Abstract
Background
Fundoplication is known to improve allograft outcomes in lung transplant recipients by reducing retrograde aspiration secondary to gastroesophageal reflux disease, a modifiable risk factor for chronic allograft dysfunction. Laparoscopic Nissen fundoplication has historically been the anti-reflux procedure of choice, but the procedure is associated with discernable rates of postoperative dysphagia and gas-bloat syndrome. Laparoscopic Toupet fundoplication, an alternate anti-reflux surgery with lower rates of foregut complications in the general population, is the procedure of choice on our institution’s lung transplant protocol. In this work, we evaluated the efficacy and safety of laparoscopic Toupet fundoplication in our lung transplant recipients.
Methods
A prospective case series of 44 lung transplant recipients who underwent laparoscopic Toupet fundoplication by a single surgeon between September 2018 and November 2020 was performed. Preoperative and postoperative results from 24-h pH, esophageal manometry, gastric emptying, and pulmonary function studies were collected alongside severity of gastroesophageal reflux disease and other gastrointestinal symptoms.
Results
Median DeMeester score decreased from 25.9 to 5.4 after fundoplication (p < 0.0001), while percentage of time pH < 4 decreased from 7 to 1.1% (p < 0.0001). The severity of heartburn and regurgitation were also reduced (p < 0.0001 and p = 0.0029 respectively). Overall, pulmonary function, esophageal motility, gastric emptying, severity of bloating, and dysphagia were not significantly different post-fundoplication than pre-fundoplication. Patients with decreasing rates of FEV1 pre-fundoplication saw improvement in their rate of change of FEV1 post-fundoplication (p = 0.011). Median follow-up was 32.2 months post-fundoplication.
Conclusions
Laparoscopic Toupet fundoplication provides objective pathologic acid reflux control and symptomatic gastroesophageal reflux improvement in lung transplant recipients while preserving lung function and foregut motility. Thus, laparoscopic Toupet fundoplication is a safe and effective antireflux surgery alternative in lung transplant recipients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Retrograde chronic microaspiration secondary to gastroesophageal reflux disease (GERD) is associated with acute rejection episodes [1], may contribute to the development of bronchiolitis obliterans (BOS) after LTx, and may lead to the development of chronic lung allograft dysfunction (CLAD) [2]. As such, GERD can threaten lung transplant (LTx) outcomes, especially long-term graft and recipient survival.
GERD has a known association with the development of end-stage lung disease of various etiologies of lung disease [3], including chronic BOS or obstructive CLAD, especially when compounded by the concurrent presence of esophageal dysmotility [4]. Given that GERD and esophageal dysmotility are prevalent pre- and post-LTx [3], anti-reflux surgery with fundoplication has been proposed to prevent this GERD-induced damage [5, 6].
Laparoscopic Nissen fundoplication (LNF), a full 360° circumferential wrap, has been the anti-reflux surgery (ARS) procedure of choice for over 60 years; however, it is often associated with gas-bloat and higher rates of short-term dysphagia [7, 8]. A less restrictive posterior 270° laparoscopic Toupet fundoplication (LTF), reported to be as effective for controlling GERD in the general patient population [9], can be offered to patients with severe esophageal dysmotility to lower the risk of developing postoperative dysphagia and gas-bloat syndrome [10].To date, limited data is available describing LTF in post-LTx patients in terms of efficacy of controlling GERD and the rate of postoperative complications and adverse symptoms.
To address this knowledge gap, this study was conducted to assess the effects of LTF on reflux control and symptom reduction in LTx recipients.
Methods
Patient selection
After obtaining institutional review board (IRB) approval, lung transplant recipients for whom ARS was indicated and planned were recruited to participate in this prospective case series. After obtaining informed consent, total of 44 patients who underwent LTF following lung transplant between September 2018 and November 2020 by a single surgeon who has performed over 400 LTFs at the University of Florida Shands Hospital (Gainesville, FL) was included. Data were collected until June 17, 2022. This study complies with the ISHLT Ethics guidelines.
