Abstract
Background
Avoidance of vaccine-preventable infections in paediatric renal allograft recipients is of utmost importance. However, the development and maintenance of protective vaccination titres may be impaired in this patient population owing to their need for immunosuppressive medication.
Methods
In the framework of the Cooperative European Paediatric Renal Transplant Initiative (CERTAIN), we therefore performed a multi-centre, multi-national study and analysed vaccination titres pre- and post-transplant in 155 patients with serial titre measurements in comparison with published data in healthy children.
Results
The percentage of patients with positive vaccination titres before renal transplantation (RTx) was low, especially for diphtheria (38.5%, control 75%) and pertussis (21.3%, control 96.3%). As few as 58.1% of patients had a hepatitis B antibody (HBsAb) titre >100 IU/L before RTx. 38.1% of patients showed a vaccination titre loss post-transplant. Patients with an HBsAb titre between 10 and 100 IU/L before RTx experienced a significantly (p < 0.05) more frequent hepatitis B vaccination titre loss post-transplant than patients with an HBsAb titre >100 IU/L. The revaccination rate post-transplant was low and revaccination failed to induce positive titres in a considerable number of patients (27.3 to 83.3%). Treatment with rituximab was associated with a significantly increased risk of a vaccination titre loss post-transplant (odds ratio 4.26, p = 0.033).
Conclusions
These data show a low percentage of patients with positive vaccination titres pre-transplant, a low revaccination rate post-transplant with limited antibody response, and a high rate of vaccination titre losses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infections constitute a major cause of morbidity and mortality in paediatric renal transplant recipients whose susceptibility to infections is increased owing to their need for life-long immunosuppressive treatment [1, 2]. Prevention of systemic infections is therefore of vital importance in this vulnerable patient population. Vaccination is an essential strategy for reducing the rate of specific, potentially life-threatening, bacterial and viral infections. We have recently reported in a large multi-centre, multi-national study that the vaccination coverage in paediatric kidney transplant candidates is incomplete [3]. In addition, as a consequence of pre-transplant uraemia and/or post-transplant immunosuppression, paediatric renal transplant recipients may build up an inadequate immune response against vaccine antigens and/or may be at an increased risk of vaccination titre loss. So far, data published on pre- and post-transplant vaccination titres in paediatric renal transplant patients are scarce and mostly restricted to small, single-centre studies [4, 5]. We therefore analysed, in a multi-centre, observational, non-interventional cohort study, the pre- and post-transplant vaccination titres and the antibody losses and efficacy of revaccinations after renal transplantation in a sub-cohort of the recently published multi-centre, multi-national study [3].
Materials and methods
Study design and patient population
This is a multi-centre, multi-national cohort analysis of data reported to the Cooperative European Paediatric Renal Transplant Initiative (CERTAIN) Registry (www.certain-registry.eu), which, owing to its detailed and comprehensive data capture, allows an in-depth characterisation of specific patient cohorts [6]. Vaccination-specific data collection for this particular analysis was performed based on a defined protocol (see Supplementary Material). Inclusion criteria were:
-
1.
Paediatric patients who had undergone renal transplantation between 1999 and 2013
-
2.
Kidney allograft recipients with a complete and validated data set, aged ≤21 years at the time of transplantation
-
3.
A time interval of at least 4 weeks between (re)vaccination and serum sampling for titre measurement
In total, 155 paediatric renal transplant recipients with serial titre measurements were included in the study. Patient and transplant characteristics, including initial immunosuppressive therapy, are shown in Table 1. In addition, 14 patients received rituximab at 5.5 ± 4.2 months (range, 0.8–11.7 months) post-transplant because of post-transplant lymphoproliferative disease (n = 3) or acute or chronic antibody-mediated rejection (n = 11).
The CERTAIN Registry has been approved by the ethics committee of each contributing centre and is kept in full accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. Written informed consent to participate in the registry was obtained from all parents or guardians and patients when appropriate for their age. The study was designed, analysed and reported according to the STROBE guidelines (https://www.strobe-statement.org).
Analysis of vaccination titres
Vaccination certificates and patient records were used to provide information about types and timing of the following vaccinations: Bacillus Calmette–Guerin (BCG), diphtheria, Haemophilus influenzae type B (HiB), hepatitis A, hepatitis B, human papillomavirus (HPV), influenza, measles, mumps, meningococci, pertussis, pneumococci, polio, rotavirus, rubella, tetanus and varicella. Country-, era-specific and age-appropriate vaccination coverage was obtained for each patient. For this purpose, the country-specific vaccination recommendation applicable for the respective patient age at the time of a specifically recommended vaccination was taken as the reference. The patient’s vaccination schedule was defined as “complete” if the specific vaccine had been given according the country-, era- and age-appropriate vaccination recommendation. Vaccine types, dosages, and mean time period between the last vaccine dose and titre measurement before renal transplantation are given in Supplementary Table 1.
Vaccination titres were annually determined post-transplant in local laboratories of the participating transplant centres, and the results were usually available within 2 weeks of measurement. Commercial assays used for measurement of the following vaccination titres are contained in Supplementary Table 2: hepatitis A, hepatitis B, measles, mumps, rubella, and varicella. Besides evaluating the percentage of patients with a positive HBsAb titre >10 IU/L, we also analysed the rate of patients with HBsAb titres above 100 IU/L, as suggested by the European Consensus Group of Kidney Disease: Improving Global Outcomes (KDIGO) [7, 8]. In a subset of patients, additional vaccination titres, not routinely determined in patient aftercare, were measured in a cross-sectional manner by the following assays: diphtheria (Novagnost® Diphtheria Toxin 5S IgG; Siemens Healthcare, Germany; positive titre >1.0 IU/L), pertussis (anti-Bordetella pertussis toxin ELISA (IgG); EUROIMMUN, Germany; positive titre >100 IU/mL), pneumococci (VaccZyme™ Anti-PCP IgG Enzyme Immunoassay Kit; The Binding Site, Germany; positive titre >3.3 mg/L; this assay measures a total IgG pneumococcal response to the following pneumococcal capsular polysaccharide antigens: 1–5, 6B, 7F, 8, 9 N, 9 V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, 33F), and tetanus (Novagnost® Tetanus Toxin 5S IgG; Siemens Healthcare, Germany; positive titre >1.0 IU/L). These assays were performed in a central laboratory (Department of Infectious Diseases, Virology, University Hospital Heidelberg, Germany); the results were only available at the end of the study.
