Abstract
Objective
Pudendal Nerve Entrapment (PNE) may determine chronic pelvic pain associated with symptoms related to its innervation area. This study aimed to present the technique and report the outcomes of the first series of robot-assisted pudendal nerve release (RPNR).
Patients and methods
32 patients, who were treated with RPNR in our centre between January 2016 and July 2021, were recruited. Following the medial umbilical ligament identification, the space between this ligament and the ipsilateral external iliac pedicle is progressively dissected to identify the obturator nerve. The dissection medial to this nerve identifies the obturator vein and the arcus tendinous of the levator ani, which is cranially inserted into the ischial spine. Following the cold incision of the coccygeous muscle at the level of the spine, the sacrospinous ligament is identified and incised. The pudendal trunk (vessels and nerve) is visualized, freed from the ischial spine and medially transposed.
Results
The Median duration of symptoms was 7 (5, 5–9) years. The median operative time was 74 (65–83) minutes. The median length of stay was 1 (1–2) days. There was only a minor complication. At 3 and 6 months after surgery, a statistically significant pain reduction has been encountered. Furthermore, the Pearson correlation coefficient reported a negative relationship between the duration of pain and the improvement in NPRS score, − 0.81 (p = 0.01).
Conclusions
RPNR is a safe and effective approach for the pain resolution caused by PNE. Timely nerve decompression is suggested to enhance outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
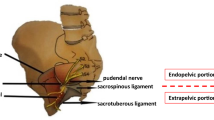
Pudendal nerve entrapment (PNE) is caused by the compression of the nerve at different levels along its course. The three critical points for PNE are: between the sacrotuberous and sacrospinous ligaments, Alcock’s canal and the falciform process of the sacrotuberous ligaments. The most common compression point is the ischial spine, at the attachment site of the sacrospinous ligament [1]. Clinically, we would suspect entrapment in this region when perineal and lower limb pain occurs, The latter may also be associated with motor deficits [2].
Given the absence of pathognomonic radiological or electrophysiological findings, the diagnosis of PNE is mainly clinical and exclusionary [1]. Due to the low success rate of medical therapy, decompressive surgery is frequently advocated, especially in patients with pain for less than six years [3]. Several approaches have been described, such as transgluteal [4], transischiorectal [5], transperineal [6], and laparoscopic [7]. In 2015, the first report on robotic decompression of the pudendal nerve was presented [8].
To our knowledge, no series of robotic pudendal nerve release (RPNR) has been published.
Objective
To describe the technique and present the outcomes of the first series of RPNR.
Materials and methods
Patients
Between 2016 and 2021, 32 patients were submitted to RPNR for PNE causing chronic perineal pain for over, which lasted for more than 6 months. The pudendal neuralgia diagnosis was established through a process of differential diagnosis, which involved ruling out other organic pathologies, such as abscesses, fistula, infections or tumors.
Surgery was adopted in all cases if when the conservative treatment (i.e. medical and physical therapy) was ineffective and the anesthetic pudendal nerve block provided symptom were ineffective. Only patients who met the Nantes criteria were included [9]. They are based on five elements: a- painful symptom in the pudendal nerve innervation area; b- pain worsening in the sitting position; c- the awakenings absence for pain during the night; d- no sensory impairment on physical examination; e- pain relief after Pudendal Nerve Block.
Database
A customized database, including baseline demographic data, pain characteristics [such as primary site, side, numeric pain rating scale (NPRS)], and symptoms duration, was developed. We gathered the perioperative data, including operative time (OT), console time (CT), length of stay, and postoperative complications ranked according to the Clavien-Dindo Classi [10]. The patient global impression of change (PGIC) scale data was also registered.
Follow-up
The first two clinical evaluations were conducted at 3 and 6 months after surgery, respectively. NPRS and PGIC were recorded for each time point.
Ethics
The study was conducted in accordance with the Good Clinical Practice rules and with the ethical principles contained in the Declaration of Helsinki as amended in Hong Kong. Each patient gave a written informed consent for the surgical procedure and for the collection of their data for research purposes. Institutional board review approval was obtained by the Groupement de Coopération Sanitaire ELSAN #01, France (#2022–04-Dr-PIERQUET-01).
Surgical technique
Under general anesthesia, the patient is placed in dorsal decubitus. Pneumoperitoneum is established at 12 mm Hg through closed access. The trocars are placed such as for a robot-assisted radical prostatectomy with one 5 mm assistant port.
The iliac vessels and the ipsilateral medial umbilical ligament are the first landmarks to identify.
The peritoneum is incised laterally to the umbilical ligament. Careful attention should be paid to males for the vas deferens that should be preserved. The dissection proceeds into the plane between the bladder and the external iliac vein targeting the obturator fossa After the obturator nerve recognition, the plane medial to it is dissected; the obturator vein and, subsequently, the internal obturator muscle are identified. Eventual fat hernias of the obturator fossa should be reduced to maximize the working space. The endopelvic fascia and the arcus tendinous of the levator ani are visualized. A deeper dissection at the level of the cranial insertion of the arcus tendinous reveals the ischial spine. The coccygeal muscle fibers are identified and cut with cold scissors close to the ischial spine; the sacrospinous ligament is evidenced and incised. The pudendal vessels and nerve are identified, released from the ischial spine and medially transposed. The final step of the decompression consists of the section of the roof of the proximal end of Alcock’s canal to free the PN entirely. Usually, the restoration of visible arterial pulsations confirms the decompressive effect of the surgery. In the case of bleeders, the hemostasis is guaranteed with 5-mm metallic clips or simple compression, avoiding the extensive use of energy.
