Abstract
Background
Serum chloride derangements are associated with poor clinical outcomes, including acute kidney injury (AKI) and mortality. We sought to determine the association between persistent hyperchloremia and renal recovery in critically ill children with AKI.
Methods
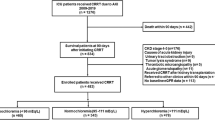
We performed a retrospective cohort study of all patients with day 2 AKI admitted to a large academic pediatric intensive care unit from January 2014 to December 2015. After applying exclusion criteria, 348 patients were categorized as (1) hyperchloremia on both day 2 and day 7 (PersistentCl), (2) hyperchloremia on day 2 with normochloremia on day 7 (RecoveredCl), (3) normochloremia on day 2 with hyperchloremia on day 7 (DelayedCl), and (4) no hyperchloremia on day 2 nor day 7 (NormalCl). Hyperchloremia was defined as ≥ 110 mEq/L. The primary outcome was renal recovery on day 7, defined as the absence of AKI criteria. Secondary outcomes included discharge renal recovery, mortality, duration of mechanical ventilation, and hospital length of stay.
Results
Day 7 renal recovery rates for PersistentCl, RecoveredCl, DelayedCl, and NormalCl were 37%, 66%, 71%, and 52% respectively. PersistentCl had lower odds of day 7 renal recovery (aOR = 0.29; 95% CI, 0.14 to 0.60; p = 0.0009), lower odds of discharge renal recovery (aOR = 0.22; 95% CI, 0.11 to 0.48; p = 0.0001), and higher odds of mortality (aOR = 3.50; 95% CI, 1.11 to 11.10; p = 0.03) when compared with RecoveredCl after adjusting for confounders.
Conclusions
Persistent hyperchloremia is independently associated with impaired renal recovery as well as higher mortality. Prospective studies are indicated to determine if serum chloride represents a modifiable risk factor for poor outcomes.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) is common in critically ill children with roughly 25% of all patients admitted to the pediatric intensive care unit (PICU) being affected [1]. It is well established that AKI is associated with poor in-hospital clinical outcomes, including a higher mortality rate [1]. Beyond the in-hospital risks, there is growing evidence that AKI is associated with worse long-term outcomes, including the development of chronic kidney disease (CKD) [2,3,4]. To better define the AKI to CKD transition, the term acute kidney disease (AKD) was recently introduced and includes patients with persistent kidney dysfunction between 7 days and 3 months post AKI [5]. AKD has been shown to be associated with worse clinical outcomes compared to patients with recovery of renal function within 7 days of the development of AKI, including higher rates of CKD [2,3,4], increased infection risk [6], and higher mortality rates [7]. In one study, patients were stratified by their renal recovery pattern. Those with renal recovery within 7 days had a 1-year mortality of 9.8%, those with recovery by hospital discharge had a 29% 1-year mortality, and those without recovery had a 59.8% 1-year mortality [7]. Given these risks, renal recovery and AKD are outcomes of growing clinical interest.
Serum chloride derangements have been increasingly shown to be associated with poor outcomes, including higher rates of AKI and mortality in both pediatric and adult populations [8,9,10,11,12,13,14,15]. The proposed mechanism for this effect is thought to be related to the impact of hyperchloremia on renal afferent arteriolar vasoconstriction [16,17,18]. It is physiologically plausible that if patients with hyperchloremia have higher levels of renal afferent arteriolar vasoconstriction, they may be predisposed to impaired renal recovery and higher rates of AKD.
The purpose of this study was to determine if persistent hyperchloremia is associated with impaired renal recovery in critically ill children with AKI. We hypothesized that among patients with AKI, those with persistent hyperchloremia in the first week of their PICU course would be associated with worse rates of renal recovery after accounting for other clinical measures known to be associated with AKI.
