veno-venous hemodialysis using the cardio-renal pediatric dialysis emergency MachineTM: first clinical experiences. Blood Purif 47:149–155
Garzotto F, Zaccaria M, Vidal E, Ricci Z, Lorenzin A, Neri M, Murer L, Nalesso F, Ruggeri A, Ronco C (2019) Choice of catheter size for infants in continuous renal replacement therapy: bigger is not always better. Pediatr Crit Care Med 20:e170–e179
Selewski DT, Charlton JR, Jetton JG, Guillet R, Mhanna MJ, Askenazi DJ, Kent AL (2015) Neonatal acute kidney injury. Pediatrics 136:e463–e473
Pollack MM, Ruttimann UE, Getson PR (1988) Pediatric risk of mortality (PRISM) score. Crit Care Med 16:1110–1116
Goldstein SL, Currier H, Graf C, Cosio CC, Brewer ED, Sachdeva R (2001) Outcome in children receiving continuous venovenous hemofiltration. Pediatrics 107:1309–1312
Lorenzin A, Garzotto F, Alghisi A, Neri M, Galeano D, Aresu S, Pani A, Vidal E, Ricci Z, Murer L, Goldstein SL, Ronco C (2016) CVVHD treatment with CARPEDIEM: small solute clearance at different blood and dialysate flows with three different surface area filter configurations. Pediatr Nephrol 31:1659–1665
Vidal E, Garzotto F, Parolin M, Manenti C, Zanin A, Bellettato M, Remuzzi G, Goldstein SL, Murer L, Ronco C (2017) Therapeutic plasma exchange in neonates and infants: successful use of a miniaturized machine. Blood Purif 44:100–105
Ricci Z, Guzzi F, Tuccinardi G, Di Chiara L, Clark W, Goldstein SL, Ronco C (2017) Dose prescription and delivery in neonates with congenital heart diseases treated with continuous veno-venous hemofiltration. Pediatr Crit Care Med 18:623–629
Hackbarth R, Bunchman TE, Chua AN, Somers MJ, Baum M, Symons JM, Brophy PD, Blowey D, Fortenberry JD, Chand D, Flores FX, Alexander SR, Mahan JD, McBryde KD, Benfield MR, Goldstein SL (2007) The effect of vascular access location and size on circuit survival in pediatric continuous renal replacement therapy: a report from the PPCRRT registry. Int J Artif Organs 30:1116–1121
Westrope C, Morris KP, Kee CY, Farley M, Fleming S, Morrison G (2016) Experience of circuit survival in extracorporeal continuous renal replacement therapy using small-calibre venous cannulae. Pediatr Crit Care Med 17:e260–e265
Bunchman TE (2019) Vascular access for extracorporeal therapies: how do we evaluate them? Pediatr Crit Care Med 20:305–306
Ricci Z, Guzzi F, Tuccinardi G, Romagnoli S (2016) Dialytic dose in pediatric continuous renal replacement therapy patients. Minerva Pediatr 68:366–373
Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, Hackbarth R, Somers MJ, Baum M, Symons JM, Flores FX, Benfield M, Askenazi D, Chand D, Fortenberry JD, Mahan JD, McBryde K, Blowey D, Goldstein SL (2010) Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis 55:316–325
Symons JM, Brophy PD, Gregory MJ, McAfee N, Somers MJ, Bunchman TE, Goldstein SL (2003) Continuous renal replacement therapy in children up to 10 kg. Am J Kidney Dis 41:984–989
Tal L, Angelo JR, Akcan-Arikan A (2016) Neonatal extracorporeal renal replacement therapy-a routine renal support modality? Pediatr Nephrol 31:2013–2015
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Prof. Ronco received speakers’ honoraria from GE Healthcare, Fresenius, ESTOR, and B. Braun. He also received compensation and consulted for Astute Medical, OCD, Biomérieux, Baxter, Jafron Biomedical Co., and Asahi Medical. All other authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PPTX 73.5 kb)
Rights and permissions
About this article
Cite this article
Garzotto, F., Vidal, E., Ricci, Z. et al. Continuous kidney replacement therapy in critically ill neonates and infants: a retrospective analysis of clinical results with a dedicated device. Pediatr Nephrol 35, 1699–1705 (2020). https://doi.org/10.1007/s00467-020-04562-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04562-y