Abstract
Beta-trace protein (BTP) is a novel marker of glomerular filtration rate (GFR). To date, no pediatric formula for calculating GFR based on BTP has been developed. We measured GFR, serum creatinine and BTP in 387 children who underwent 474 99mTc-diethylene triamine pentaacetic acid renal scans. A BTP-based formula for estimating GFR was derived using stepwise linear regression analysis. A separate control group of 116 measurements in 99 children was used to validate the novel formula. A formula was also developed for each gender. The novel formula is: \( {\text{GFR}} = {\text{1}}{0^\wedge }\left({\text{1}}.{\text{9}}0{\text{2}} + \left( {0.{\text{9515 x LOG}}\left( {{\text{1}}/{\text{BTP}}} \right)} \right) \right) \). The Spearman rank correlation coefficient between the BTP-derived GFR estimate and the measured GFR was 0.80 [95% confidence interval (CI) 0.76–0.83], which is substantially better than that derived with the Schwartz formula (r = 0.70, 95% CI 0.65–0.74). The Bland–Altman analysis revealed a mean bias of 1.21% [standard deviation (SD) 28%] in the formula development dataset, which was virtually identical to the 1.03% mean bias (29.5% SD) in the validation group and no different from the Schwartz formula bias. The percentage of values within 10% (33.0 vs. 28.3%) and 30% deviation (76.8 vs. 72.6%) were better for BTP-based formula than for the Schwartz formula. Separate formulas according to gender did not perform better than that for the pediatric population. This BTP-based formula was found to estimate GFR with reasonable precision and provided improved accuracy over the Schwartz GFR formula.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inulin clearance forms the gold standard for measuring glomerular filtration rate (GFR). However, due to the lack of availability of inulin, this practice has been replaced by nuclear medicine GFR scans [1]. Although accurate, the latter are expensive and cumbersome, involve radiation exposure, and can only be performed infrequently. Consequently, for frequent monitoring, surrogate markers of GFR have to be utilized [2]. The most commonly used marker of GFR is serum creatinine, although more recently cystatin C (CysC) has been gaining popularity. In children, serum creatinine level varies with muscle mass. To account for this variability, creatinine-based GFR estimates are usually calculated using height/creatinine ratios, as muscle mass correlates very closely with height. The most commonly used formula, the Schwartz formula [3], is hampered by several limitations, including the non-renal elimination of creatinine and the substantial over-estimation of GFR in patients with advanced renal failure [4]. The Schwartz formula also becomes inaccurate in patients with altered muscle mass, such as children with spina bifida [5].
Beta-trace protein (BTP), also known as prostaglandin D synthase, is a 23- to 29-kDa enzyme that has been traditionally used as a marker of cerebrospinal fluid leakage [6, 7]. It is expressed in all tissues except the ovaries [8], and its biological actions include vasodilatation, bronchoconstriction, inhibition of platelet aggregation, and recruitment of inflammatory cells. Beta-trace protein has recently been presented as a promising surrogate marker for the GFR measurement [9, 10]. As BTP is a small molecular weight protein that is freely filtered by the glomerulus, its serum concentration depends on the GFR; consequently, it reflects the GFR status. Preliminary studies have confirmed a good correlation between serum BTP levels and the GFR measurement based on inulin clearance and nuclear medicine methods [10, 11].
As a tool for GFR measurement, BTP has been found to have a few distinct advantages. It has been reported that serum BTP levels do not have a significant relationship with C-reactive protein [12] and that they are unaffected by body composition [11, 13]. During the third trimester of pregnancy, BTP, but not CysC, has been shown to adequately reflect the GFR [14]. Unlike CysC, thyroid function [15] has not been reported to affect the concentration of BTP. Another possible advantage would be the lack of effect of corticosteroid administration on BTP concentrations. However, there is conflicting evidence on this property [16, 17].