As no healthy control group was available, we compared the rates of positive vaccination titres in this paediatric renal transplant patient cohort with those published for healthy children and adolescents [9,10,11,12,13,14,15,16]. We therefore performed an article search via the MEDLINE literature database. The following predefined inclusion criteria for the selection of data obtained in healthy controls were used to avoid an arbitrary selection of articles: articles published within the last 10 years to obtain contemporary data, long-term data reported on a large cohort of children and adolescents from Europe or other Western countries. Supplementary Table 3 contains the assays chosen for the determination of vaccination titres in healthy controls. Identical serological assays for vaccination titres against hepatitis A, hepatitis B, measles, mumps, rubella and varicella were applied in the transplant cohort and in healthy controls. For detection of vaccination titres against diphtheria, pneumococci, pertussis and tetanus, similar serological assays were used for patients and controls. We also analysed the impact of the primary renal disease (immune-mediated disease requiring immunosuppressive treatment before transplantation, nephrotic syndrome with loss of vaccine-specific IgGs) and an incompleteness of the vaccination schedule as potential risk factors for a negative pre-transplant vaccination titre.
A post-transplant titre loss was defined as a switch from a positive to a negative titre for a specific vaccination. We analysed risk factors for a vaccination titre loss post-transplant. In terms of hepatitis B vaccination, we also investigated the association between a higher vaccine dose, ≥ 20 μg (− 40 μg; adult or dialysis dose) vs 5–10 μg (paediatric dose), and the ability to obtain an HBsAb titre above 100 IU/L, in addition to the association between a higher vaccine dose and the risk of an HBsAb titre loss. We also analysed the impact of rituximab therapy and other immunosuppressants and nephrotic-range proteinuria (≥ 1,000 mg/m2 per day) as potential risk factors for a titre loss after immunisation with live and inactivated vaccines. Post-transplant revaccination rates were reported as percentages of patients receiving a revaccination scheduled for the 1st, 2nd, or 3rd year after transplantation. Revaccinations were performed depending on local standard procedures. Hence, they were given not only to patients with titre loss, but also to previous non-responders and to patients without serial titre measurement.
Data validation process
After automatic validation during the data entry process, all documented data in the CERTAIN Registry undergo an additional manual quality assurance process, because the documented data set needs to be locally approved by the on-site supervising physician and to pass a plausibility check by the data quality manager in the registry headquarters before incorporation into the CERTAIN data repository. For the present study, additional source data verification was carried out by one of the investigators (MA).
Statistical analysis
Data were analysed with PASW (SPSS) Statistics 24.0. If not stated otherwise, categorical parameters are presented as number and percentage of patients, whereas results for continuous variables are expressed as mean ± standard deviation. Normal distribution of data was evaluated by means of the Shapiro–Wilks test. Uni- and multivariate analyses (logistic regression) were used to describe the association of potential risk factors such as age at transplantation, immunosuppressive medication, transplant function, anti-rejection therapy and completeness of the vaccination schedule with a titre loss post-transplant. A two-tailed p value <0.05 was regarded as statistically significant. All analyses were of an explorative, non-confirmatory nature.
Results
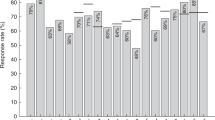
Table 2 shows the pre-transplant rates of positive and negative vaccination titres in patients, stratified according to the completeness of the applicable vaccination schedule. For most vaccines, the rate of pre-transplant positive titres was low. We compared the rates of positive vaccination titres in this patient cohort with those published for healthy children and adolescents (Table 2) [9,10,11,12,13,14,15]. With the exception of HBsAb titres, transplant patients developed a positive vaccination titre less often than healthy children and adolescents (Table 2). The association of a negative pre-transplant vaccination titre with an incomplete vaccination schedule (OR 1.70, p = 0.122) or a nephrotic syndrome such as primary renal disease (OR 2.23, p = 0.113) was weak and statistically not significant. In addition, there was no association between a negative pre-transplant vaccination titre and an immune-mediated primary renal disease (OR 1.26, p = 0.664) or pre-transplant dialysis treatment vs pre-emptive transplantation (OR 1.13, p = 0.667).
Figure 1 depicts the flowchart of patients with vaccine titre measurement before and after renal transplantation. In the 1st year after renal transplantation, 40 out of 105 (38.1%) of patients with pre- and post-transplant vaccine titre measurement experienced an antibody loss (Table 3). Post-transplant revaccinations failed to induce positive titres in a considerable number of patients (Table 4). In addition, the revaccination rate post-transplant was generally low: depending on the type of vaccination, only 1.0% (1 out of 98) (pneumococci) to 21.2% (11 out of 52) (tetanus, diphtheria) of patients received their scheduled revaccinations during the first 3 years post-transplant. It would have been interesting to analyse whether there was a difference in the immune response to revaccination between prior vaccine non-responders and patients with a post-transplant titre loss. However, the number of patients in this study was too low to make a valid statement.
Inactivated vaccines
Hepatitis A
Forty out of 62 paediatric renal transplant patients (64.5%) exhibited a positive vaccination titre (Table 2) in response to the hepatitis A vaccine compared with 97.9% of healthy children and adolescents (Table 2) [11, 15]. During the 1st year after transplantation, 9.8% of patients lost their vaccination titre (Table 3). The post-transplant revaccination rate was low, ranging between 3.2% (4 out of 126) and 5.3% (4 out of 75) in the first 3 years. In addition, revaccination resulted in a positive hepatitis A vaccination titre in only 50.0% of patients (Table 4). In neither unvaccinated nor vaccinated transplant recipients was a hepatitis A virus (HAV) infection observed during the 3 years post-transplant.
Hepatitis B
Most patients, namely 83.3%, had positive pre-transplant HBsAb titres above 10 IU/L (Table 2), a rate that even exceeds that observed in healthy children and adolescents (72.6%, Table 2). However, only 58.1% developed HBsAb titres above 100 IU/L before transplantation (Table 2), a percentage comparable with that seen in healthy individuals (Table 2) [9]. A significant association was observed between the use of a higher vaccine dose (adult or dialysis dose, ≥ 20 μg) and the ability to obtain a pre-transplant HBsAb titre above 100 IU/L (OR 2.31, p = 0.031). Post-transplant HBsAb titre measurements were available for 91 patients (Fig. 1), 75 of whom showed a positive titre above 10 IU/L pre-transplant. Fourteen patients (18.7%) lost their HBsAb titre post-transplant (Table 3).