Methods
We performed a single-center retrospective cohort study of all patients with AKI on day 2 of admission to the PICU at Children’s Hospital Colorado from January 2014 to December 2015. The University of Colorado Multiple Institutional Review Board approved the study with a waiver of informed consent. Patients were excluded for the following reasons: (1) age < 90 days or ≥ 18 years, (2) admission to the separate cardiac intensive care unit for children with primary cardiac disease and/or following cardiac surgery, (3) no PICU admission laboratory values, (4) history of end-stage renal disease (need for maintenance dialysis or receipt of a renal transplant), (5) a disorder of chloride transport, and (6) admission for diabetic ketoacidosis (DKA). DKA was excluded because of the electrolyte derangements due to hyperglycemia and its management. Patient demographics, comorbidities, pertinent laboratory tests, and outcomes were extracted from the electronic health record (EHR). All laboratory tests were performed in the clinical laboratory and assessed in calendar days from the day of PICU admission. Pediatric Risk of Mortality (PRISM) III score was determined on admission [19].
Outcomes
The primary outcome was day 7 renal recovery. Secondary outcomes included day 50 or discharge renal recovery, mortality, duration of mechanical ventilation among survivors, and hospital length of stay among survivors.
Definitions
For the inclusion criteria, AKI was defined using the Kidney Disease Improving Global Outcomes (KDIGO) serum creatinine criteria [20]. Baseline creatinine was defined as the lowest serum creatinine in the 3 months prior to admission. If no baseline creatinine was available, one was calculated using the bedside Schwartz equation assuming a glomerular filtration rate (GFR) of 120 mL/min per 1.73 m2 as previously described and validated [1, 21, 22].
For the outcome of interest, renal recovery was defined as the absence of AKI serum creatinine criteria on the day of interest [5, 23]. Specifically, renal recovery was defined as a normalization of creatinine to a level < 150% of the baseline creatinine.
Hyperchloremia was defined as a maximum serum chloride level ≥ 110 mEq/L on the day of interest. Patients were categorized by derangements in serum chloride concentrations into four phenotypes: (1) persistent hyperchloremia (PersistentCl), (2) recovery of chloride derangement (RecoveredCl), (3) delayed hyperchloremia (DelayedCl), and (4) no derangements in chloride (NormalCl), based upon their day 2 and day 7 levels. Hyperchloremia on day 2 and persistent hyperchloremia on day 7 defined PersistentCl. Hyperchloremia on day 2 with a return to normochloremia on day 7 defined RecoveredCl. Normochloremia on day 2, but with hyperchloremia on day 7 defined DelayedCl. Normochloremia on day 2 and on day 7 defined NormalCl. Patients who developed hyperchloremia after day 2 but recovered by day 7 were included in the NormalCl group.
Increase in sodium (↑Na) was the difference between the peak in the first 24 h of the PICU admission and the admission sodium level. Percent fluid overload was calculated as: ([total PICU intake − total PICU output] / PICU admission weight in kg) × 100 [24]. All diagnosis categories (sepsis, traumatic brain injury (TBI), respiratory failure, history of oncologic diagnosis, history of organ or bone marrow transplant) were based upon International Classification of Diseases 9 (ICD-9) and ICD-10 diagnosis codes. Diagnosis codes for respiratory failure for non-pulmonary reasons were included.
Statistical analysis
Clinical and demographic information were summarized and compared between groups. Non-normally distributed continuous variables were reported as median with interquartile range and compared using Wilcoxon rank sum and Kruskal-Wallis tests as appropriate. Categorical variables were summarized as number and percent, and were compared using the Fisher exact test, Cochran-Mantel-Haenszel (CMH) test, or chi-square analysis as appropriate. Bonferroni corrected p values were calculated to control for multiple pairwise comparisons on the outcomes of day 7 renal recovery, discharge renal recovery, and mortality.