In clinical practice, the applicability of a surrogate marker needs an appropriate formula by which to calculate the estimated GFR (eGFR) from a corresponding serum concentration. At the time this manuscript was being prepared, a formula for the estimation of GFR based on serum BTP had been established only in adults [17]. Here, we report on the development and the validation of a novel formula for the estimation of GFR in children based on BTP. We hypothesized that BTP-based GFR estimates would determine the GFR more accurately than serum creatinine measurements or the Schwartz formula.
Subjects and methods
Patients and methods
After obtaining approval from the Institutional Review board, we analyzed 474 nuclear GFR scans in 387 pediatric patients (172 girls, 44.4%; mean age 10.7 ± 7.1 years) with various renal pathologies who had been referred for a nuclear renal scan between July 1999 and September 2002. The data were derived in association with a previously published study [11], which continued to enroll patients after the initial publication. We included patients from the Children′s Hospital of Eastern Ontario who underwent a 99m technetium diethylene triamine pentaacetic acid (99mTc DTPA) GFR scan, with a three-point sampling approach at 2, 3, and 4 h post-injection according to Russell [18]. To ensure a homogenous patient selection in our analysis, we omitted the patients enrolled from Berlin in the previous study. In total, 298 patients were included in the study protocol, with 387 patients available for the generation of the data set. Body surface area was calculated using the Haycock formula [19], and GFR was normalized to a body surface area (BSA) of 1.73 m2. This set of measurements will be referred to hereafter as the formula generation dataset.
Upon completion of the initial study, BTP became available for routine clinical practice. To validate the formula derived from the generation dataset, one of the authors (RN) retrieved a new dataset based on simultaneously determined serum BTP levels and 99mTc DTPA renal scans. From the initial list of 264 BTP measurements, we compiled a new validation dataset based on 125 BTP measurements in 103 patients with concomitant nuclear GFR and serum creatinine estimations. The validation group had a similar gender and age distribution as the formula generation dataset (40 females, 38.8%, not significantly different from the formula generation group, p = 0.28, Fisher′s exact test; mean gold standard GFR 95.6 ± 44.6, not significantly different from the formula generation group, p = 0.052; mean age 10.3 ± 5.1 years, not significantly different from the original group, p = 0.94, unpaired t test), and the validation of the formulas was performed between December 2002 and July 2006.
The methods for the determination of BTP (Dade Behring, Milton Keynes, UK) and creatinine (Ortho Clinical Diagnostics, Tilburg, the Netherlands) have been described in our previous study [11]. The creatinine-GFR/BSA value was calculated with the Schwartz formula [3]:
The constants used for the Schwartz formula were 49.9 for adolescent boys and 46.2 for all other children [20].
To address the question of whether separate formulas are required for boys and girls, we also developed separate formulas for both genders and compared the agreement between GFR and estimated GFR (eGFR) using gender-specific formulas, with the agreement for the formula derived for the group as a whole.
Statistics
Wherever possible, simple descriptive statistics were used. Contiguous data were tested for normal distribution using the Shapiro–Wilks normality test. Normally distributed data were analyzed using parametric methods (mean, standard deviation, t test, Pearson correlation); in all other cases, non-parametric methods were used (median, range, Wilcoxon′s matched pairs test and Spearman rank correlation). For the development of the BTP formula, we used a similar multiple stepwise linear regression analysis as in our previous study after log–log transformation [21]. For the evaluation of the newly derived formula for the estimation of GFR based on BTP, we used Bland–Altman analysis [22]. All statistical analyses were performed using the commercially available software GraphPad Prism software, ver. 4.02 (GraphPad, San Diego, CA).
Results
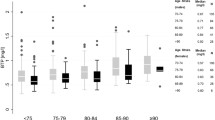
Testing by the Shapiro–Wilk test revealed that all of the parameters under study were not normally distributed. Following log transformation of the data, however, all log-transformed variables became normally distributed. The median age of patients was 11.01 years (range 0.21–18.9), median height was 137.0 cm (range 55.00–192.6), median weight was 34.70 kg (range 5.1–116.2), median GFR was 105.5 mL/min/1.73 m2 (range 7.0–414.0), median creatinine level was 61 μmol/L (range 23–530), and median BTP level was 0.775 mg/L (range 0.24–5.56).
