Abstract
It is common practice to estimate glomerular filtration rate (GFR) from the Schwartz formula (a height creatinine/ratio), although it has its limitations. Cystatin C was found to be a superior marker of GFR. No formula has been validated to estimate GFR from cystatin C in children. Children (aged 1.0–18 years, n=536) with various renal pathologies undergoing nuclear medicine GFR clearance studies (99mTc-DTPA single-injection technique) were tested. Cystatin C was measured with a nephelometric assay. The Schwartz GFR was calculated using enzymatically determined serum creatinine in micromoles per liter using the constant 48 for adolescent males and 38 otherwise. Using multiple stepwise regression analysis on log/log-transformed data, we derived the following relationship between the cystatin C concentration and GFR: log(GFR)=1.962+[1.123*log(1/Cystatin C)]. Using the Bland and Altman analysis to test agreement between the Schwartz formula and gold standard GFR showed considerable bias, with a mean difference of +10.8% and a trend towards overestimation of the GFR by the Schwartz formula with lower GFRs. In contrast, the Bland and Altman analysis applied on the GFR estimate derived from cystatin C showed the mean difference to be negligible at +0.3% and no trend towards overestimation of the GFR with lower GFRs. In the regression analysis of the estimate and the GFR, the Schwartz estimate showed significant deviation from linearity, whereas the cystatin C estimate did not. In conclusion, the data suggest that this novel cystatin C-based GFR estimate shows significantly less bias and serves as a better estimate for GFR in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Serum creatinine is the most widely used marker to predict glomerular filtration rate (GFR). Nevertheless, serum creatinine remains a crude marker of GFR. Creatinine concentrations are insensitive for detection of mild-to-moderate reductions in GFR. In childhood, there is age, gender, and muscle mass dependency of serum creatinine, and assessing a normal GFR accurately, even with the use of body height/creatinine ratios, remains difficult [1, 2, 3]. To estimate GFR more accurately, low molecular weight proteins have been suggested to replace serum creatinine. β2-Microglobulin has been advocated as a better predictor of GFR [4, 5]. However, the serum concentration can increase as an acute-phase protein in disorders such as lupus nephritis, which clearly require adequate assessment of GFR. More recent studies have suggested that serum or plasma cystatin C (CysC) may be better markers for GFR than serum creatinine (meta-analysis in reference [6]). CysC offers an advantage over creatinine because of its independence from age and gender [7]. Of all small molecular weight protein markers, CysC was superior to any other surrogate marker of GFR in children [8]. While there is increasing evidence that CysC is indeed a better marker of GFR, clinicians want an estimation of GFR from cystatin C and not simply a serum concentration. To date no study has established a CysC-based formula for the evaluation of GFR in children based on a large patient cohort. There is hope that CysC measurement may yield a more accurate formula for the estimation of GFR. We therefore embarked on a study aimed at the comparison of the currently used Schwartz formula and a novel formula based on CysC in children and adolescents who underwent a gold stand nuclear medicine GFR study.
Patients and methods
Study groups
The study received full approval of the local ethics boards and was in accordance with the ethical standards of the Helsinki declaration of 1975 (revised in 1983). Written consent was obtained in each case from the parents and in the case of a consenting minor, from the patient as well. Venous blood samples were obtained from 536 children with various renal pathologies, referred for nuclear medicine GFR study. Patients were recruited consecutively unless peers refused participation in the study. The patients were attending the Pediatric Nephrology Outpatient Clinic in Ottawa. Their ages ranged from 1.0 to 18.0 years, with a mean of 11.2±4.5 years; 41% of patients were females. Mean height was 136.7±28.4 cm (range 62.3–189.1 cm), mean weight was 40.2±20.0 kg (range 6.5–104.0 kg), and mean body surface area covered 1.22±0.42 m2 (range 0.33–2.20 m2). The main indications for the GFR measurements were: various forms of glomerulonephritis (44.7%), obstructive uropathy (19.9%), reflux nephropathy (13.6%), condition after renal transplantation (5.4%), and others (16.4%, including post hemolytic uremic syndrome, steroid-sensitive syndrome, cystinosis, orthostatic proteinuria, etc.).
Methods
GFR was determined using a 99mTc-DTPA single-injection technique with a three-point sampling approach at 2, 3, and 4 h post injection according to Russell [9], with the minor modification that no 10-min sample was obtained.
Serum creatinine was measured with an enzymatic assay (Ortho Clinical Diagnostics), and the constants of 38 for children above 1 year of age and 48 for adolescent males (Schwartz estimate [1] with a 20% correction for enzymatic measurement of creatinine) were used to calculate the GFR estimate according to Schwartz. We validated the correction factor of the Schwartz formula in our patient cohort. For adolescent males, the correction factor was 49.4±10.5, not significantly different from 48 (P=0.3271, one-sample t-test). For all the others, the factor was 40.3±7.7. The published factor 38 was within one standard deviation (SD). We therefore used the factors 38 and 48. The formula reads:
Determination of CysC was performed using the N Latex Cystatin C kit (Dade Behring) on a Behring BN ProSpec analyzer.