Figure 2 illustrates the course of HBsAb titres over the first 3 years post-transplant, stratified according to the baseline titre between 10 and 100 IU/L or above 100 IU/L. Patients with a titre above 100 IU/L before transplantation developed an HBsAb titre loss post-transplant significantly less often than those with a baseline titre ranging between 10 and 100 IU/L (Fig. 2). A higher hepatitis B vaccine dose tended to be associated with a less frequent loss of the post-transplant HBsAb titre (OR 0.47, p = 0.065).
The revaccination rate post-transplant was low and amounted to only 18.4% (7 out of 38), 6.7% (1 out of 15) and 12.5% (1 out of 8) of scheduled hepatitis B vaccinations during the 1st, 2nd and 3rd post-transplant years respectively. Moreover, revaccination failed to induce positive titres in a remarkable number of patients: only 47.6% developed a sufficient HBsAb titre after post-transplant revaccination (Table 4).
Diphtheria, tetanus, pertussis
The rates of patients with a positive pre-transplant vaccination titre against diphtheria, tetanus and pertussis (DTP) were noticeably low, amounting to only 38.5%, 60.0% and 21.3% respectively (Table 2). In contrast, healthy children and adolescents develop more often a positive vaccination titre against these infectious agents (diphtheria, 75.0%, tetanus, 89.5%, pertussis, 96.3%; Table 2) [12, 13]. The percentages of patients with a post-transplant vaccination titre loss were 46.7% for diphtheria, 29.2% for tetanus and 75.0% for pertussis (Table 3). Revaccination rates were low, ranging between 3.1% (3 out of 98) and 21.2% (11 out of 52) within the first 3 years post-transplant. Additionally, revaccinations failed to induce positive titres in a considerable number of patients: only 66.7% developed a sufficient diphtheria titre, 72.7% a positive tetanus titre and as few as 16.7% exhibited a positive pertussis titre in response to revaccination (Table 4).
Pneumococci
Some 69.4% of patients exhibited a positive vaccination titre against pneumococci serotypes before transplantation. In healthy children and adolescents, the rates of a positive titre range between 63.2% and 99.5% (Table 2), dependent on the type of vaccine [14]. During the 1st year post-transplant, 46.2% of patients with serial pneumococci vaccine titre measurements experienced a titre loss (Table 3). The revaccination rate after transplantation was low, namely 1.0% (1 out of 98), 2.9% (2 out of 70) and 2.8% (2 out of 71) during the 1st, 2nd and 3rd post-transplant years respectively.
Live vaccines
Measles, mumps, rubella
Before transplantation, only 77.4% of patients had a positive measles titre, despite a complete vaccination schedule (Table 2). Hence, paediatric patients with advanced CKD developed a positive measles titre in response to vaccination less often than healthy children and adolescents (97.0%, Table 2) [10]. The same difference was observed for vaccine titres against mumps, which were positive in 73.0% of transplant recipients (Table 2) and in 93.8% of healthy individuals (Table 2). Although the rate of patients with a positive pre-transplant rubella vaccine titre was high (89.8%, Table 2), it was still lower than that found in healthy children and adolescents (100%, Table 2). Figure 3 illustrates the post-transplant course of measles, mumps and rubella (MMP) titres. Although most patients with a positive pre-transplant measles or rubella titre still exhibited positive titres after transplantation, about 30% of patients had lost their positive vaccine titre against mumps within 3 years post-transplant, this pointing to a lower immunogenicity of the mumps vaccine than that of the measles or rubella vaccine (Fig. 3).
Varicella
The pre-transplant rate of positive varicella titres was 79.2%. Hence, 20.8% of patients did not develop positive vaccination titres before transplantation (Table 2). Compared with healthy individuals (Table 2) [10], the rate of patients with a positive varicella titre was 17.6% lower. Moreover, 25.0% of patients with serial varicella vaccine titre measurement (12 out of 48) lost their positive titre over the 1st year post-transplant. During the observation period of up to 3 years post-transplant, 3 unvaccinated (3.9%) and 2 vaccinated recipients (2.6%) contracted varicella (p = 0.660). Vaccination titres were available for the latter 2 patients who had been vaccinated against varicella. Although one of them developed varicella infection despite a positive vaccination titre of 400 IU/L, the other one had lost her vaccination titre 3.3 years after vaccination, before acquiring varicella infection.
Risk factors associated with a post-transplant vaccination titre loss
Although according to univariate analysis rituximab therapy (OR 4.23, p = 0.022) and a TAC-based immunosuppression (OR 3.23, p = 0.005) were significantly associated with an increased rate of a vaccination titre loss (Table 5), the use of rituximab, according to multivariate analysis, was the only independent risk factor associated with a 4 times higher risk of a titre loss (p = 0.033; Table 5). No significant association was observed between the use of rituximab and a titre loss after a specific vaccination (live vaccines: OR 1.12, p = 0.880; inactivated vaccines: OR 1.64, p = 0.454). Data about B cell recovery after rituximab treatment were available for 7 patients over a period of 3 years post-transplant. Three patients showed no B cell recovery during the first 3 years post-transplant, and 4 patients had no rebound of vaccination titres, despite B cell recovery.
A trend towards a higher risk of a vaccination titre loss became apparent under an MMF-based immunosuppression (OR 2.29), but without statistical significance (p = 0.089; Table 5). Immunosuppressive score, IL-2R antagonist induction, steroid-based immunosuppression, transplant function (defined as estimated glomerular filtration rate [eGFR] [17]), anti-rejection therapy and incompleteness of the vaccination schedule were not associated with a vaccination titre loss (Table 5).
Discussion
To our knowledge, this is the largest study published so far investigating the course of vaccination titres and the impact of immunosuppressive therapy on vaccination titres in paediatric renal transplant recipients. The main findings are that:
-
1.
For most vaccines, pre-transplant titres were low, especially in comparison with healthy children.