The Spearman rank correlation coefficient between measured GFR and 1/BTP was 0.80 with a 95% confidence interval (CI) of 0.76–0.83, which was significant at p < 0.0001 (Fig. 1). The correlation coefficient for BTP was superior to that between measured GFR and the Schwartz formula (correlation coefficient 0.70, 95% CI 0.65–0.74).
Relationship between measured glomerular filtration rate (GFR) and GFR based on the novel beta-trace protein (BTP) formula \( {\text{GFR}} = {\text{1}}{0^\wedge }\left( {{\text{1}}.{\text{9}}0{\text{2}} + \left( {0.{\text{9515 x LOG}}\left( {{\text{1}}/{\text{BTP}}} \right)} \right)} \right) \) in 474 GFR measurements from the formula generation dataset used to derive the formula. The regression correlation coefficient was 0.7994 with a 95% confidence interval of 0.7632 to 0.8306, which was significant (p < 0.0001)
To generate the formula for the estimation of GFR based on BTP, we used a previously proposed modeling method [21] with log–log transformation of both parameters in the formula generation dataset:
(Fig. 1).
The Bland–Altman analysis, which was then applied to compare the nuclear GFR and the BTP-based eGFR from the generating dataset, revealed a mean bias of 1.21%, a standard deviation (SD) of 27.97%, and a 95% CI of −53.61 to 56.01% (Fig. 2). Using the same approach, the formula was then validated in the validation dataset, revealing a mean bias of 1.03%, which is almost identical to that of the generating set, with a SD of 29.51% and a 95% CI of −56.81 to 58.87%. Bland–Altman analysis for the comparison between nuclear GFR and Schwartz GFR in the generating set further revealed a bias of −0.97%, a SD of 35.11% and a 95% CI of −69.79 to 67.85%. The Bland–Altman results using the validating data set revealed a bias of −7.17%, a SD of 27.85% and a 95% CI of −61.75 to 47.42%. The results for each separate dataset are given in Table 1. We also compared the percentage error for BTP and for the Schwartz GFR. There were consistently more measurements within 10% (33.0 vs. 28.3%) and 30% (76.8 vs. 72.6%) using the BTP-based eGFR than using the Schwartz GFR. The results of the percentage error in the formula generation dataset and the validation dataset are given in Table 2.
The Bland–Altman analysis to test agreement between the newly derived formula for calculating GFR from the serum BTP concentration using \( {\text{GFR}} = {10^ \wedge }\left( {1.902 + \left( {0.9515{\text{xLOG}}\left( {1/{\text{BTP}}} \right)} \right)} \right) \). Differences were plotted as percentages of the average. The mean difference was 1.21% with a standard deviation (SD) of 27.97%. The 95% confidence interval (CI) was –53.6 to 56.0% and is indicated by the dashed lines on the graph. The slope of the regression line was not significantly non-zero. This analysis was performed on the formula generation dataset (n = 474)
Thereafter, we analyzed the formula generation dataset separately for males (n = 258) and females (n = 216). The two new gender-specific datasets did not differ significantly for age, height, weight, BSA, creatinine, Schwartz GFR, GFR, BTP, and 1/BTP. The formula for boys reads:
Similarly, the formula for girls reads:
The Bland–Altman analysis for agreement between the specific male formula and the formula derived for both genders revealed a slight underestimation of the bias at 4.46% with a SD of 1.50% and a 95% CI 1.50–7.38%. Conversely, the Bland–Altman analysis between the specific female formula and the formula derived for both genders shows a slightly overestimating bias of −0.04% with a standard deviation of 1.61% and a 95% CI 3.20–3.11%. We also compared the percentage error using both the formula for both genders as well as the gender-specific formulas. The differences were not significant (Table 3).