Three multivariable logistic regression models were used to test for an association between (1) hyperchloremia phenotype and day 7 renal recovery, (2) hyperchloremia phenotype and discharge renal recovery, and (3) hyperchloremia phenotype and mortality after adjusting for potential confounders. The RecoveredCl phenotype was chosen as the reference to highlight the effect of serum chloride on the outcomes of interest. Covariates for adjustment were identified via a stepwise model selection algorithm at an entry level cutoff of 0.35 and a final inclusion cutoff of 0.30. Final covariates included peak stage of AKI in the first 7 days of the PICU admission, PRISM III score, age, ↑Na, history of oncologic diagnosis, history of organ or bone marrow transplant, and respiratory failure and diagnoses of sepsis. Although the lowest serum bicarbonate level in the first 24 h and percent fluid overload on PICU day 2 were not selected as covariates in the stepwise selection, we felt that given their importance in relation to chloride, we included these covariates in the model. Bicarbonate was chosen as a reflection of the acidosis of the patient. Fluid overload was chosen as a reflection of the fluid given to the patient as well as a potential surrogate for the chloride load, given our hospital’s consistent use of 0.9% saline for both resuscitation and maintenance. Thus, the final list of covariates used in the models included PRISM III score, age, ↑Na, stage of AKI, history of oncologic diagnosis, history of organ or bone marrow transplant, respiratory failure, diagnoses of sepsis, lowest serum bicarbonate level in the first 24 h, and percent fluid overload on PICU day 2. Interactive effects of ↑Na and chloride phenotypes were assessed in multiple logistic regression models. We tested the two-way interactions to evaluate if there was improvement in the model fit. No interactions were indicated. The statistical significance level was set at alpha < 0.05. For multiple group comparisons, p values were Bonferroni corrected. Data analysis was performed using SAS software 9.4, Cary, NC, USA, and JMP (copyright © 2002–2012 SAS Institute Inc).
Results
A total of 348 children with AKI were included in the study. Four chloride phenotypes were identified: 20% (n = 68) in the PersistentCl group, 26% (n = 91) in the RecoveredCl group, 4% (n = 14) in the DelayedCl group, and 50% (n = 175) in the NormalCl group. One hundred twenty-eight patients (37%) had baseline creatinine in the medical record and 220 patients (63%) had baseline creatinine calculated based on their height. The number of patients with baseline creatinine from the medical record for each chloride phenotype was 40% (n = 36) for RecoveredCl, 24% (n = 16) for PersistentCl, 21% (n = 3) for DelayedCl, and 42% (n = 73) for NormalCl. All patients with calculated baseline creatinine had heights.
For the entire study cohort, renal recovery on day 7 of the PICU stay was 53% (n = 184), renal recovery at discharge was 58% (n = 202), and mortality was 13% (n = 45). Thirteen patients died after day 7 in their PICU stay. Sixty-nine percent (n = 9/13) of them lacked renal recovery on day 7. Table 1 summarizes the patient demographics and clinical characteristics among the different chloride phenotypes.
In univariate analyses, the PersistentCl phenotype was associated with the highest proportion of patients with ↑Na ≥ 5 mEq/L, 50% (n = 34), and the lowest serum bicarbonate in the first 24 h, 17 mEq/L (IQR, 15–21 mEq/L). Other demographic and clinical differences are summarized in Table 1. The PersistentCl phenotype had a lower proportion of patients with renal recovery at day 7 (37%) compared with the RecoveredCl group (66%, p = 0.002). There was no difference between the PersistentCl phenotype and either of the NormalCl (51%) or DelayedCl (71%) phenotypes (Fig. 1). The PersistentCl phenotype had a lower proportion of patients with renal recovery at discharge (41%) compared with RecoveredCl (75%, p = 0.0001) and NormalCl (51%, p = 0.009). There was no difference between PersistentCl and DelayedCl (Fig. 1) phenotypes.