-
2.
A significant number of patients (38.1%) experienced a titre loss after transplantation.
-
3.
Post-transplant revaccination rates were insufficient and failed to induce positive titres in a noticeable number of patients.
-
4.
Treatment with the anti-B-cell antibody rituximab was significantly associated with the loss of positive vaccination titres after transplantation.
In the present study, the rates of patients with positive vaccination titres differed widely between 21.3% and 83.3%, depending on the type of vaccine and the completeness of vaccination schedules. Previous, mostly retrospective, single-centre studies in paediatric transplant candidates and recipients have yielded similar heterogeneous results [4, 18, 19]. Regarding hepatitis B, most patients (83.3%) in this study developed a positive HBsAb titre above 10 IU/L before transplantation; a value that is considered to afford protection against hepatitis B [7]. Only one other study examined the rate of paediatric renal transplant candidates with HBsAb titres >10 IU/L and reported a similar percentage (84.3%) [5]. It is noteworthy that the rate of positive vaccination HBsAb titres among the paediatric renal transplant population of the present study was even higher than that observed in healthy children and adolescents [9]. This is probably attributable to the fact that dialysis patients and renal transplant candidates are tested regularly for hepatitis B and HBsAb titres and get revaccinated if their titre falls below 10 IU/L. However, concerns have been expressed whether an HBsAb titre between 10 and 100 IU/L is sufficient to fully protect against hepatitis B, especially against the background of a potential titre loss under post-transplant immunosuppressive treatment. Hence, the European Consensus Group of KDIGO has suggested aiming at HBsAb titres >100 IU/L in renal allograft recipients [8].
In the present study, only 58.1% of paediatric patients developed HBsAb titres above 100 IU/L before transplantation. Two small, single-centre studies found even lower rates of 42.9% and 32% respectively [4, 18]. We observed a significant association between higher hepatitis B vaccine doses (≥ 20 μg) and the ability to achieve HBsAb titres >100 IU/L. As suboptimal response to the hepatitis B vaccine and a more rapid decline in HBsAb levels after immunisation have been well-documented among adult dialysis patients, the Advisory Committee on Immunisation Practices (ACIP) recommends that these patients receive augmented hepatitis B vaccine doses of 40 μg [20, 21], whereas for paediatric transplant candidates a comparable recommendation has not yet been phrased.
Although an earlier study did not show a clear gradient of higher seroconversion rates with increasing hepatitis B vaccine doses in paediatric dialysis patients [22], other studies suggested higher vaccination response rates in children receiving booster vaccinations with augmented vaccine doses [23, 24]. To date, the ACIP has not made specific recommendations for higher hepatitis B vaccine dosage use in paediatric patients on dialysis treatment [20]. Our findings, however, support the rationale to administer higher vaccine doses to achieve HBsAb titres above 100 IU/L. This issue is especially relevant in light of our observation that 18.7% of patients experienced an HBsAb titre loss (< 10 IU/L) over the 1st year post-transplant. An important result of our study is that the loss of protective HBsAb titres was significantly more frequent in patients with HBsAb titres between 10 and 100 IU/L than in those with titres above 100 IU/L before transplantation. A similar observation was made in a single-centre study among adult kidney allograft recipients [25]. As post-transplant revaccinations failed to induce protective HBsAb titres in 52.4% of patients in the present study, aiming for higher (> 100 IU/L) vaccination titres before transplantation by administering augmented vaccine doses appears advisable.
Of course, it should be remembered that host protection upon vaccination usually results from the complex interplay between humoral and cellular components of the immune system. Indeed, a cross-sectional study among 51 adult kidney allograft recipients revealed that a proportion of patients lacking an adequate humoral vaccine response (HBsAb titre <10 IU/L) was nevertheless able to mount a hepatitis B vaccine-specific T cell response [25]. A small study among 31 paediatric liver transplant recipients arrived at the same conclusions [26]. It is noteworthy that in the present study, none of the patients who had received a hepatitis B vaccine contracted the disease.
Our data indicate an inadequate humoral immune response against hepatitis A in paediatric renal transplant patients, as only 64.5% developed a positive HAV vaccine titre before transplantation, compared with 97.9% of healthy individuals [11, 15]. In addition, one tenth of patients lost their positive titre during the 1st year post-transplant, and revaccination resulted in positive HAV vaccine titres in only 72.7% of the patients. In a former study among adult kidney allograft recipients, as few as 24% of patients exhibited anti-HAV seroconversion after a primary dose of HAV vaccine, rising to 72% after a second booster dose [27]. A small study investigated the immune response to HAV vaccination in 13 paediatric heart transplant recipients, also showing a low humoral response in only 25% of patients [28]. To date, the American Society of Transplantation (AST) recommends routine HAV vaccination for all paediatric transplant candidates and recipients, whereas monitoring of HAV vaccination titres is currently advised only in the case of an ongoing risk of exposure [29].
Immunocompromised patients carry an increased risk of invasive pneumococci infections [30, 31]. The AST therefore recommends regular vaccine titre monitoring, and according to KDIGO guidelines, revaccination should be considered every 3–5 years post-transplant [7, 29]. We observed that only two thirds of paediatric kidney allograft recipients developed positive titres against certain pneumococci serotypes. Also, 46.2% lost their vaccination titre during the 1st year post-transplant. Hence, our findings support the recommendations of the AST to regularly monitor titres and initiate timely revaccinations. Two main formulations of pneumococcal vaccines are currently available: a 13-valent pneumococcal protein-conjugate vaccine and a 23-valent polysaccharide vaccine. Protein-conjugated vaccines may produce antibodies of higher avidity and also lead to the formation of memory B cells. In healthy infants under 2 years of age, the protein-conjugated vaccine proved to be more immunogenic. However, studies in adult and paediatric solid organ transplant (SOT) recipients did not show the superiority of one vaccine type [32,33,34]. Yet, it should be borne in mind that the conferred protection against pneumococci diseases remains unknown and may vary by serotype. Assessment of the response to pneumococcal vaccines is difficult because of the large numbers of serotypes contained in the vaccines. Additionally, different serological assays have yielded discrepant results for pneumococci vaccine titres in a prospective study among adult renal transplant recipients [35]. In the present study, only the pneumococcal 23vELISA (VaccZyme™) was used to determine a total IgG pneumococcal response. The main limitation of this assay is its inability to distinguish between high responses to a few individual serotypes and a good overall vaccine response. Hence, no statement can be made about protective titres against specific serotypes. However, a recent study by Dziadzio et al. found a good (approximately 80%) diagnostic agreement for an abnormal test-vaccination response when comparing a total pneumococcal IgG threshold of 4-fold rise and a serotype-specific IgG threshold of ≥0.35 mg/L [36]. According to the current literature, a repeated and sequential use of both the conjugated and the polysaccharide vaccines is recommended to achieve optimal protection against invasive pneumococci infections in SOT recipients [21, 29, 37].