Discussion
The main objective of our study was to develop a formula for estimating GFR based on serum BTP levels. Utilizing the GFR measurements obtained with 99mTc DTPA renal scans and simultaneous serum BTP levels, we were able to derive a novel formula after logarithmic transformation of the measured GFR and the reciprocal of the serum BTP level.
The GFR based on the nuclear renal scan showed a better agreement with the BTP-estimated GFR than with the creatinine-based GFR, based on calculations with the Schwartz formula. The agreement was acceptable, with a mean bias of −1.4% (SD 28%) for the entire group. In addition, validation in a reasonably sized control group generated almost identical results.
In terms of the validation, it has recently been suggested that a reasonably sized control group should comprise at least 40 measurements [23], as a smaller control group can introduce a bias. By these standards, our observations from a sample size of 125 measurements in 103 patients improved the validity of our observation.
For the clinical applicability of a formula, the degree of agreement that should be considered acceptable remains an important question. The literature provided us with the guidelines. For serum creatinine, an agreement of ±0.3 mg/dL or 26.5 µmol/L is considered to be acceptable; for other markers, an acceptable variation is 25–30% [23]. The agreement of 28% with our BTP-estimated GFR formula met these criteria. Of note, the BTP-based formula also performed better than the Schwartz formula. Therefore, our results provide evidence that BTP is a better surrogate marker for GFR estimation than serum creatinine, even when the old Schwartz formula is used.
However, our derived BTP formula may not perform as well as a recently derived formula that uses urea, creatinine and CysC measurements [24]. With this so-called “CKiD” method, 87.7% of the eGFR was within 30% of the isotopic (i)GFR, and 45.6% was within 10%. These results are superior to those found using the BTP-based formula derived here. Thus, while this newly derived BTP formula performs better than the creatinine-based Schwartz formula, it may be inferior to formulas based on both creatinine and CysC. As urea was only collected in a subset of the patients who participated in our study, we were unable to compare the diagnostic performance of the modified Schwartz formula in our own data. There are some concerns with CysC, especially in pregnancy [14] while the BTP-based formula was accurate in predicting eGFR in pregnancy. The verdict on whether or not CysC is affected by inflammation remains to be made. BTP is independent of inflammation [12]. As such, there may be a clinical role for a BTP-based eGFR. Furthermore, the performance of the CKiD formula has not yet been tested under conditions that affect serum creatinine levels, such as spina bifida and muscle disorders, while it is known that serum BTP levels are not affected under such clinical conditions.
A BTP-based estimation is more expensive than serum creatinine testing. At this point in time, a single BTP estimation costs CDN$22 and therefore compares unfavorably with the creatinine-based estimation of CDN$4.00, although it is comparable to the costs of a CysC-based estimation. However, it is expected that with more frequent utilization, the costs of the BTP tests will decrease.
Of interest, the estimated BTP-GFR showed a better correlation with the measured GFR in our male pediatric cohort than in our female one. This observation matches the data on CysC, where a better correlation between measured GFR and estimated CysC-GFR was observed in males [25]. Creatinine-based formulas, such as the modification of diet in renal disease (MDRD), modified MDRD, and Cockcroft–Gault formula, in adults also perform better in males than in females [26–28]. The need of a different formula in girls is not unexpected considering their higher fat mass across all ages [29, 30]. However, we found an acceptable agreement between the eGFR based on the formula for both genders and each of the gender-specific formulas. As such, we feel that a differentiation for gender is not necessary in young children and adolescents. This is in contrast to the BTP eGFR formula that we recently published for adult patients where the results have to be multiplied by 0.88 for females [17]. However, Pöge et al. recently published an adult BTP eGFR formula that was also independent of gender [31].
Importantly, the Schwartz-derived GFR accounted for 70% of the variability of the GFR, whereas BTP alone accounted for 80% of the variability of the GFR. This was slightly better than the 75.6% variability that was reported for BTP alone in adult patients [17]. The data of Pöge et al. [31] and from our study are in contrast to the data of Solichova et al. who, based on their measurement of BTP in 25 volunteers, concluded that BTP was not a useful tool for estimating GFR [32]. This difference is likely explained by the much larger number of subjects in our study.