Lung transplant protocol
Per the University of Florida Lung Transplant Protocol, all pre-and post-lung transplant recipients undergo universal foregut evaluation. A barium esophagram and gastric emptying study (GES) are performed pre-transplant. Patients with severe esophageal dysmotility depicted by fluoroscopy require high-resolution esophageal manometry (HREM). Post-transplant: HREM and 24-h pH off proton pump inhibitor (PPI) therapy are performed at 3 months. Pulmonary function tests are performed routinely every 1–3 months during clinic visits. If patients have typical GERD symptoms and pathologic acid exposure during the 24-h pH study, they are placed on anti-secretory therapy (PPI). LTF is performed post-transplant if the graft is impacted negatively by acute cellular rejection or documented positive amylase or pepsin obtained during the bronchoscopy. Otherwise, anti-reflux surgery was planned once the patient has reached an FEV1 plateau phase after the peak FEV1 is reached. As indicated by this approach, no patient in this study underwent LTF with a rapid lung function decline. Indications for LTF were symptomatic GERD with or without hiatal hernia, and silent GERD (with abnormal DeMeester score). Hiatal hernias were repaired at time of fundoplication. At our institution, patients with ‘mild’ gastroparesis (retention of 10–20% at 4 h) did not undergo additional intervention. Patients with ‘moderate’ (20–30% at 4 h) or ‘severe’ (> 30% at 4 h) gastroparesis underwent pyloroplasty at the same time as their anti-reflux operation.
Gastrointestinal studies
Reflux status was defined by the presence of abnormal DeMeester score. Pre-operative and postoperative results of the following tests were collected: 24-h ambulatory pH study, high-resolution esophageal manometry, and 4-h gastric emptying study. The most recent pre-LTF findings were compared to the most recent findings at the time of data collection (see Fig. 1).
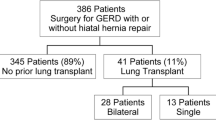
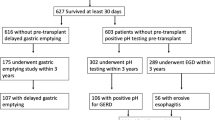
Flowchart depicting the inclusion and exclusion criteria, data collected, and time points used for this study. Forty-four lung transplant recipients who were to undergo laparoscopic Toupet fundoplication (LTF) were consented. Results of tests in the lung transplant protocol including pulmonary function test (PFT), 24-h pH, manometry, and gastric emptying study results were collected, and severities of patient symptoms were obtained from clinic provider notes. Pre-LTF data was compared post-LTF data
DeMeester score was determined from 24-h ambulatory pH studies. Pathologic acid exposure based on the DeMeester score was > 14.7 [11]. All ambulatory pH studies were conducted off PPI therapy. The result of a gastric emptying study was considered normal, mild, moderate, or severe if retained gastric contents at 4 h were < 10%, 10 to < 20%, 20 to < 30%, or ≥ 30% respectively. Esophageal motility was studied using high-resolution esophageal manometry. Integrated relaxation pressure (IRP), distal contractile integral (DCI), and lower esophageal sphincter pressure (LESP) were collected. Esophageal motility disorders were diagnosed based on the Chicago 4.0 classification [12]. We classified axial diameter of 1–3 cm as a ‘small’ hiatal hernia, 3–5 cm as a ‘medium’ hiatal hernia, and > 5 cm as a ‘large’ hiatal hernia.
Symptom severity
At the GI clinic, patients were asked to rate the severity of symptoms on our GI clinic symptom inventory: heartburn, regurgitation, bloating, dysphagia, and nausea, while distension was noted on physical exam. Severity was rated on Likert scale: 0 = never present, 1 = mild (symptoms present but not affecting daily activities/meals), 2 = moderate (symptoms affecting some daily activities/meals), and 3 = severe (symptoms affecting most daily activities/meals). Preoperative and postoperative symptom severities were compared.
Lung function
Lung function was assessed using forced expiratory volume in 1 s (FEV1) and percentage of best FEV1 value from pulmonary function tests (PFT). Pre-LTF baseline was calculated by taking the average of the 3 most recent FEV1 values prior to fundoplication. The FEV1 values at 6 weeks, 3 months, 6 months, 12 months post-LTF, and the most recent at time of data collection were compared to baseline. Best FEV1 is determined by the highest 2 FEV1 values taken at least 3 weeks apart. Percentage of best FEV1 at 6 weeks, 3 months, 6 months, 12 months post-LTF, and the most recent at time of data collection were compared to the pre-LTF values. According to our protocol, PFTs were measured 4, 5, 7, 9, 11, and 12-weeks post LTx, then monthly for the first year, every 3 months for 2 years, then every 4 months thereafter. To assess rate of change of FEV1, FEV1 values were plotted over time. The pre-LTF rate was defined as the gradient between the last 3 FEV1 values pre-LTF, while the post-LTF rate was gradient calculated using all available FEV1 since LTF. The pre-LTF and post-LTF gradients were reported as the pre-LTF and post-LTF rates of change of FEV1 in milliliters per day, respectively. A subset of patients who had decreasing rate of change of FEV1 prior to LTF was further analyzed.