The rates of protective vaccination titres against diphtheria and tetanus were low in this paediatric renal transplant population before transplantation, namely 38.5% and 60.0% respectively. Former small single-centre studies reported protective titre rates ranging between 38.0% and 64.7% for diphtheria, and percentages of 81.5% to 90.0% for tetanus [4, 18, 19, 21, 37,38,39,40]. Loss of diphtheria and tetanus vaccination titres occurred in 29.2% to 46.7% of our patient population, and booster revaccinations failed to induce a protective titre in one third of recipients. Our study is the 1st that reported immunity against pertussis vaccine in paediatric kidney allograft recipients. As few as 21.3% of patients developed a protective pertussis vaccine titre, compared with 96.3% of healthy children and adolescents [12]. In addition, post-transplant revaccination induced protective titres in only 16.7% of patients. This finding is important, as there is an increase in Bordetella pertussis infection in the general population, especially in adults [41]. For this reason, regular booster vaccination against pertussis is of special importance for transplant candidates and recipients. It should be noted that all patients in this study had received acellular pertussis vaccines (and not wild-type vaccines, which exhibit a higher reactogenicity). It has been shown that healthy individuals vaccinated with different pertussis vaccines may exhibit different courses of antibody loss and changes in cellular immunity [42]. To date, there are no universally accepted correlates of pertussis protection. In this study, only the anti-pertussis-toxin ELISA (with a high specificity) was used to determine positive vaccine titres. Hence, IgGs specific for other pertussis antigens, probably contributing to pertussis-specific immunity and even protection from pertussis disease, may have been missed. In addition, avidity testing was not performed.
It is a matter of concern that only 77.4% and 73.0% of patients developed protective vaccination titres against measles and mumps respectively. The rate of protective rubella vaccine titres was higher (89.9%), but one tenth of patients still showed inadequate humoral immunity. Moreover, 30% of patients had lost their mumps vaccine titre during the first 3 years post-transplant. An explanation for the poor maintenance against the mumps component could be the fact that 15.7% of patients had received only one dose of the mumps vaccine. Even in healthy individuals, a significant decline in mumps titres has been observed after a single dose of vaccine, whereas two doses resulted in a higher percentage of vaccine responders [43]. Another reason for the observed decrease in mumps titres may be the use of vaccines containing the Jeryl Lynn strain, which has been shown to be less immunogenic than other live virus strains [44]. Previous single-centre studies revealed comparable rates of positive vaccination titres in paediatric kidney allograft recipients [4, 45, 46]. In a small single-centre study, only 80.0%, 68.6% and 74.3% of 35 paediatric renal transplant candidates showed protective pre-transplant vaccination titres against measles, mumps and rubella respectively [4]. Even though, fortunately, none of the patients in our study suffered measles, mumps or rubella during the observation period, our data indicate the necessity of regular vaccine titre monitoring to ensure passive immunisation in the case of contact with an infected person, where feasible.
Before transplantation, 20.8% of patients in the present study had no protective varicella titre, thus making them susceptible to this potentially life-threatening infectious disease. This rate is comparable with that observed in a former study among 35 paediatric renal transplant recipients (80.0%) [4]. Additionally, 25.0% lost their protective titre during the 1st year post-transplant. In fact, one patient with a vaccine titre loss suffered varicella infection, whereas another patient developed chickenpox, despite a positive vaccine titre. A recent study investigated the sustainability of the humoral response to varicella vaccine in paediatric transplant recipients following a pre-transplant immunisation strategy [47]. Eighty per cent of 14 patients showed waning immunity against varicella-zoster virus (VZV) with a positive correlation between lymphocyte counts and varicella titres [47]. It is crucial, however, that the absence of high varicella vaccination titres does not imply a loss of protection, as cell-mediated immunity may still be intact [48]. Although varicella vaccination has been shown to be immunogenic and safe in paediatric liver transplant recipients under low immunosuppressive therapy, post-transplant vaccination remains a controversial issue, as fatal courses of vaccine varicella infection after transplantation have been described [49,50,51]. Our data extend current experiences with pre- and post-transplant varicella vaccine titres and support the concept of regular titre monitoring, as recommended by the AST [29].
Our study is the first to demonstrate a significant association between immunosuppressive treatment with rituximab and an increased vaccination titre loss in paediatric kidney allograft recipients. The risk of titre loss was 4 times higher in patients who had received rituximab. Only one previous study investigated the impact of rituximab administration on the response to tetanus toxoid vaccination in 39 adult renal transplant recipients (13 on rituximab treatment, 26 controls) [52]. Rituximab impaired the immune response after tetanus toxoid vaccination, but did not obliterate it in all patients [52]. It is plausible that rituximab-induced diminishing of B cells and a lack of IgG production lead to a reduction or even loss of vaccine titres. Therefore, it would be interesting to know whether vaccination titres do rebound after B cell recovery. However, as data on B cell recovery were only available for a subset of patients, we cannot make a valid statement regarding a potential titre rebound after B lymphocyte reconstitution.
