Abstract
Background
High-dose methotrexate therapy (HDMTX) is a common form of chemotherapy used in children with high-grade malignancy such as osteosarcoma. Treatment with HDMTX requires careful monitoring of drug levels with folinic acid (leucovorin) rescue therapy. Toxicity from methotrexate is not uncommon and sometimes causes significant morbidity and mortality.
Case-diagnosis/treatment
We report an 11-year-old child whose 24-h post-HDMTX serum level was 651.8 μmol/L (recommended level <20 μmol/L), which was complicated by septic shock and progressive renal and liver failure. As carboxypeptidase (glucarpidase) was not available locally, she was treated with the sequential use of charcoal hemoperfusion (CHP) and single-pass albumin dialysis (SPAD). The patient recovered without complications. Both liver and renal function recovered with no significant late sequelae.
Conclusion
CHP and SPAD are effective extracorporeal methods of removing methotrexate. They provide alternative treatment options for critical care nephrologists in the management of methotrexate toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High-dose methotrexate therapy (HDMTX) is a common form of chemotherapy used in children with high-grade malignancy such as osteosarcoma. Treatment with HDMTX requires careful monitoring of drug levels with folinic acid (leucovorin) rescue therapy. Toxicity from methotrexate is not uncommon and sometimes causes significant morbidity and mortality.
Case report
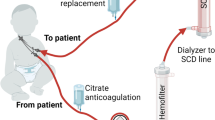
An 11-year-old girl (body weight 38 kg) with left hip osteosarcoma was treated with HDMTX at 12 g/m2 according to the Hong Kong Paediatric Haematology Oncology Study Group Chemotherapy protocol (HKPSOSG Osteosarcoma 2009). She was pre-hydrated with fluid at 3 L/m2/h. Urine alkalinization was achieved with intravenous sodium bicarbonate infusion at 40 mmol/L to keep urinary pH >7. She received intravenous calcium folinate (leucovorin) bolus for rescue therapy as according to the protocol. Five hours after commencement of HDMTX, she developed septic shock, with blood pressure dropping to 76/33 mmHg and a pulse rate of 100 bpm. She was stabilized with several normal saline boluses. Intravenous vancomycin, piperacillin/tazobactam (Tazocin®), and amikacin were empirically commenced after sepsis workup. Her serum MTX level 5 h post-infusion was 1,121 μmol/L. She was treated using leucovorin infusion, but serum MTX remained at a high level of 651.8 μmol/L at 24 h post-therapy: the recommended serum methotrexate level at 24 h post-HDMTX is <20 μmol/L. Her antibiotics were replaced by meropenem and the dose was adjusted to renal function. Her serum creatinine rose from a baseline of 41 μmol/L to 289 μmol/L at 32 h post-HDMTX infusion, her estimated glomerular filtration rate (eGFR) dropped from 130 to 18.6 mL/min/1.73 m2 and she became oliguric. Her serum alanine transferase increased to 5,877 IU/L (normal: <20 IU/L) and aspartate aminotransferase rose to 5,853 IU/L (normal <20 IU/L). Leucovorin infusion was increased from 2.3 g/day to 4 g/day without a significant drop in serum MTX level or improvement in liver and renal function. As carboxypeptidase was not readily available locally, she was treated with single-pass albumin dialysis (SPAD) with albumin dialysate at 44 g/L using the usual continuous renal replacement therapy (CRRT) machine (Gambro Prismaflex™) and ST100 hemofilter (AN69, 0.9 m2). This albumin dialysate was prepared by removing 1,100 mL of Hemosol BO solution from its usual 5-L bag and replacing it with 1,100 mL of 20 % albumin (220 g). This dialysate has a final albumin concentration of 44 g/L (4.4 % albumin dialysate). The blood flow rate was set at 100–130 mL/min, the replacement fluid flow rate at 1,000 mL/h, and dialysate flow at 1,500 mL/h. Two sessions (4 h/session) of charcoal hemoperfusion using the Adsorba Cartridge 300C (Prismaflex Adsorba Kit™) were added to SPAD. We tried to stop SPAD after 51 h of dialysis and hemoperfusion (HP) treatment; however, the serum MTX level rebounded from 5.23 μmol/L to 6.89 μmol/L eighteen hours later and serum creatinine increased from a nidus of 88 μmol/L to 262 μmol/L. SPAD was restarted 20 h after it was stopped and continued for another 46 h. MTX clearance was measured by calculating:
Figure 1a shows the drop in serum MTX level with different treatment options. The sequential use of CHP and SPAD was shown to lower MTX faster than leucovorin alone (Fig. 1b). The MTX clearance was up to 70 mL/kg/h at the commencement of therapy (Fig. 1c). Urine MTX clearance increased dramatically 140 h later as renal function and urine output improved. Serum creatinine normalized on day 14 of treatment. Her serum creatinine was 48 μmol/L with eGFR of 112 mL/min/1.73 m2 upon discharge from the intensive care unit. No major complications, such as severe marrow failure or mucositis, were detected, despite exposure to such toxic levels of MTX. After this episode, HDMTX was replaced by ifosfamide and etoposide in the chemotherapy protocol.