Complications
Postoperative courses were reviewed from patient charts. Thirty-day complications were noted and classified according to the modified Clavien-Dindo classification system [13].
Statistical analyses
Categorical data were reported as proportions. Continuous data were analyzed using two-tailed Wilcoxon test. The confidence intervals for relative risk (RR) of abnormal motility and GI symptoms after LTF was calculated by the Koopman asymptotic score. PFTs were analyzed using mixed-effects analysis. p values < 0.05 and confidence intervals not including the null value (RR = 1) were considered significant. R Studio Version 2022.12.0 + 353 [14, 15] was used to calculate effect size, all other statistical analyses were performed using Prism 9 Version 9.3.1 for macOS, GraphPad Software, San Diego, California USA, www.graphpad.com.
Results
Patient demographics
Of the 44 lung transplant recipients who were included in this study, the median time (and interquartile range [IQR]) between transplantation and fundoplication was 12.8 (7.5–21.8) months. Median time to last follow-up from fundoplication and LTx were 32.2 (28.2–35.4) months and 45.1 (38.8–53.7) months, respectively. Patient demographics and operative details are summarized in Table 1.
Reflux control
After LTF, the median DeMeester score decreased from 25.9 to 5.4 [p < 0.0001] (Fig. 2a). This is accompanied by a decrease in percentage of total time with pH < 4.0 from 7.0 to 1.1% [p < 0.0001] (Fig. 2b). In addition, the relative risk (CI) of having an abnormal DeMeester score after LTF was 0.304 (0.154, 0.529) compared to post-LTx pre-LTF (Fig. 3). Post-LTF, overall severity of heartburn and regurgitation decreased (p < 0.0001 and p = 0.0029 respectively) (Fig. 4a and b).
Pre-laparoscopic Toupet fundoplication (LTF) and post-LTF Demeester scores. A Demeester scores were obtained from 24-h ambulatory 24-h pH studies. B Total percentage of time in reflux. Boxes represent median and interquartile range, while whiskers represent minimum to maximum values. Two-tailed Wilcoxon test was used to determine significance
Relative risk of abnormal gastrointestinal (GI) test results or GI symptoms post-laparoscopic Toupet fundoplication. Objective measures of acid reflux, esophageal dysfunction, and gastroparesis were obtained using 24-h pH, high-resolution manometry, and gastric emptying studies, respectively. Symptoms of heartburn, regurgitation, dysphagia, and bloating were obtained from provider clinic notes. Circles represent relative risk while lines represent confidence intervals calculated using Koopman asymptotic score
Pre-laparoscopic Toupet fundoplication (LTF) post-LTF gastrointestinal symptoms: A heartburn, B regurgitation, C bloating, D distension, E dysphagia, and F nausea. Graphs show number of patients with each severity score pre- and post-LTF. Severity was ranked on a Likert scale where 0 = absence, 1 = mild, 2 = moderate, 3 = severe. P values were calculated using two-tailed Wilcoxon test
Pulmonary function
There was no significant difference in FEV1 at 6 weeks, 3 months, 6 months, 12 months, and beyond 1 year compared to pre-LTF baseline (Fig. 5a). Likewise, the percentage of best FEV1 was no different than the pre-LTF baseline levels between 6 weeks and 1 year and beyond (Fig. 5b). Overall, rate of change of FEV1 after LTF was not statistically significant compared to before (p = 0.346) (Fig. 5c). Seventeen patients had decreasing rate of FEV1 prior to LTF. Of these 17 patients, 16 saw improvement in their rate of change of FEV1 after LTF, and the median improvement for this subset was 0.93 mL/day (p = 0.0011, effect size = 0.741) (Fig. 5d).