A limitation of our study is that no data are available on the avidity of vaccine-induced antibodies or on patients’ cellular immunity. It is known, however, that on the one hand, the level of vaccination titres does not reflect cell-mediated immunity, whereas on the other hand, titres considered to be protective for healthy individuals may not prevent infection in immunosuppressed children. In addition, caution should be exercised when comparing data of transplant patients and healthy controls where vaccination titres were measured by different assays. Also, measurement of vaccine-specific IgGs by different laboratories may introduce some bias into the comparability of the data, but we aimed to reduce this potential bias by comparing only qualitative rather than quantitative titres. In addition, proficiency panels for serological assays ensured the standardisation and comparability of titres among different laboratories and served as external quality control. Furthermore, diminished cellular reactivity and failing IgG antibody avidity maturation have to be taken into account in transplant recipients when considering positive vaccine titres as protective. Indeed, a recent study investigating the humoral and cellular immune response to VZV vaccination or wild-type infection in kidney or liver transplant recipients and healthy controls has demonstrated significantly lower IgG-anti-VZV avidities and diminished numbers of VZV-specific IFN-γ-producing lymphocytes in transplant recipients despite similar VZV-specific IgG antibody concentration compared with age-matched healthy controls [48]. Also, we did not check the vaccination status of household contacts, which is an important element of so-called herd protection for immunocompromised patients. The strength of our study is that it investigated serial pre- and post-transplant vaccination titre measurement in a large cohort of paediatric renal transplant recipients. Therefore, we think that this registry analysis provides valuable information about vaccination titres in paediatric renal transplant recipients and the impact of immunosuppressive treatment on waning immunity.
To conclude, our data show a low percentage of patients with protective vaccination titres pre-transplant, a low revaccination rate post-transplant with limited antibody response and a high rate of vaccination titre losses. Post-transplant measurement of vaccination titres at regular intervals is advisable to document protection against vaccine-preventable diseases and to guide the indication and timing of revaccinations post-transplant. In addition, reduced antibody avidity and waning cellular immunity have to be considered in transplant recipients. Particular emphasis should be laid on patients receiving rituximab treatment, as this therapy was significantly associated with post-transplant vaccination titre losses. Moreover, closer adherence to vaccination schedules is requested to provide sufficient immunity through timely booster vaccinations. Further studies are needed to clarify whether augmenting the vaccine dosage, reducing booster intervals and/or using adjuvant vaccines are appropriate measures for increasing the immunity of transplant patients against vaccine-preventable diseases. Also, future mechanistic studies should be undertaken to clarify why in transplant recipients particular immune responses to specific vaccines are impaired and others are not.
References
Samuel SM, Tonelli MA, Foster BJ, Alexander RT, Nettel-Aguirre A, Soo A, Hemmelgarn BR (2011) Survival in pediatric dialysis and transplant patients. Clin J Am Soc Nephrol 6:1094–1099
https://web.emmes.com/study/ped/annlrept/annualrept2014.pdf North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Annual Transplant Report 2014. Accessed on 17 January 2017
Hocker B, Aguilar M, Schnitzler P, Pape L, Dello Strologo L, Webb NJA, Bald M, Genc G, Billing H, Konig J, Buscher A, Kemper MJ, Marks SD, Pohl M, Wigger M, Topaloglu R, Rieger S, Krupka K, Bruckner T, Fichtner A, Tonshoff B (2017) Incomplete vaccination coverage in European children with end-stage kidney disease prior to renal transplantation. Pediatr Nephrol. https://doi.org/10.1007/s00467-017-3776-3
Prelog M, Pohl M, Ermisch B, Fuchshuber A, Huzly D, Jungraithmayr T, Forster J, Zimmerhackl LB (2007) Demand for evaluation of vaccination antibody titers in children considered for renal transplantation. Pediatr Transplant 11:73–76
Genc G, Ozkaya O, Aygun C, Yakupoglu YK, Nalcacioglu H (2012) Vaccination status of children considered for renal transplants: missed opportunities for vaccine preventable diseases. Exp Clin Transplant 10:314–318
Plotnicki L, Kohl CD, Hocker B, Krupka K, Rahmel A, Pape L, Hoyer P, Marks SD, Webb NJ, Soylemezoglu O, Topaloglu R, Szabo AJ, Seeman T, Marlies Cornelissen EA, Knops N, Grenda R, Tonshoff B (2013) The CERTAIN registry: a novel, web-based registry and research platform for pediatric renal transplantation in Europe. Transplant Proc 45:1414–1417
Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group (2009) KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9 [Suppl 3]:S1–S155
Kasiske BL, Zeier MG, Chapman JR, Craig JC, Ekberg H, Garvey CA, Green MD, Jha V, Josephson MA, Kiberd BA, Kreis HA, RA MD, Newmann JM, Obrador GT, Vincenti FG, Cheung M, Earley A, Raman G, Abariga S, Wagner M, Balk EM (2010) KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int 77:299–311
Stroffolini T, Guadagnino V, Caroleo B, De Sarro G, Foca A, Liberto MC, Giancotti A, Barreca GS, Marascio N, Lombardo FL, Staltari O, Sersale’s Study G (2012) Long-term immunogenicity of hepatitis B vaccination in children and adolescents in a southern Italian town. Infection 40:299–302
Knuf M, Zepp F, Helm K, Maurer H, Prieler A, Kieninger-Baum D, Douha M, Willems P (2012) Antibody persistence for 3 years following two doses of tetravalent measles-mumps-rubella-varicella vaccine in healthy children. Eur J Pediatr 171:463–470
Van Der Wielen M, Vertruyen A, Froesner G, Ibanez R, Hunt M, Herzog C, Van Damme P (2007) Immunogenicity and safety of a pediatric dose of a virosome-adjuvanted hepatitis a vaccine: a controlled trial in children aged 1–16 years. Pediatr Infect Dis J 26:705–710