Statistical analysis
All statistical analysis was performed using GraphPad Prism Software for Science Version 3.0 (San Diego, Calif., USA) or Medcalc, Version 6.14.000 (Mariakerke, Belgium). Standard regression and correlation analysis was applied. Deviation from linearity was tested with the Cusum test. Agreement between methods was tested with the Bland and Altman plot method [10]. The Bland and Altman plot is a statistical method used to compare two measurement techniques. In this graphical method the differences (or alternatively the ratios) between the two techniques are plotted against the averages of the two techniques. Horizontal lines are drawn at the mean difference, and at the mean difference±1.96 times the SD of the differences. If the differences within mean±1.96 SD are not clinically important, the two methods may be used interchangeably. To compare the Bland and Altman analysis plots derived from the two formulae we used a mountain plot. A mountain plot (or "folded empirical cumulative distribution plot") is created by computing a percentile for each ranked difference between a new method and a reference method (here the GFR). To obtain a folded plot, the following transformation is performed for all percentiles above 50: percentile=100−percentile. These percentiles are then plotted against the differences between the two methods [11].
Results
In total, 536 patients were studied. Each received a GFR scan with simultaneous determination of height, serum creatinine, and CysC. The 99Tc-DTPA GFR ranged from 7 (severe renal failure) to 209 ml/min per 1.73 m2 (significant hyperfiltration). The mean GFR was 103±41 ml/min per 1.73 m2. GFR was normally distributed as determined by the Kolmogorov-Smirnov test.
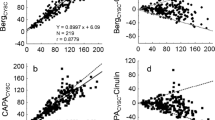
The mean Schwartz GFR was 90±32 ml/min per 1.73 m2 with a range of 12–234 ml/min per 1.73 m2. The Schwartz GFR was also normally distributed. The correlation coefficient between the GFR and the Schwartz estimate was 0.7686 [95% confidence interval (CI) 0.7315–0.8011, P<0.0001, Pearson r 2=0.5907]. A regression line between GFR and Schwartz GFR could be expressed as y=11.88+0.75x GFR. The 95% CI for the intercept in that formula was 8.5–15.5 and for the slope it was 0.71–0.79. The Cusum test for linerarity showed significant deviation from zero (Fig. 1).
Passing and Bablok regression between the Schwartz estimate and the glomerular filtration rate (GFR). The equation of the linear regression line reads: y=11.8853+0.7490*x, whereby the intercept was 11.8853 [95% confidence interval (CI) 8.5038–15.5476], and the slope was 0.7490 (95% CI 0.7095–0.7923). The Cusum test for linearity showed significant deviation from linearity (P<0.05)
Serum CysC ranged from 0.36 mg/l to 7.44 mg/l, and CysC was not normally distributed when analyzed by the Kolmogorov-Smirnow test. The median CysC was 0.89 mg/l. The reciprocal of CysC ranged from 0.13 to 2.77 l/mg and was normally distributed with a mean of 1.09±0.37 l/mg. The reciprocal of CysC correlated with GFR, with a correlation coefficient of 0.8180 (95% CI 0.7879–0.8442, P<0.0001, Pearson r 2=0.6692). However, a plot of the reciprocal of CysC versus GFR revealed that the data did not follow a straight line, but rather a log/log transformation was required in order to obtain a straight line (Fig. 2). From the log/log-transformed data we used multiple stepwise regression analysis to obtain the formula: log(GFR)=1.962+[1.123*log(1/CysC)].
Scatter plot of the reciprocal of serum cystatin C (CysC) concentration versus the GFR determined by 99Tc-DTPA clearance. Also given is the non-linear regression line after log-log transformation and the 95% CI and the 95% prediction interval. The latter represents two curves drawn parallel to the regression line. These curves represent the 95% prediction interval for the regression curve. The 95% prediction interval is much wider than the 95% CI. For any given value of the independent variable, this interval represents the 95% probability for the values of the dependent variable
The GFR derived from this novel formula ranged from 9.6 to 288 ml/min per 1.73 m2. The mean was 101±38 ml/min per 1.73 m2. The correlation coefficient between the GFR and the CysC estimate was 0.8107 (95% CI 0.7796–0.8379, P<0.0001, Pearson r 2=0.6572). A regression line between GFR and CysC estimate could be expressed as y=10.12+0.87x GFR. The 95% CI for the intercept in that formula was 6.4–14.1 and for the slope it was 0.83–0.91. The Cusum test for linerarity showed no significant deviation from zero (Fig. 3).