An adult formula was very recently evaluated in children [33] in a study involving a mixed cohort of adult and pediatric patients. The BTP formula performed well in the relatively small cohort of 54 pediatric patients. We applied this formula to our dataset but found its performance somewhat inferior to our specific pediatric formula (data not included in the Results). While 95% of the BTP-based estimated GFR were within a 50% error interval and 68% were within a 30% error window, only 18.3% were within a 10% error window. It is therefore not surprising that a specifically developed pediatric formula would perform better in the pediatric setting.
There are some limitations to this study. It is important to note that we did not control for corticosteroid use. A recent study suggests that high levels of corticosteroids may influence BTP concentrations [16]. In contrast, other studies have not demonstrated any effect of high-dose glucocorticoid therapy on BTP concentrations [9, 16].
In summary, the BTP formula proposed here performed significantly better than the Schwartz formula. We conclude that this BTP formula, which has been validated, can be used to predict the true GFR with a higher accuracy than the Schwartz formula. Until more studies test the performance of BTP-based estimated GFR, BTP could have a role in the clinical conditions that affect serum CysC levels.
References
Sambataro M, Thomaseth K, Pacini G, Robaudo C, Carraro A, Bruseghin M, Brocco E, Abaterusso C, DeFerrari G, Fioretto P, Maioli M, Tonolo GC, Crepaldi G, Nosadini R (1996) Plasma clearance rate of 51Cr-EDTA provides a precise and convenient technique for measurement of glomerular filtration rate in diabetic humans. J Am Soc Nephrol 7:118–127
Filler G (2006) How to measure renal function in children—what is the role of cystatin C? Curr Pediatr Rev 2:225–231
Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A (1976) A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58:259–263
Seikaly MG, Browne R, Bajaj G, Arant BS Jr (1996) Limitations to body length/serum creatinine ratio as an estimate of glomerular filtration in children. Pediatr Nephrol 10:709–711
Pham-Huy A, Leonard M, Lepage N, Halton J, Filler G (2003) Measuring glomerular filtration rate with cystatin C and beta-trace protein in children with spina bifida. J Urol 169:2312–2315
Clausen J (1961) Proteins in normal cerebrospinal fluid not found in serum. Proc Soc Exp Biol Med 107:170–172
Hoffmann A, Nimtz M, Conradt HS (1997) Molecular characterization of β-trace protein in human serum and urine: a potential diagnostic marker for renal diseases. Glycobiology 7:499–506
Olsson JE, Sandberg M (1975) Demonstration of synthesis of beta-trace protein in different tissues of squirrel monkey. Neurobiology 5:270–276
Pöge U, Gerhardt TM, Stoffel-Wagner B, Palmedo H, Klehr HU, Sauerbruch T, Woitas PR (2005) β-Trace protein is an alternative marker for glomerular filtration rate in renal transplantation patients. Clin Chem 51:1531–1533
Priem F, Althaus H, Birnbaum M, Sinha P, Conradt HS, Jung K (1999) Beta-trace protein in serum: a new marker of glomerular filtration rate in the creatinine-blind range. Clin Chem 45:567–568
Filler G, Priem F, Lepage N, Sinha P, Vollmer I, Clark H, Keely E, Matzinger M, Akbari A, Althaus H, Jung K (2002) Beta-Trace protein, cystatin C, β2-microglobulin, and creatinine compared for detecting impaired glomerular filtration rates in children. Clin Chem 48:729–736
Kanaoka Y, Urade Y (2003) Hematopoietic prostaglandin D synthase. Prostaglandins Leukot Essent Fatty Acids 69:163–167
Huber AR, Risch L (2005) Recent developments in the evaluation of glomerular filtration rate: is there a place for beta-trace? Clin Chem 51:1329–1330