Embree J, Law B, Voloshen T, Tomovici A (2015) Immunogenicity, safety, and antibody persistence at 3, 5, and 10 years postvaccination in adolescents randomized to booster immunization with a combined tetanus, diphtheria, 5-component acellular pertussis, and inactivated poliomyelitis vaccine administered with a hepatitis B virus vaccine concurrently or 1 month apart. Clin Vaccine Immunol 22:282–290
John T, Voysey M, LM Y, McCarthy N, Baudin M, Richard P, Fiquet A, Kitchin N, Pollard AJ (2015) Immunogenicity of a low-dose diphtheria, tetanus and acellular pertussis combination vaccine with either inactivated or oral polio vaccine compared to standard-dose diphtheria, tetanus, acellular pertussis when used as a pre-school booster in UK children: a 5-year follow-up of a randomised controlled study. Vaccine 33:4579–4585
Van den Bergh MR, Spijkerman J, Francois N, Swinnen K, Borys D, Schuerman L, Veenhoven RH, Sanders EA (2016) Immunogenicity, safety and reactogenicity of a booster dose of the 10-valent pneumococcal nontypeable H. influenzae protein D conjugate vaccine coadministered with DTPa-IPV-Hib in Dutch children: a randomized controlled trial. Pediatr Infect Dis J 35:e206–e219
Van Herck K, Hens A, De Coster I, Vertruyen A, Tolboom J, Sarnecki M, Van Damme P (2015) Long-term antibody persistence in children after vaccination with the pediatric formulation of an aluminum-free virosomal hepatitis a vaccine. Pediatr Infect Dis J 34:e85–e91
Wysocki J, Brzostek J, Konior R, Panzer FG, Francois NA, Ravula SM, Kolhe DA, Song Y, Dieussaert I, Schuerman L, Borys D (2017) Antibody persistence and immunologic memory in children vaccinated with 4 doses of pneumococcal conjugate vaccines: results from 2 long-term follow-up studies. Hum Vaccin Immunother 13:661–675
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Beil S, Kreuzer M, Pape L (2012) Course of immunization titers after pediatric kidney transplantation and association with glomerular filtration rate and kidney function. Transplantation 94:e69–e71
Balloni A, Assael BM, Ghio L, Pedrazzi C, Nebbia G, Gridelli B, Melada E, Panuccio A, Foti M, Barbi M, Luraschi C (1999) Immunity to poliomyelitis, diphtheria and tetanus in pediatric patients before and after renal or liver transplantation. Vaccine 17:2507–2511
https://www.cdc.gov/dialysis/pdfs/vaccinating_dialysis_patients_and_patients_dec2012.pdf Centers for Disease Control and prevention (CDC). Recommendations of the Advisory Committee on Immunization Practices (ACIP). Accessed on 23 August 2017
Neu AM (2012) Immunizations in children with chronic kidney disease. Pediatr Nephrol 27:1257–1263
Misurac JM, VanDeVoorde RG, Kallash M, Iorember FM, Luckritz KE, Rheault MN, Jetton JG, Turman MA, Kapur G, Twombley KE, Hashmat S, Weaver DJ, Leiser JD, Nailescu C (2017) Immunogenicity of augmented compared with standard dose hepatitis B vaccine in pediatric patients on dialysis: a Midwest pediatric nephrology consortium study. Clin J Am Soc Nephrol 12:772–778
Laube GF, Berger C, Goetschel P, Leumann E, Neuhaus TJ (2002) Immunization in children with chronic renal failure. Pediatr Nephrol 17:638–642
Watkins SL, Alexander SR, Brewer ED, Hesley TM, West DJ, Chan IS, Mendelman P, Bailey SM, Burns JL, Hogg RJ, Southwest Pediatric Nephrology Study G (2002) Response to recombinant hepatitis B vaccine in children and adolescents with chronic renal failure. Am J Kidney Dis 40:365–372
Moal V, Motte A, Vacher-Coponat H, Tamalet C, Berland Y, Colson P (2015) Considerable decrease in antibodies against hepatitis B surface antigen following kidney transplantation. J Clin Virol 68:32–36
Ni YH, Ho MC, JF W, Chen HL, YM W, RH H, Lee PH, Chang MH (2008) Response to booster hepatitis B vaccines in liver-transplanted children primarily vaccinated in infancy. Transplantation 86:1531–1535
Stark K, Gunther M, Neuhaus R, Reinke P, Schroder K, Linnig S, Bienzle U (1999) Immunogenicity and safety of hepatitis a vaccine in liver and renal transplant recipients. J Infect Dis 180:2014–2017
Martin K, Drabble A, Manlhiot C, Dipchand AI (2012) Response to hepatitis a and B vaccination after pediatric heart transplant. Pediatr Transplant 16:699–703
Danziger-Isakov L, Kumar D (2013) Vaccination in solid organ transplantation. Am J Transplant 13 [Suppl 4]:311–317
Schutze GE, Mason EO Jr, Wald ER, Barson WJ, Bradley JS, Tan TQ, Kim KS, Givner LB, Yogev R, Kaplan SL (2001) Pneumococcal infections in children after transplantation. Clin Infect Dis 33:16–21
Tran L, Hebert D, Dipchand A, Fecteau A, Richardson S, Allen U (2005) Invasive pneumococcal disease in pediatric organ transplant recipients: a high-risk population. Pediatr Transplant 9:183–186
Barton M, Wasfy S, Dipchand AI, Hebert D, Ng V, Solomon M, Fecteau A, Stephen D, Allen U (2009) Seven-valent pneumococcal conjugate vaccine in pediatric solid organ transplant recipients: a prospective study of safety and immunogenicity. Pediatr Infect Dis J 28:688–692
Lin PL, Michaels MG, Green M, Mazariegos GV, Webber SA, Lawrence KS, Iurlano K, Greenberg DP (2005) Safety and immunogenicity of the American Academy of Pediatrics-recommended sequential pneumococcal conjugate and polysaccharide vaccine schedule in pediatric solid organ transplant recipients. Pediatrics 116:160–167
Kumar D, Welsh B, Siegal D, Chen MH, Humar A (2007) Immunogenicity of pneumococcal vaccine in renal transplant recipients—three year follow-up of a randomized trial. Am J Transplant 7:633–638
Fishman JA, Ikle DN, Wilkinson RA (2017) Discrepant serological assays for Pneumococcus in renal transplant recipients—a prospective study. Transpl Int 30:689–694
Dziadzio M, Morales G, Harvey J, Smith R, Lukawska J (2017) Comparison of 23-valent pneumococcal IgG ELISA with multiplex 13-valent serotype-specific antibody assay as diagnostic tools in subjects with suspected antibody deficiency. J Mol Immunol 2:2
Campbell AL, Herold BC (2005) Immunization of pediatric solid-organ transplantation candidates: immunizations in transplant candidates. Pediatr Transplant 9:652–661
Enke BU, Bokenkamp A, Offner G, Bartmann P, Brodehl J (1997) Response to diphtheria and tetanus booster vaccination in pediatric renal transplant recipients. Transplantation 64:237–241