Day 7 and discharge renal recovery rates by chloride phenotypes. Patients with PersistentCl had a significantly lower rate of day 7 renal recovery compared with RecoveredCl (p = 0.002), and significantly lower rate of discharge renal recovery compared with RecoveredCl (p = 0.0001) and NormalCl (p = 0.009). PersistentCl, hyperchloremia on day 2 and day 7; RecoveredCl, hyperchloremia on day 2 with normochloremia on day 7; DelayedCl, no hyperchloremia on day 2, but with hyperchloremia on day 7; and NormalCl, no hyperchloremia on day 2 nor day 7. p values are Bonferroni corrected for multiple comparisons
The PersistentCl phenotype was associated with a higher mortality rate (29%) compared with RecoveredCl (8%, p = 0.002) and NormalCl (8%, p = 0.009) (Fig. 2). There was no difference between PersistentCl and the DelayedCl (29%) phenotypes. There was no difference in the duration of hospital length of stay among survivors (p = 0.06). The DelayedCl had significantly longer duration of mechanical ventilation among survivors (19.5 days; IQR, 7.5–27; p = 0.04).
Mortality rates by chloride phenotypes. Patients with PersistentCl had a significantly higher rate of mortality compared with those with RecoveredCl (p = 0.002) and NormalCl (p = 0.009). PersistentCl, hyperchloremia on day 2 and day 7; RecoveredCl, hyperchloremia on day 2 with normochloremia on day 7; DelayedCl, no hyperchloremia on day 2, but with hyperchloremia on day 7; and NormalCl, no hyperchloremia on day 2 nor day 7. p values are Bonferroni corrected for multiple comparisons
In a multivariable analysis, the PersistentCl phenotype was associated with lower odds of day 7 renal recovery (aOR = 0.29; 95% CI, 0.14 to 0.60; p = 0.009). Unadjusted and adjusted odds ratios of day 7 renal recovery are summarized in Table 2. In a second multivariable analysis, the PersistentCl phenotype was associated with a lower odds of discharge renal recovery (aOR = 0.22; 95% CI, 0.11 to 0.48; p = 0.0001). In this analysis, the NormalCl phenotype was significantly different from the RecoveredCl phenotype (aOR = 0.39; 95% CI, 0.20 to 0.74; p = 0.004), and the DelayedCl group was not significantly different from the RecoveredCl group (aOR = 0.85; 95% CI, 0.20 to 3.64; p = 0.82). Unadjusted and adjusted odds ratios of renal recovery at discharge are summarized in Table 3.
In a third multivariable analysis, there was a 3.5 times increase in odds of mortality in those with PersistentCl (aOR = 3.5; 95% CI, 1.11 to 11.10; p = 0.03) (Table 4) when compared with those with RecoveredCl. The NormalCl group was not significantly different from the RecoveredCl group (aOR = 1.81; 95% CI, 0.57 to 5.76; p = 0.32). The DelayedCl group was significantly different from the RecoveredCl group (aOR = 11.35; 95% CI, 1.78 to 72.21; p = 0.01). Additionally, ↑Na ≥ 5 mEq/L had a 3.1 increased adjusted odds of mortality (aOR = 2.77; 95% CI, 1.23 to 6.21; p = 0.01).
When comparing the effects of PersistentCl in reference to the NormalCl group, the direction of the estimates indicating PersistentCl decreased the odds of recovery at both day 7 and day 50. The odds of mortality increased with PersistentCl. Adjusted odds estimates did not reach statistical significance, yet were consistent in direction with the initial inference. We also performed two post hoc sensitivity regression analyses on specific subgroups of patients. The first included only the PersistentCl group and the RecoveredCl with a total of 159 patients. The inference was consistent with the initial analysis, with significant differences observed between the two groups in the same direction for all three outcomes (data not shown). When analyzing only the PersistentCl and NormalCl phenotypes (n = 243), the direction of the estimated difference in odds was consistent with the original inference; however, the difference only attained statistical significance in the univariate analysis with a decrease in the odds of renal recovery at both day 7 and day 50 and an increase in the odds of mortality with PersistentCl.
Discussion
To our knowledge, this is the first pediatric study to evaluate the effects of chloride on AKI recovery and death in patients with known AKI. In this study, we demonstrate that persistent hyperchloremia is a common finding among critically ill children with AKI (20%) and that it is associated with impaired renal recovery and increased mortality. These findings persisted after adjustment of confounding variables, including severity of illness, degree of acidosis, and severity of AKI. Interestingly, the baseline mortality rate was high among the patients (13%) in our study. This rate was similar to that of those who developed AKI in a previously reported multi-national study [1]. Despite the high overall mortality rate in this population, patients with persistent hyperchloremia still had a 3.5 greater odds of dying compared with those with a return to normochloremia.