a This diaphragm showed the drop in methotrexate (MTX) over time. The timing of different treatment methods was shown. The blue arrow line represents the timing and duration of leucovorin treatment. The green arrow line represents the timing of single-pass albumin dialysis (SPAD) and the purple arrow line represents the timing of the two sessions of charcoal hemoperfusion (CHP). The red circles showed the drop in serum MTX after each session of CHP, i.e., the curve in the red circles showed a steeper slope, indicating a more rapid drop in serum MTX. b We use the trend line method and assume a first-order kinetic for MTX elimination to predict the drop in the MTX level with different methods of treatment. The broken line with purple dots represents the predicted clearance of MTX by leucovorin alone. The broken line with orange dots represents the predicted clearance of MTX by leucovorin and SPAD. The broken line with yellow dots represents the predicted clearance of MTX by leucovorin with SPAD and CHP. The combination of all three modalities of treatment achieves the highest elimination rate of MTX. c The graph shows the changes in serum creatinine with MTX clearance by SPAD and urine. Dialysis MTX clearance is best when serum MTX is highest and during the period of impaired renal function. Urine MTX clearance improved as renal function recovered from MTX toxicity and urine output increased
Discussion
High-dose methotrexate therapy ranging from 0.5 g/m2 to 12 g/m2 has been used in highly aggressive cancer therapy in children. Folinic acid (leucovorin) is the rescue drug that has been commonly used in most regimens to reduce MTX toxicity and has been shown to improve survival significantly. Although these regimens have been reported to be well tolerated, unpredictable life-threatening MTX toxicities unamendable by folinic acid are still occasionally encountered [1]. Rapid removal of MTX by extracorporeal dialysis therapy would be life-saving in these circumstances.
The choice of extracorporeal method for removing a toxic substance depends on its molecular size, charge, volume of distribution, and binding characteristics. Low-molecular-weight, water-soluble substances are readily removed by hemodialysis (HD), whereas larger size molecules require high-flux or high-efficacy filters with larger pores to remove them. The size of the molecule, however, seems not to be a major concern in HP, as it is an adsorptive treatment. Toxic substances that are highly protein- or lipid-bound are less readily removed by HD or hemofiltration as lower free levels are available in the circulating plasma for removal. However, in situations of acute toxicity, at least in the early phase, there is a larger proportion of free drug unbound in the serum available for removal by most extracorporeal techniques. The volume of distribution (Vd) of a substance represents the dispersion of a substance in various body compartments. Substances with Vd exceeding 1 L/kg are considered to have a large volume of distribution and exhibit multi-compartmental kinetics. A continuous method of toxic substance removal would be desirable and a continuous therapy would be a better option for addressing the issue of rebound phenomenon [2, 3].
Methotrexate has a molecular size of 454 Da and a volume of distribution of 0.18 L/kg in acute use and 0.4–0.8 L/kg in chronic use [3]. It is highly protein bound, with 50 % plasma protein binding at therapeutic plasma concentrations; hence, conventional HD with a low flux filter would not be a good treatment option. To enhance MTX removal, various dialysis modalities and combinations have been reported, with conflicting results [4, 5]. Widemann and Adamson [5] reported on their 49 patients with HDMTX-induced nephrotoxicity. Conventional HD, hemodiafiltration, high-flux HD, CHP, peritoneal dialysis, and combinations of therapies were used with variable effectiveness. Apart from the influence of physicochemical and pharmacokinetic properties of the toxic substance, the availability of extracorporeal techniques in the local setting also affects the choice of treatment.