Akbari A, Lepage N, Keely E, Clark HD, Jaffey J, MacKinnon M, Filler G (2005) Cystatin-C and beta trace protein as markers of renal function in pregnancy. Br J Obstet Gynaecol 112:575–578
Manetti L, Pardini E, Genovesi M, Campomori A, Grasso L, Morselli LL, Lupi I, Pellegrini G, Bartalena L, Bogazzi F, Martino E (2005) Thyroid function differently affects serum cystatin C and creatinine concentrations. J Endocrinol Invest 28:346–349
Abbink FC, Laarman CA, Braam KI, van Wijk JA, Kors WA, Bouman AA, Spreeuwenberg MD, Stoffel-Wagner B, Bökenkamp A (2008) Beta-trace protein is not superior to cystatin C for the estimation of GFR in patients receiving corticosteroids. Clin Biochem 41:299–305
White CA, Akbari A, Doucette S, Fergusson D, Hussain N, Dinh L, Filler G, Lepage N, Knoll GA (2007) A novel equation to estimate glomerular filtration rate using beta-trace protein. Clin Chem 53:1965–1968
Russell CD (1993) Optimum sample times for single-injection, multi-sample renal clearance methods. J Nucl Med 34:1761–1765
Haycock GB, Schwartz GJ, Wisotsky DH (1978) Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr 93:62–66
Filler G, Foster J, Acker A, Lepage N, Akbari A, Ehrich JH (2005) The Cockcroft-Gault formula should not be used in children. Kidney Int 67:2321–2324
Filler G, Lepage N (2003) Should the Schwartz formula for estimation of GFR be replaced by cystatin C formula? Pediatr Nephrol 18:981–985
Bland JM, Altman DG (1999) Measuring agreement in method comparison studies. Stat Methods Med Res 8:135–160
Westgard JO, Ehrmeyer SS, Darcy TP (2004) CLIA final rules for quality systems. Westgard QC, Madison
Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Sharma AP, Kathiravelu A, Nadarajah R, Yasin A, Filler G (2009) Body mass does not have a clinically relevant effect on cystatin C eGFR in children. Nephrol Dial Transplant 24:470–474
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 130:461–470
Levey AS, Greene T, Kusek J, Beck GJ (2000) A simplified equation to predict glomerular filtration rate from serum creatinine (abstract). J Am Soc Nephrol 11:A0828
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
van der Sluis IM, de Ridder MA, Boot AM, Krenning EP, de Muinck Keizer-Schrama SM (2002) Reference data for bone density and body composition measured with dual energy x ray absorptiometry in white children and young adults. Arch Dis Child 87:341–347
Boot AM, Bouquet J, de Ridder MA, Krenning EP, de Muinck Keizer-Schrama SM (1997) Determinants of body composition measured by dual-energy X-ray absorptiometry in Dutch children and adolescents. Am J Clin Nutr 66:232–238
Pöge U, Gerhardt T, Stoffel-Wagner B, Palmedo H, Klehr HU, Sauerbruch T, Woitas RP (2008) Beta-trace protein-based equations for calculation of GFR in renal transplant recipients. Am J Transplant 8:608–615
Solichova P, Novackova L, Ochmanova R, Stejskal D (2006) Assessment of serum beta-trace protein (BTP) measurement in the prediction of glomerular filtration rate. Comparison with serum cystatin C. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 150:83–84
White CA, Akbari A, Doucette S, Fergusson D, Hussain N, Dinh L, Filler G, Lepage N, Knoll GA (2009) Estimating GFR using serum beta trace protein: accuracy and validation in kidney transplant and pediatric populations. Kidney Int 76:784–791
Funding
The study was supported by a restricted research grant from Dade Behring GmbH, Marburg, Germany, and Dade Behring, Inc., Mississauga, Ontario, Canada (no grant number available).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Benlamri, A., Nadarajah, R., Yasin, A. et al. Development of a beta-trace protein based formula for estimation of glomerular filtration rate. Pediatr Nephrol 25, 485–490 (2010). https://doi.org/10.1007/s00467-009-1355-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-009-1355-y