Passing and Bablok regression between the CysC GFR estimate and the GFR. The equation of the linear regression line reads: y=10.1203+0.8654*x, whereby the intercept was 10.1203 (95% CI 6.3961–14.1027), and the slope was 0.8654 (95% CI 0.8252–0.9061). As the 95% CIs for the slope do not overlap, it can be assumed that the formula for the estimation of GFR based on CysC was significantly better than the Schwartz formula. The Cusum test for linearity showed no significant deviation from linearity (P>0.05)
Bland and Altman analysis was performed to test for agreement between the two GFR estimates {Schwartz GFR and CysC estimate using log(GFR)=1.962+[1.123*log(1/CysC)]} and the gold standard GFR. The mean of the difference between the Schwartz GFR and the GFR showed a mean deviation of +10.8% (Fig. 4), whereas the mean deviation between the mean of the difference between the CysC estimate and GFR was only +0.3% (Fig. 5). The span between + and –1.96 SD for the Schwartz GFR in the Bland and Altman analysis was 95.1%, whereas the same parameter for the novel CysC estimate was only 87% (Figs. 4 and 5). Regression analysis of the values obtained form the Bland and Altman analysis for the Schwartz formula and the gold standard GFR showed a slope that was significantly non-zero. This did not apply for the novel CysC estimate. The comparison of the two Bland and Altman analyses was performed using a mountain plot. This showed a systematic error when applying the Schwartz formula compared with the novel estimate from CysC (Fig. 6).
Bland and Altman analysis to test agreement between the Schwartz formula using the enzymatically determined serum creatinine in micromoles per liter and the factor 38, except for adolescent males in whom factor 48 was used. We chose to plot differences as percentages of averages. This option is useful when there is an increase in variability of the differences as the magnitude of the measurement increases, as is indeed the case for supra-normal GFRs. There was considerable bias, with a mean difference of +10.8% and the slope of the regression line of the plots was significantly non-zero
Bland and Altman analysis to test agreement between the newly derived formula for calculating GFR from the serum CysC concentration using log(GFR)=1.962+[1.123*log(1/CysC)]. Again, differences were plotted as percentage of the averages. The mean difference was negligible at +0.3% and, unlike with the Schwartz formula, the regression analysis revealed no trend towards overestimation of the GFR by the CysC formula with lower GFRs
In order to compare the Bland and Altman plots for the Schwartz formula (open squares) and the newly derived GFR estimate based on the serum CysC (full circles), we performed a mountain plot (or "folded empirical cumulative distribution plot"), which is created by computing a percentile for each ranked difference between a new method and a reference method. To obtain a folded plot, the following transformation is performed for all percentiles above 50: percentile=100−percentile. These percentiles are then plotted against the differences between the two methods. One can clearly identify the bias induced by the Schwartz formula
Discussion
The clinician needs an accurate assessment of GFR. Gold standard tests for assessment of GFR frequently cannot be applied, they are expensive, cumbersome, and often involve radiation. Therefore the current practice is to use the Schwartz estimate [1]. The shortcomings of the Schwartz estimate are well established, especially the substantial overestimation of GFR in those patients who have a GFR of under 20 ml/min per 1.73 m2 [3]. However, the Schwartz estimate remains the most widely used surrogate marker of GFR. Recently, CysC has been demonstrated to be a superior marker of GFR than serum creatinine [6], both in adults and in children. It was therefore overdue to derive a formula for the determination of GFR from 1/CysC similar to the Schwartz formula. Such a study has not yet been forthcoming and to the best of our knowledge this is the first such formula for children to be validated. Using stepwise multiple regression from the log/log-transformed data, we derived the following formula for the estimation of GFR from the serum CysC concentration: log(GFR)=1.962+[1.123*log(1/CysC)].
We compared the diagnostic performance of this formula with the currently used Schwartz formula. Three main findings could be established. Firstly, the regression line between GFR and the Schwartz estimate shows significant deviation from linearity. This did not apply for the novel estimate. Secondly, the Bland and Altman analysis of the Schwartz formula versus the gold standard GFR revealed significant bias. Thirdly, the comparison of the Bland and Altman analysis as performed by mountain plot analysis between the Schwartz GFR estimate and the novel CysC estimate showed a better agreement of the CysC estimate with the gold standard GFR. One has to conclude from these data that the Schwartz GFR estimate should be replaced by the novel CysC-based GFR estimate.