Hemoperfusion using charcoal is an adsorptive treatment. Early use of HP was focused on the clearance of toxin or poisons for which antidotes were not available. With the advancement in HD techniques using high-flux and high-efficiency dialysis filters, the role of HP in nephrology has been declining. HP has several disadvantages: it is costly, the charcoal cartridge is saturated in 3–4 h and requires changing, and it does not normalize electrolyte disturbances or manage fluid balance. For earlier HP cartridges, the blood came into direct contact with the charcoal, frequently causing hypersensitivity reactions and charcoal embolization. These complications have been overcome by newer HP cartridges that are coated in thin porous membranes, such as cellulose acetate in the CHP cartridge we have used. Other serious complications such as thrombocytopenia, leukocytopenia, hypocalcemia, hypothermia, and reduction of fibrinogen and hypoglycemia have been reported [6, 7]. However, these complications were not observed in our patient. The risk of hypoglycemia in CHP has also been minimized by priming the cartridge with dextrose solution, as in our patient. Despite having these possible complications, CHF has several advantages in terms of removal of toxic substances. Toxin clearance is not affected by its molecular size. HP can clear both water-soluble and lipophilic toxins. It may be more effective in removing highly protein-bound toxins as it competes with plasma protein to bind the toxins. These advantages render it an important extracorporeal removal technique and its use is still prevalent in some parts of the world such as Asia, where pesticide poisoning represents a significant reason for dialysis [7]. We have chosen CHP as one of our extracorporeal circuits for removing MTX. To overcome its short therapy duration, the need to correct azotemia and the fluid and electrolyte balance in our patient, and to deal with the rebound phenomenon, we augmented our dialysis regimen by adding a continuous therapy, SPAD, as the backbone for extracorporeal renal support and to enhance the clearance of MTX.
With albumin dialysis, albumin on the other side of the membrane binds to free sites on toxins, which are then filtered from the blood. In general, we can remove any toxin, just by selecting the correct “lock-protein” to use [8, 9]. SPAD is set up by a simple modification to the usual CRRT circuit. Albumin is added to the dialysate, but it does not pass through the recycle process as in the molecular absorbent recirculating system (MARS) for toxin removal in liver dialysis. SPAD not only provides a continuous therapy, but adds extra strength to toxin removal by using albumin as the dialysate [9]. Askenazi et al. [10] first reported using continuous venovenous hemodialysis (CVVHD) to treat carbamazepine intoxication in a 30-kg patient. The authors used CVVHD with a 4.5 % albumin dialysate with an AN69 M60 dialyzer, and serum carbamazepine declined substantially. The concentration of albumin dialysate and hemofilter used were very similar to those in our patient. However, this was only a case report and carbamazepine has different pharmacokinetic properties from MTX. SPAD is theoretically a feasible alternative for toxic drug removal; however, more questions have to be answered before we can comment further on its effectiveness. What is the optimal concentration of albumin dialysate? Does the concentration of albumin dialysate affect the clearance, or is it the dose of CRRT itself that affects the outcome?
Churchwell et al. [11] compared the clearance of valproic acid, carbamazepine and phenytoin by using a control dialysate, 2.5 % albumin, and 5 % albumin dialysate with different blood flow and dialysate flow rates in a continuous HD model using 1 L of bovine blood. Polysulfone or an AN69 hemodialyzer was used. They showed that carbamazepine and valproic acid clearance can be enhanced by albumin dialysate, but not phenytoin. The 5 % albumin dialysate apparently further increased the extraction coefficient of carbamazepine and valproic acid. The change in dialytic clearance was not affected by the change in blood flow rate or dialysate flow rate. However, these anticonvulsants have different pharmacokinetic profiles from MTX: they have smaller molecular weight, are highly protein-bound, and have a smaller volume of distribution. Vilay et al. [12] reported their experience in treating a 13-year-old girl with MTX toxicity (serum MTX level 446 μmol/L). Several extracorporeal therapies were used sequentially in their patient and they showed that greater MTX elimination may be achieved with standard dialysate run at a higher effluent flow rate, as observed during the CVVHDF treatment period. SPAD did not demonstrate an appreciable increase in MTX clearance compared with albumin-free dialysate. However, different modalities were used at different times after MTX intoxication. The timing from the onset of intoxication affects the amount of free drug in serum and the degree of compartmentation, both of which change the pharmacokinetics of the drug and affect the effectiveness of clearance by different methods. Furthermore, their patient was non-oliguric, and renal excretion is still the most effective method of MTX elimination, as shown in our patient.