Ghio L, Pedrazzi C, Assael BM, Panuccio A, Foti M, Edefonti A (1997) Immunity to diphtheria and tetanus in a young population on a dialysis regimen or with a renal transplant. J Pediatr 130:987–989
Pedrazzi C, Ghio L, Balloni A, Panuccio A, Foti M, Edefonti A, Assael BM (1999) Duration of immunity to diphtheria and tetanus in young kidney transplant patients. Pediatr Transplant 3:109–114
Esposito S, Principi N (2016) Immunization against pertussis in adolescents and adults. Clin Microbiol Infect 22 [Suppl 5]:S89–S95
Prelog M, Almanzar G, Rieber N, Ottensmeier B, Zlamy M, Liese J (2013) Differences of IgG antibody avidity after an acellular pertussis (aP) booster in adolescents after a whole cell (wcP) or aP primary vaccination. Vaccine 31:387–393
Boulianne N, De Serres G, Ratnam S, Ward BJ, Joly JR, Duval B (1995) Measles, mumps, and rubella antibodies in children 5-6 years after immunization: effect of vaccine type and age at vaccination. Vaccine 13:1611–1616
Miller E, Hill A, Morgan-Capner P, Forsey T, Rush M (1995) Antibodies to measles, mumps and rubella in UK children 4 years after vaccination with different MMR vaccines. Vaccine 13:799–802
Flynn JT, Frisch K, Kershaw DB, Sedman AB, Bunchman TE (1999) Response to early measles-mumps-rubella vaccination in infants with chronic renal failure and/or receiving peritoneal dialysis. Adv Perit Dial 15:269–272
Schulman SL, Deforest A, Kaiser BA, Polinsky MS, Baluarte HJ (1992) Response to measles-mumps-rubella vaccine in children on dialysis. Pediatr Nephrol 6:187–189
Barton M, Wasfy S, Melbourne T, Hebert D, Moore D, Robinson J, Marchese RD, Allen UD (2009) Sustainability of humoral responses to varicella vaccine in pediatric transplant recipients following a pretransplantation immunization strategy. Pediatr Transplant 13:1007–1013
Prelog M, Schonlaub J, Jeller V, Almanzar G, Hofner K, Gruber S, Eiwegger T, Wurzner R (2013) Reduced varicella-zoster-virus (VZV)-specific lymphocytes and IgG antibody avidity in solid organ transplant recipients. Vaccine 31:2420–2426
Verolet CM, Posfay-Barbe KM (2015) Live virus vaccines in transplantation: friend or foe? Curr Infect Dis Rep 17:472
Chaves Tdo S, Lopes MH, de Souza VA, Dos Santos SS, Pereira LM, Reis AD, David-Neto E (2005) Seroprevalence of antibodies against varicella-zoster virus and response to the varicella vaccine in pediatric renal transplant patients. Pediatr Transplant 9:192–196
Weinberg A, Horslen SP, Kaufman SS, Jesser R, Devoll-Zabrocki A, Fleckten BL, Kochanowicz S, Seipel KR, Levin MJ (2006) Safety and immunogenicity of varicella-zoster virus vaccine in pediatric liver and intestine transplant recipients. Am J Transplant 6:565–568
Puissant-Lubrano B, Rostaing L, Kamar N, Abbal M, Fort M, Blancher A (2010) Impact of rituximab therapy on response to tetanus toxoid vaccination in kidney-transplant patients. Exp Clin Transplant 8:19–28
Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y et al (1999) The Banff 97 working classification of renal allograft pathology. Kidney Int 55:713–723
Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Baldwin WM 3rd, Bracamonte ER, Broecker V, Cosio F, Demetris AJ, Drachenberg C, Einecke G, Gloor J, Glotz D, Kraus E, Legendre C, Liapis H, Mannon RB, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Rodriguez ER, Seron D, Seshan S, Suthanthiran M, Wasowska BA, Zachary A, Zeevi A (2010) Banff '09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant 10:464–471
Hocker B, Fickenscher H, Delecluse HJ, Bohm S, Kusters U, Schnitzler P, Pohl M, John U, Kemper MJ, Fehrenbach H, Wigger M, Holder M, Schroder M, Billing H, Fichtner A, Feneberg R, Sander A, Kopf-Shakib S, Susal C, Tonshoff B (2013) Epidemiology and morbidity of Epstein-Barr virus infection in pediatric renal transplant recipients: a multicenter, prospective study. Clin Infect Dis 56:84–92
Acknowledgements
Our thanks are due to study nurse Annette Mechler for her continuous excellent contribution to the CERTAIN Registry. BH is an awardee of the “DZIF Clinical Leave Stipend” by the German Center for Infection Research (DZIF).
Funding
We gratefully acknowledge the support of the CERTAIN Registry by a grant from the Dietmar Hopp Stiftung and by grants from the pharmaceutical companies Astellas and Novartis.
Author information
Authors and Affiliations
Contributions
BH and BT took part in the study design, performance of the study, data analysis and writing of the manuscript. MA and PS participated in the performance of the study, data analysis and writing of the manuscript. KK took part in the performance of the study and data analysis. MB, JK, SDM, GG, AB, MJK, HB, MP, LDS, NJAW, SR, AM and AF participated in the performance of the study and writing of the manuscript. TB took part in data analysis and writing of the manuscript. All authors reviewed the manuscript, believe that it represents valid work and approved it for submission.
Corresponding author
Ethics declarations
The CERTAIN Registry has been approved by the ethics committee of each contributing centre and is kept in full accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. Written informed consent to participate in the registry was obtained from all parents or guardians and patients when appropriate for their age.
Conflicts of interest
BH received travel grants from and participated in advisory boards for Astellas, Novartis and Roche. SDM received research grants from Astellas and Novartis. NJAW has participated in advisory boards for Astellas, AbbVie, Alexion and Raptor. BT received research grants, travel grants, lecture fees from and participated in advisory boards for Astellas, Bristol–Myers Squibb, Novartis and Roche. MA, PS, LP, MB, JK, GG, AB, MJK, HB, MP, LDS, SR, KK, AF, TB and AM declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Höcker, B., Aguilar, M., Schnitzler, P. et al. Vaccination titres pre- and post-transplant in paediatric renal transplant recipients and the impact of immunosuppressive therapy. Pediatr Nephrol 33, 897–910 (2018). https://doi.org/10.1007/s00467-017-3868-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-017-3868-0