These effects of hyperchloremia on outcomes in this study are consistent with prior studies in both adult and pediatric populations irrespective of AKI diagnosis. We previously demonstrated that all critically ill children with perturbations in chloride homeostasis had worse clinical outcomes, including mortality [9]. Similarly, Stenson et al. demonstrated that in pediatric patients with septic shock, hyperchloremia was independently associated with a complicated course and increased mortality [10]. Moreover, the subpopulation in the study by Stenson et al. who had the worst odds for both a complicated course and mortality, was those with a minimum chloride level ≥ 110 mEq/L during the first 7 days of their PICU stay [10]. This subpopulation of septic patients would meet our definition of persistent hyperchloremia.
The proposed mechanism for this association with worse clinical outcomes is the possible effect of hyperchloremia on glomerular filtration rate (GFR). Increases in the extracellular chloride levels have been shown to lead to vasoconstriction of the afferent arteriole and decreased GFR in animal models [16]. This vasoconstriction was further illustrated by Chowdhury et al. in their experiment with healthy volunteers in which an infusion of 0.9% saline led to decreased renal blood flow and cortical tissue perfusion [18]. It is therefore plausible that hyperchloremia may cause decreases in renal perfusion which may lead to new AKI, increased AKI severity, or impaired renal recovery. The etiology of the hyperchloremia is unclear. Our working hypothesis is that there is a phenotype of AKI in which the kidneys are unable to maintain chloride homeostasis and when those patients are given a large chloride load, they develop hyperchloremia.
The high frequency of impaired renal recovery in the patients with persistent hyperchloremia—only 41% having recovered upon discharge—is very concerning. It has previously been shown that a lack of renal recovery increases a patient’s risk for developing CKD [2] or dying by the 1-year follow-up [7]. With such significant risks, every effort to promote renal recovery and prevent potential on-going harm should be pursued. Therefore, further evaluation of whether serum chloride is a potential modifiable risk factor is warranted. Critically ill children may benefit from a decreased chloride load with the use of balanced fluids for both resuscitation and maintenance. Research into this approach is currently underway.
Two other phenotypes worth discussing are DelayedCl and NormalCl. First, the sample size of the DelayedCl phenotype is small (n = 14), but the group had a similar absolute mortality rate to the PersistentCl group. Moreover, in the adjusted regression model, the DelayedCl group had an 11.4 increased odds of mortality. This further demonstrates that hyperchloremia is associated with increased mortality. Second, the NormalCl cohort had a low renal recovery rate that was similar to patients with persistently deranged chloride homeostasis, but with a relatively lower mortality rate. These findings may suggest that AKI with vs. without disruption in chloride homeostasis may represent different phenotypes of AKI. Though further investigation would be needed to evaluate this hypothesis, these facts highlight the on-going need for better ways to measure and categorize AKI beyond creatinine.
Our study has several strengths and limitations. One strength is that we have a large population of critically ill children with AKI. Another is that we have controlled for serum sodium, serum bicarbonate, and severity of AKI. There are several limitations that warrant discussion. First, this is a retrospective study, and thus we can only establish associations and not causality. Second, we were unable to assess the total chloride load of the patients prior to admission to the PICU. The chloride content of the fluids may have varied significantly. However, anecdotally, 0.9% saline is used almost exclusively at our institution and referring hospitals. Therefore, the chloride load given to patients is likely reflected in the fluid volume delivered. Third, the fluid overload assessment is based on admission weight. Some patients may have been volume depleted on admission and thus result in an over-estimation of the degree of fluid overload in these patients. Fourth, misclassification of non-recovery from unknown pre-existing CKD may have occurred in patients for which there was no baseline creatinine measurement and one was calculated based on a normative GFR. Fifth, we did not evaluate for other potential causes of ongoing renal injury, including nephrotoxic medication burden. Sixth, while the cohort included in this study is from nearly 5 years ago, we do not believe there have been significant changes in management that would impact chloride load and AKI across many pediatric centers, making our results relevant to the care of critically ill children. Lastly, creatinine is an imperfect biomarker and may overestimate recovery by ignoring a decrease in muscle mass that occurs during critical illness and hospitalizations in general [25].