Pre-laparoscopic Toupet fundoplication (LTF) and post-LTF pulmonary function test results. A FEV1 B percentage of best at timepoints post-LTF were compared to pre-LTF using mixed-effects analysis. C, D Rates of change of FEV1 pre- and post-LTF were plotted against time. Wilcoxon matched-pairs test was used for comparison. C All patients were analyzed. D 17 patients with decreasing rates of FEV1 pre-LTF were sub-analyzed. Box shows median and interquartile range. Whiskers show minimum to maximum values
Esophageal and gastric motility
There was no difference between pre-and-post-LTF IRP, DCI, and LESP [medians of 12.9 vs 9.2 mmHg, 2025 vs 1643 mmHg.s.cm, 26.7 vs 25.6 mmHg and p = 0.100, 0.799, 0.478 respectively] (Fig. 6a–c). Post-LTF, patients had no significant change in percentage of retained gastric contents after 4 h or in the severity of gastroparesis [median 14.6% vs. 5.5%, median mild vs. none and p = 0.832, p = 0.204, respectively] (Fig. 6d and e) assessed by 4-h GES. More patients saw a reduction in the severity of gastroparesis than worsening or development of gastroparesis (Fig. 6f). In addition, the relative risk of having a diagnosis according to the Chicago Classification 4.0 and abnormal gastric emptying were not significantly different pre-and-post-LTF (Fig. 3).
Pre-laparoscopic Toupet fundoplication (LTF) and post-LTF high resolution manometry and 4-h gastric emptying studies. Pre- and post-LTF A integrated relaxation pressure (IRP), B distal contractile integral (DCI), C lower esophageal pressure (LESP), D gastric retention after 4 h, were compared. Boxes represent median and interquartile range. Whiskers represent minimum to maximum values. E Severity of gastroparesis: 0-normal, 1-mild. 2-moderate, or 3-severe for < 10%, 10 to < 20%, 20 to < 30%, or ≥ 30% retained respectively. Percentages of patients experiencing no, mild, moderate, or severe gastroparesis were plotted. F Change in severity of gastroparesis from before LTF. P values were calculated using Wilcoxon matched-pairs test
Other gastrointestinal symptoms
The severity of bloating, distension, nausea, and dysphagia were not significantly different pre- and post-LTF (Fig. 4c–f). In addition, the relative risk for dysphagia, nausea, bloating, and abdominal distension was not increased following LTF (Fig. 3).
Surgical complications
Thirty-day overall complication rate was 27% (Table 1). There were no deaths associated with the LTF procedure. Of the 44 patients, only 1 (2%) patient required intensive care unit stay for altered mental status and shortness of breath, another 2 (5%) were treated for severe complications according to the Modified Clavien-Dindo classification [13] (1 diagnostic laparoscopy, 1 thoracentesis), 1 patient had a left heart catheterization, and 8 (20%) had minor complications, most of which were for pain control. There were no differences in operative time, blood loss, and complication rate between small and large hiatal hernias.
Discussion
This study shows LTF effectively controls acid exposure and reflux symptoms in LTx patients. We demonstrated that LTF significantly reduces and normalizes DeMeester score and percentage of total time where esophageal pH < 4.0 post-LTF from 25.9 to 5.4 and 7.0 to 1.1% respectively. Showing the decrease in these two objective measures afforded by LTF is important because GERD symptoms are non-specific and frequently absent in the LTx population [3]. Even though GERD symptoms do not accurately predict acid reflux and aspiration, we also showed that LTF reduces symptomatic GERD, decreasing the severity of heartburn and regurgitation experienced by our patients.
We also showed the presence and severity of bloating, distension, dysphagia, and nausea were no different before and after LTF. As such, LTF in LTx patients is not associated with increased incidence of post-op dysphagia and gas/bloat syndrome, which have been reported to be associated with LNF [16]. This is consistent with the lower prevalence of these symptoms described in the general population in the short-term after LTF [7, 8, 10]. In addition, we found that LTF did not significantly alter objective measures of esophageal and gastric motility in LTx recipients with HREM and 4-h gastric emptying studies, respectively. The relative risks of abnormal manometry and gastroparesis (Fig. 3) post-LTF were not significantly different, suggesting that LTF neither treated nor caused development of esophageal or gastric dysmotility. It is reassuring that most patients did not see an exacerbation of esophageal and gastric dysmotility after LTF because impaired esophageal and gastric motility are known to exacerbate GERD after lung transplantation [5]. Although some patients with normal esophageal function and gastric emptying prior to LTF developed esophageal dysmotility or impaired gastric emptying, a greater number of patients saw resolution or decrease in severity of their esophageal and gastric baseline abnormalities. This is important in the context of relative contraindication for fundoplication in patients with severe esophageal dysmotility and moderate to severe gastroparesis [17], both prevalent after LTx [18].