However, several issues will have to be considered. There is substantial variability with the serum creatinine determination. In essence, the constants of the Schwartz estimate have to be validated for each individual center. We used a 20% correction factor when compared with the original paper [1], because we measured serum creatinine enzymatically. The factors 38 and 48 are most widely used when measuring creatinine in SI units [12]. We validated these factors for enzymatic creatinine measurements against 51Cr-EDTA clearances [13]. There is good agreement between the 99Tc-DTPA clearance methods used in this study and the 51Cr-EDTA clearance [8]. It may be possible to eliminate some of the systematic error observed when using the Schwartz formula by recalculating the constants for special patient groups. However, the large variability of serum creatinine determinations and the need for large studies in every single center renders such approaches largely unusable. Currently, only a single assay for CysC has been approved by the FDA, namely the nephelometric test used in our study. The costs for this assay remain substantially higher than those for serum creatinine determination. In Canada, we currently pay CDN $ 0.20 for an enzymatic creatinine determination and CDN $ 2.40 for a CysC determination (12 times the cost of serum creatinine). There is considerable hope that the price for a CysC assay will come down with an increased usage. Apart from the differences in the price of the assay, substantial start-up costs in those centers that do not have equipment that allows the usage of the Dade Behring kit, such as the BN Prospect or others, may serve as an additional impediment to the implementation of CysC-based monitoring of GFR. The very fact that currently only one CysC assay has FDA approval could result in universal standardization of the determination of GFR in children. However, the use of a single assay would be unfavorable to competition, which would help to further decrease the price of CysC assays. The initial problem of lack of calibration between the different CysC assays [14] seems to have improved [15, 16]. However, standardization of CysC still requires reference preparations to allow reproducible comparison between automated immunoassays.
The other major limitation lies in the very term GFR estimate. While the deviation from the reference GFR method is improved with our current formula compared with the Schwartz estimate, there still is an error of up to 44%. In particularly important clinical situations, such as the accurate determination of GFR when considering a patient for preemptive renal transplantation, a full GFR scan cannot be replaced by a GFR estimate. However, our results clearly favor a move towards a CysC-based GFR estimation in children and call for a standardization of CysC assays worldwide.
References
Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A (1976) A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58:259–263
Schwartz GJ, Brion LP, Spitzer A (1987) The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children and adolescents. Pediatr Clin North Am 34:571–590
Seikaly MG, Browne R, Bajaj G, Arant BS Jr (1996) Limitations to body length/serum creatinine ratio as an estimate of glomerular filtration in children. Pediatr Nephrol 10:709–711
Nolte S, Mueller B, Pringsheim W (1991) Serum alpha 1-microglobulin and beta 2-microglobulin for the estimation of fetal glomerular renal function. Pediatr Nephrol 5:573–577
Filler G, Witt I, Priem F, Ehrich JHH, Jung K (1997) Are cystatin C and beta-2-microglobulin better markers than serum creatinine for prediction of a normal glomerular filtration rate in pediatric subjects? Clin Chem 43:1077–1078
Dharnidharka VR, Kwon C, Stevens G (2002) Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis 40:221–226
Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J (1998) Cystatin C—a new marker of glomerular filtration rate in children independent of age and height. Pediatrics 101:875–881
Filler G, Priem F, Lepage N, Sinha P, Vollmer I, Clark H, Keely E, Matzinger M, Akbari A, Althaus H, Jung K (2002) Beta-trace protein, cystatin C, beta(2)-microglobulin, and creatinine compared for detecting impaired glomerular filtration rates in children. Clin Chem 48:729–736
Russell CD (1993) Optimum sample times for single-injection, multisample renal clearance methods. J Nucl Med 34:1761–1765
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet I:307–310
Krouwer, JS, Monti KL (1995) A simple, graphical method to evaluate laboratory assays. Eur J Clin Chem Clin Biochem 33:525–527
Dalton RN, Haycock GB (1999). Laboratory investigation. In: Barratt TM, Avner ED, Harmon W (eds) Pediatric nephrology, 4th edn. p 355
Filler G, Priem F, Vollmer I, Gellermann J, Jung K (1999) Diagnostic sensitivity of serum cystatin for impaired glomerular filtration rate. Pediatr Nephrol 13:501–505
Finney H, Newman DJ, Gruber W, Merle P, Price CP (1997) Initial evaluation of cystatin C measurement by particle-enhanced immunonephelometry on the Behring nephelometer systems (BNA, BN II). Clin Chem 43:1016–1022
Erlandsen EJ, Randers E, Kristensen JH (1999) Evaluation of the Dade Behring N Latex cystatin C assay on the Dade Behring Nephelometer II system. Scand J Clin Lab Invest 59:1–8
Randers E, Erlandsen EJ (1999) Serum cystatin C as an endogenous marker of the renal function—a review. Clin Chem Lab Med 37:389–395
Acknowledgements
The study was funded by a limited educational grant from Dade Behring GmbH, Marburg, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by a research grant from Dade Behring GmbH, Germany. There is no conflict of interest.
Rights and permissions
About this article
Cite this article
Filler, G., Lepage, N. Should the Schwartz formula for estimation of GFR be replaced by cystatin C formula?. Pediatr Nephrol 18, 981–985 (2003). https://doi.org/10.1007/s00467-003-1271-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-003-1271-5