Conclusions
Persistent hyperchloremia is independently associated with impaired renal recovery as well as increased mortality among children with AKI early in the course of critical illness. Given these important risks, prospective studies are indicated to determine whether serum chloride levels represent a modifiable risk factor for AKI development, AKI recovery, and death.
References
Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL (2017) Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376:11–20. https://doi.org/10.1056/NEJMoa1611391
Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, Matsell DG (2012) Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis 59:523–530. https://doi.org/10.1053/j.ajkd.2011.10.048
Hollander SA, Montez-Rath ME, Axelrod DM, Krawczeski CD, May LJ, Maeda K, Rosenthal DN, Sutherland SM (2016) Recovery from acute kidney injury and CKD following heart transplantation in children, adolescents, and young adults: a retrospective cohort study. Am J Kidney Dis 68:212–218. https://doi.org/10.1053/j.ajkd.2016.01.024
Helgadottir S, Sigurdsson MI, Palsson R, Helgason D, Sigurdsson GH, Gudbjartsson T (2016) Renal recovery and long-term survival following acute kidney injury after coronary artery surgery: a nationwide study. Acta Anaesthesiol Scand 60:1230–1240. https://doi.org/10.1111/aas.12758
Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, Bittleman D, Cruz D, Endre Z, Fitzgerald RL, Forni L, Kane-Gill SL, Hoste E, Koyner J, Liu KD, Macedo E, Mehta R, Murray P, Nadim M, Ostermann M, Palevsky PM, Pannu N, Rosner M, Wald R, Zarbock A, Ronco C, Kellum JA (2017) Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 13:241–257. https://doi.org/10.1038/nrneph.2017.2
Griffin BR, You Z, Holmen J, SooHoo M, Gist KM, Colbert JF, Chonchol M, Faubel S, Jovanovich A (2019) Incident infection following acute kidney injury with recovery to baseline creatinine: a propensity score matched analysis. PLoS One 14:e0217935. https://doi.org/10.1371/journal.pone.0217935
Kellum JA, Sileanu FE, Bihorac A, Hoste EA, Chawla LS (2017) Recovery after acute kidney injury. Am J Respir Crit Care Med 195:784–791. https://doi.org/10.1164/rccm.201604-0799OC
Barhight MF, Lusk J, Brinton J, Stidham T, Soranno DE, Faubel S, Goebel J, Mourani PM, Gist KM (2018) Hyperchloremia is independently associated with mortality in critically ill children who ultimately require continuous renal replacement therapy. Pediatr Nephrol 33:1079–1085. https://doi.org/10.1007/s00467-018-3898-2
Barhight MF, Brinton J, Stidham T, Soranno DE, Faubel S, Griffin BR, Goebel J, Mourani PM, Gist KM (2018) Increase in chloride from baseline is independently associated with mortality in critically ill children. Intensive Care Med 44:2183–2191. https://doi.org/10.1007/s00134-018-5424-1
Stenson EK, Cvijanovich NZ, Anas N, Allen GL, Thomas NJ, Bigham MT, Weiss SL, Fitzgerald JC, Checchia PA, Meyer K, Quasney M, Hall M, Gedeit R, Freishtat RJ, Nowak J, Raj SS, Gertz S, Grunwell JR, Wong HR (2018) Hyperchloremia is associated with complicated course and mortality in pediatric patients with septic shock. Pediatr Crit Care Med 19:155–160. https://doi.org/10.1097/PCC.0000000000001401
de Vasconcellos K, Skinner DL (2018) Hyperchloraemia is associated with acute kidney injury and mortality in the critically ill: a retrospective observational study in a multidisciplinary intensive care unit. J Crit Care 45:45–51. https://doi.org/10.1016/j.jcrc.2018.01.019