Our study showed that lung function remained stable within the time frame of analysis, evidenced by no significant changes in FEV1 and % best FEV1 before and after LTF (Fig. 5). The lack of difference or improvement could be because recipients undergo LTF when their lung function is near their peak. While we did not see an improvement of lung function, we were encouraged that lung function was preserved closed to their peaks at a median of 32.2 months post-LTF. More importantly, of the patients who had already shown decline in FEV1 after peaking but before LTF, LTF had a large effect (r = 0.741) reversing that decline. This suggests that LTF could preserve lung function and potentially improve longevity of the grafts.
In our study of LTF in 44 LTx patients, there were no deaths and only one intensive care unit stay. Of the other three patients who had severe complications defined by Modified Clavien-Dindo Grade [13], one required a diagnostic laparoscopy for gastrostomy leak, one required thoracentesis for pleural effusion, and one underwent left heart catheterization for cardiomyopathy. Most of the other minor complications were pain control. Together, with insignificant changes in esophageal and gastric motility-associated symptoms, and stability in FEV1, we show that LTF is safe in the LTx population.
We acknowledge that a limitation of this study is the small sample size; however, to our knowledge, this is the largest study to date evaluating efficacy and side effects of LTF as an ARS in LTx recipients, looking at objective measures of GI function and symptoms. Furthermore, this is the only prospective study that includes lung function after LTF. BOS, the most common phenotype of chronic rejection [19] develops in 57% LTx recipients within five years of transplantation [20]. Only five patients of our patients have follow-up test results 5 years post-LTx. Despite all five having stable PFTs, longer follow-up of more patients will be needed to determine the real impact of LTF on lung function. However, we are assured that LTF has not caused deterioration in FEV1 at a median of 32.2 (28.2–35.4) months post-LTF or 45.1 (38.8–53.7) months post-LTx. In addition, a recent randomized control trial showed well-controlled reflux symptoms, with no difference between LTF or LNF 15 years post-ARS in the general population [21], suggesting that LTF could potentially be just as effective in LTx patients long-term. We intend to follow these patients for up to 10 years postoperatively to assess both symptom control and durability of the surgical repair.
Finally, our study did not use validated symptom questionnaires (GERD-Q). However, it is well-known (also observed in our study) that GERD symptoms are non-specific and frequently silent in the LTx population [2]. Hence, in LTx recipients, treatment decisions to prevent acid reflux (both medical and surgical) should not be based on symptoms. Instead, treatment should be based on impedance-pH monitoring—the most sensitive and gold standard test for the diagnosis of GERD.
We would like to highlight the timing of fundoplication in our study. All our patients underwent LTF after their lung function had peaked (determined by an FEV1 plateau phase after the peak FEV1 was reached), with a median of 12.8 months between transplantation and fundoplication. This was in accordance with the University of Florida Lung Transplant Protocol, which also indicated for LTF if the graft was impacted negatively by acute cellular rejection or documented positive amylase or pepsin obtained during bronchoscopy, but no patient in our study underwent LFT for this latter indication of rapid lung function decline. While it has been proposed by Roy et al. that early fundoplication within 6 months after lung transplantation might protect against GERD-induced lung damage based on higher FEV1 5 years after transplantation [6], the retrospective nature of their study with selection bias limits its broad application. It can be argued that patients who received early fundoplication (within 6 months of transplant) were healthier overall compared to those who had to delay timing of fundoplication (to after 6 months from transplant) due to possible unforeseen post-transplant complications unrelated to GERD [22]. In comparison, our study standardized timing of fundoplication after transplant per our institution’s protocol.