There are more questions to be answered before concluding the effectiveness of SPAD. Preparing the albumin dialysate is labor-intensive and the cost cannot be underestimated. To prepare a 4.4 % albumin dialysate and run it at 1,500 mL/h for 24 h costs around US$ 5,000/day, which is 30 times more expensive than using Hemosol® alone. The choice of modality of toxin elimination technique depends on the local situation, including the availability of facilities, machines, and expert support. If high-flux/high-efficiency CRRT is a readily available technique, clinicians should proceed with drug elimination with no delay. Both HP and SPAD provide alternative options for clinicians, and these techniques widen the spectrum of dialytic clearance of highly protein-bound drugs for the critical care nephrologist. Lastly, we would like to comment that if carboxypeptidase G2 (glucarpidase) were a readily available drug, it might be a safer option for treatment of MTX toxicity. It is unfortunate that the drug is not readily available in most countries and we have no experience in using it.
Conclusion
Sequential and combination therapy of CHP with SPAD is a practical and safe procedure for treating MTX toxicity. It may be an option for the removal of highly protein-bound drugs/toxins that are traditionally regarded as “non-dialyzable.” These treatment modalities widen the scope of blood purification techniques for the critical care nephrologist.
References
Picci P, Mercuri M, Ferrari S, Alberghini M, Briccoli A, Ferrai C (2010) Survival in high-grade osteosarcoma: improvement over 21 years at a single institution. Ann Oncol 21:1366–1373
Bayliss G (2010) Dialysis in the poisoned patient. Hemodial Int 14:158–167
Buchmann TE, Ferris ME (2011) Management of toxic ingestions with the use of renal replacement therapy. Pediatr Nephrol 26:535–541
Saland J, Leavey P, Bash R, Hansch E, Arbus G, Quingley R (2002) Effective removal of methotrexate by high flux hemodialysis. Pediatr Nephrol 112:825–829
Widemann BC, Adamson PC (2006) Understanding and managing methotrexate nephrotoxicity. Oncologist 11:694–703
Ghannoum M, Bouchard J, Nolin TD, Quellet G, Roberts DM (2014) Hemoperfusion for the treatment of poisoning: technology, determinants of poison clearance, and application in clinical practice. Semin Dial 27:350–361
Gil HW, Kim SJ, Yang JO, Lee EY, Hong SY (2010) Clinical outcome of hemoperfusion in poisoned patients. Blood Purif 30:84–88
Karvellas CJ, Gibney N, Kutsogiannis D, Wendon J, Bain VG (2007) Bench-to-bedside review: current evidence for extracorporeal albumin dialysis systems in liver failure. Crit Care 11:215
Sauer IM, Goetz M, Steffen I, Walter G, Kehr DC, Schwartlander R, Hwang YJ, Pascher A, Gerlach JC, Neuhaus P (2004) In vitro comparison of the molecular adsorbent recirculation system (MARS) and single‐pass albumin dialysis (SPAD). Hepatology 39:1408–1414
Askenazi DJ, Goldstein SL, Chang IF, Elbenberg E, Feig DI (2004) Management of a severe carbamazepine overdose using albumin enhanced continuous venovenous hemodialysis. Pediatrics 113:406–409
Churchwell MD, Pasko DA, Smoyer WE, Mueller BA (2009) Enhanced clearance of highly protein-bound drugs by albumin-supplemented dialysate during modeled continuous hemodialysis. Nephrol Dial Transplant 24:231–238
Vilay AM, Mueller BA, Haines H, Atlen JA, Askenazi DJ (2010) Treatment of methotrexate intoxication with various modalities of continuous extracorporeal therapy and glucarpidase. Pharmacotherapy 30:111
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Financial disclosure
None.
Rights and permissions
About this article
Cite this article
Chan, W.K.Y., Hui, W.F. Sequential use of hemoperfusion and single-pass albumin dialysis can safely reverse methotrexate nephrotoxicity. Pediatr Nephrol 31, 1699–1703 (2016). https://doi.org/10.1007/s00467-016-3389-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-016-3389-2