Neyra JA, Canepa-Escaro F, Li X, Manllo J, Adams-Huet B, Yee J, Yessayan L (2015) Association of hyperchloremia with hospital mortality in critically ill septic patients. Crit Care Med 43:1938–1944. https://doi.org/10.1097/CCM.0000000000001161
McCluskey SA, Karkouti K, Wijeysundera D, Minkovich L, Tait G, Beattie WS (2013) Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: a propensity-matched cohort study. Anesth Analg 117:412–421. https://doi.org/10.1213/ANE.0b013e318293d81e
Suetrong B, Pisitsak C, Boyd JH, Russell JA, Walley KR (2016) Hyperchloremia and moderate increase in serum chloride are associated with acute kidney injury in severe sepsis and septic shock patients. Crit Care 20:315. https://doi.org/10.1186/s13054-016-1499-7
Stenson EK, Cvijanovich NZ, Allen GL, Thomas NJ, Bigham MT, Weiss SL, Fitzgerald JC, Jain PN, Meyer K, Quasney M, Hall M, Gedeit R, Freishtat RJ, Nowak J, Lutfi R, Gertz S, Grunwell JR, Wong HR, Anas N (2018) Hyperchloremia is associated with acute kidney injury in pediatric patients with septic shock. Intensive Care Med 44:2004–2005. https://doi.org/10.1007/s00134-018-5368-5
Yamamoto T, Hayashi K, Matsuda H, Kubota E, Tanaka H, Ogasawara Y, Nakamoto H, Suzuki H, Saruta T, Kajiya F (2001) In vivo visualization of angiotensin II- and tubuloglomerular feedback-mediated renal vasoconstriction. Kidney Int 60:364–369. https://doi.org/10.1046/j.1523-1755.2001.00808.x
Wilcox CS (1983) Regulation of renal blood flow by plasma chloride. J Clin Invest 71:726–735
Chowdhury AH, Cox EF, Francis ST, Lobo DN (2012) A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg 256:18–24. https://doi.org/10.1097/SLA.0b013e318256be72
Pollack MM, Patel KM, Ruttimann UE (1996) PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med 24:743–752
Khwaja A (2012) KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120:c179–c184. https://doi.org/10.1159/000339789
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637. https://doi.org/10.1681/ASN.2008030287
Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL (2008) Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol 3:948–954. https://doi.org/10.2215/CJN.05431207
Forni LG, Darmon M, Ostermann M, Oudemans-van Straaten HM, Pettila V, Prowle JR, Schetz M, Joannidis M (2017) Renal recovery after acute kidney injury. Intensive Care Med 43:855–866. https://doi.org/10.1007/s00134-017-4809-x
Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, Hackbarth R, Somers MJ, Baum M, Symons JM, Flores FX, Benfield M, Askenazi D, Chand D, Fortenberry JD, Mahan JD, McBryde K, Blowey D, Goldstein SL (2010) Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis 55:316–325. https://doi.org/10.1053/j.ajkd.2009.10.048
Thongprayoon C, Cheungpasitporn W, Kashani K (2016) Serum creatinine level, a surrogate of muscle mass, predicts mortality in critically ill patients. J Thorac Dis 8:E305-311. doi:https://doi.org/10.21037/jtd.2016.03.62
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The University of Colorado Multiple Institutional Review Board approved the study with a waiver of informed consent.
Conflict of interest
Katja M. Gist, DO MSCS, is a consultant for BioPorto. The remaining authors have disclosed that they do not have any conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PPTX 98 kb)
Rights and permissions
About this article
Cite this article
Barhight, M.F., Brinton, J.T., Soranno, D.E. et al. Effects of hyperchloremia on renal recovery in critically ill children with acute kidney injury. Pediatr Nephrol 35, 1331–1339 (2020). https://doi.org/10.1007/s00467-020-04513-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04513-7