In conclusion, laparoscopic Toupet fundoplication is effective and safe in lung transplant recipients, affording objective acid reflux control, low rates of serious postoperative complications/foregut symptoms, and stable lung function. Overall, LTF could potentially improve longevity of the graft, but longer follow-up will be needed to ascertain this effect.
Abbreviations
- A1AT:
-
Alpha-1 anti-trypsin
- ARS:
-
Anti-reflux surgery
- ASA:
-
American Society of Anesthesiology
- BOS:
-
Bronchiolitis obliterans syndrome
- CLAD:
-
Chronic lung allograft dysfunction
- COPD:
-
Chronic obstructive pulmonary disease
- CF:
-
Cystic fibrosis
- CI:
-
Confidence interval
- DCI:
-
Distal contractile integral
- FEV1:
-
Forced expiratory volume in 1 s
- FVC:
-
Forced vital capacity
- GERD:
-
Gastroesophageal reflux disease
- GES:
-
Gastric emptying study
- GI:
-
Gastrointestinal
- HREM:
-
High-resolution esophageal manometry
- ILD:
-
Interstitial lung disease
- IQR:
-
Interquartile range
- IRP:
-
Integrated relaxation pressure
- ISHLT:
-
International Society for Heart and Lung Transplantation
- LARS:
-
Laparoscopic anti-reflux surgery
- LES:
-
Lower esophageal sphincter
- LESP:
-
Lower esophageal sphincter pressure
- LNF:
-
Laparoscopic Nissen fundoplication
- LTF:
-
Laparoscopic Toupet fundoplication
- LTx:
-
Lung transplant
- PFT:
-
Pulmonary function test
- PPI:
-
Proton pump inhibitor
References
Shah N, Force SD, Mitchell PO, Lin E, Lawrence EC, Easley K, Qian J, Ramirez A, Neujahr DC, Gal A, Leeper K, Pelaez A (2010) Gastroesophageal reflux disease is associated with an increased rate of acute rejection in lung transplant allografts. Transplant Proc 42:2702–2706. https://doi.org/10.1016/j.transproceed.2010.05.155
Hathorn KE, Chan WW, Lo W-K (2017) Role of gastroesophageal reflux disease in lung transplantation. World J Transplant 7:103–116. https://doi.org/10.5500/wjt.v7.i2.103
Patti MG, Vela MF, Odell DD, Richter JE, Fisichella PM, Vaezi MF (2016) The intersection of GERD, aspiration, and lung transplantation. J Laparoendosc Adv Surg Tech A 26:501–505. https://doi.org/10.1089/lap.2016.0170
Young JS, Coppolino A (2021) Esophageal disease in lung transplant patients. Ann Transl Med 9:900. https://doi.org/10.21037/atm-20-4934
Fisichella PM, Davis CS, Kovacs EJ (2012) A review of the role of GERD-induced aspiration after lung transplantation. Surg Endosc 26:1201–1204. https://doi.org/10.1007/s00464-011-2037-y
Roy SB, Elnahas S, Serrone R, Haworth C, Olson MT, Kang P, Smith MA, Bremner RM, Huang JL (2018) Early fundoplication is associated with slower decline in lung function after lung transplantation in patients with gastroesophageal reflux disease. J Thorac Cardiovasc Surg 155:2762-2771.e1. https://doi.org/10.1016/j.jtcvs.2018.02.009
Lund RJ, Wetcher GJ, Raiser F, Glaser K, Perdikis G, Gadenstätter M, Katada N, Filipi CJ, Hinder RA (1997) Laparoscopic Toupet fundoplication for gastroesophageal reflux disease with poor esophageal body motility. J Gastrointest Surg 1:301–308. https://doi.org/10.1016/s1091-255x(97)80049-2
Du X, Hu Z, Yan C, Zhang C, Wang Z, Wu J (2016) A meta-analysis of long follow-up outcomes of laparoscopic Nissen (total) versus Toupet (270°) fundoplication for gastro-esophageal reflux disease based on randomized controlled trials in adults. BMC Gastroenterol 16:88. https://doi.org/10.1186/s12876-016-0502-8
Håkanson BS, Lundell L, Bylund A, Thorell A (2019) Comparison of laparoscopic 270° posterior partial fundoplication vs total fundoplication for the treatment of gastroesophageal reflux disease: a randomized clinical trial. JAMA Surg 154:479–486. https://doi.org/10.1001/jamasurg.2019.0047
Shan C-X, Zhang W, Zheng X-M, Jiang D-Z, Liu S, Qiu M (2010) Evidence-based appraisal in laparoscopic Nissen and Toupet fundoplications for gastroesophageal reflux disease. World J Gastroenterol 16:3063–3071. https://doi.org/10.3748/wjg.v16.i24.3063
Khajanchee YS, O’Rourke RW, Lockhart B, Patterson EJ, Hansen PD, Swanstrom LL (2002) Postoperative symptoms and failure after antireflux surgery. Arch Surg 137:1008–1014. https://doi.org/10.1001/archsurg.137.9.1008
Fox MR, Sweis R, Yadlapati R, Pandolfino J, Hani A, Defilippi C, Jan T, Rommel N (2021) Chicago classification version 4.0© technical review: update on standard high-resolution manometry protocol for the assessment of esophageal motility. Neurogastroenterol Motil 33:e14120. https://doi.org/10.1111/nmo.14120
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications. Ann Surg 240:205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
R Core Team (2022) R: A language and environment for statistical computing. https://www.Rproject.org/
Wickham H, Vaughn D, Girlich M (2023) tidyr: tidy messy data. https://tidyr.tidyverse.org, https://github.com/tidyverse/tidyr.
Robertson AGN, Krishnan A, Ward C, Pearson JP, Small T, Corris PA, Dark JH, Karat D, Shenfine J, Griffin SM (2012) Anti-reflux surgery in lung transplant recipients: outcomes and effects on quality of life. Eur Respir J 39:691–697. https://doi.org/10.1183/09031936.00061811
Yodice M, Mignucci A, Shah V, Ashley C, Tadros M (2021) Preoperative physiological esophageal assessment for anti-reflux surgery: a guide for surgeons on high-resolution manometry and pH testing. World J Gastroenterol 27:1751–1769. https://doi.org/10.3748/wjg.v27.i16.1751
Castor JM, Wood RK, Muir AJ, Palmer SM, Shimpi RA (2010) Gastroesophageal reflux and altered motility in lung transplant rejection. Neurogastroenterol Motil 22:841–850. https://doi.org/10.1111/j.1365-2982.2010.01522.x
Verleden SE, Vos R, Vanaudenaerde BM, Verleden GM (2017) Chronic lung allograft dysfunction phenotypes and treatment. J Thorac Dis 9:2650–2659. https://doi.org/10.21037/jtd.2017.07.81
Hennessy SA, Hranjec T, Swenson BR, Kozower BD, Jones DR, Ailawadi G, Kron IL, Lau CL (2010) Donor factors are associated with bronchiolitis obliterans syndrome after lung transplantation. Ann Thorac Surg 89:1555–1562. https://doi.org/10.1016/j.athoracsur.2010.01.060
Analatos A, Håkanson BS, Ansorge C, Lindblad M, Lundell L, Thorell A (2022) Clinical outcomes of a laparoscopic total vs a 270° posterior partial fundoplication in chronic gastroesophageal reflux disease: a randomized clinical trial. JAMA Surg. https://doi.org/10.1001/jamasurg.2022.0805
Antonoff MB (2018) How much does early fundoplication for lung transplant recipients with gastroesophageal reflux disease truly help? Challenges in escaping the perils of retrospective review. J Thorac Cardiovasc Surg 155:2772–2773. https://doi.org/10.1016/j.jtcvs.2018.02.057
Acknowledgements
The authors would like to thank Sara H. Gray, Candice L. Rogers, and Stefanie L. Wright for their help with this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Kelly M. Herremans is funded by NHGRI T32 HG008958, but has no financial relationships with any pharmaceutical or device company. Drs. Celeste G. Yergin, Sheetal Patel, Andres Pelaez, Tiago Machuca, Alexander L. Ayzengart, and Manuel A. Amaris, MD have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yergin, C.G., Herremans, K.M., Patel, S. et al. Laparoscopic Toupet fundoplication: a safe and effective anti-reflux option in lung transplant recipients. Surg Endosc 37, 8429–8437 (2023). https://doi.org/10.1007/s00464-023-10245-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10245-0