No reconstruction of the sacrospinous ligament after the PN transposition was performed in our series. Although this maneuver may aid pelvic floor stability, its utility has not been documented. Finally, no wrapping of the decompressed PN by an omental flap has been performed that has been described by Erdogru et al. [7].
Figure 1 briefly provides a step-by-step description of the surgical steps.
A Identification of the LMUL and peritoneal incision lateral to it, sparing the LRL. LRP left round ligament of the uterus. LMUL left medial umbilical ligament. B Progressive dissection of the left latero-vesical space, till the endopelvic fascia. C Identification of the internal obturator muscle (OM), levator ani (LA), coccygeous muscle (CM) and the fascial tendinous arch (AT) of the pelvis, that stretches from the pubovesical ligaments to the ischial spine. ON obturator nerve, Bl bladder, LRL lft round ligament of the uterus, LMUL left medial umbilical ligament. D Close view. Identification and division of the fibers of the coccygeous muscle E Division of the fibers of the coccygeous muscle. F Identification of the sacrospinous ligament (SPL) and its division close to the ischial spine (IS). G The curved line corresponds to the contour of the ischial spine (IS). The pudendal nerve has been released from the surroundings tissues and transposed medially to the IS; visibly free of any compression, it enters into the Alcock’s canal (dashed arrow)
No drain was used. All patients were discharged without indication for antibiotics or anticoagulants.
Statistical analysis
The median and interquartile ranges were used to evaluate quantitative variables. Absolute frequencies and percentages were used to measure qualitative variables. Follow-up changes in NPRS were analyzed using Wilcoxon Test. The Pearson correlation coefficient was calculated to evaluate the impact of preoperative variables on the NPRS score improvement.
All statistical tests were two-tailed, and p < 0.05 was considered statistically significant. Statistical tests were performed using IBM SPPS (v 26) software.
Results
The 32 patients (19 females and 13 males) who underwent RPNR were included in this study. The median duration of symptoms was 7 (5, 5–9) years, and the median preoperative NPRS score was 8 (8–9). Two people had already undergone transgluteal PNR on the same side. Almost all patients suffered concomitant anxiety syndrome (27/32, 84%), while five diagnoses of depression occurred. Median OT and CT were 74 (65–83) and 63 (53–71) mins, respectively. The median length of stay was 1 (1–2) days. There were no major complications, while a minor one (CD-1) occurred only in one patient who reported postoperative pain relieved by analgesics. The baseline and perioperative data of the included patients are summarized in Table 1.
At 3-month and 6-month postoperative follow-up visits, the NPRS progressively reduced (8 vs 6, p < 0.001, and 6 vs 4, p < 0.001, respectively). Even an improvement in PGIC occurred: there was a "minimal improvement" in perceived pain after three months from surgery, and the overall status was "much improved" after six months. Table 2 summarizes the data of NPRS and PGIC scales. The Pearson correlation coefficient revealed a negative association of NPRS with the duration of symptoms (0.7027, p < 0.001).
Discussion
PNE syndrome is a disabling condition impacting the quality of life [1]. Besides pain, PNE may lead to urinary, sexual and anorectal disorders [11]. The clinical manifestations vary based on anatomical variants, entrapment sites, severity and duration of compression. Thus, the PNE diagnosis can be very challenging, and patients undergo a long investigation period before treatment. In addition, a concomitant anxiety-depressive syndrome may occur in several cases, as in our series (84%). The latter is a negative predictor of the success of surgery [12].
Despite the medical therapy's positive influence on the incidence and severity of chronic postsurgical pain during the perioperative period [13], nerve decompression is the only option that guarantees long-term outcomes. In a randomized controlled trial, surgical treatment determined an improvement in pain immediately after 3 months in patients compared to the control group, 57.1% vs 6.7%, and then increased the gap at 12 months of follow-up, 71.4% vs 13.3% [14].
Different surgical approaches have been described to release an entrapped PN. Several open techniques were proposed according to the site of PN branch involvement: transischiorectal and transperineal (anterior) incision, despite a limited field of view, and transgluteal (posterior) incision, with a higher rate of surgical trauma. Therefore, the site-specific open approach choice for the surgery is preferable for optimal functional outcomes [14]. Laparoscopic surgery was introduced by Possover to reduce postoperative comorbidities and minimize scarring [15]. Table 3 shows the available literature describing the use of minimally invasive PNR [7, 15,16,17]. Our series was in line with the other papers, except for a lower frequency of postoperative complications and length of stay. Similar results were achieved in improvement in Patient-Reported Outcomes.
To our knowledge, we provide the first case series of PNR performed robotically. Our study demonstrates that the procedure is quick and safe. Similarly to the laparoscopic approach, it allows for a straightforward and bloodless access to the two most common sites of PNE, being the ischial spine and the Alcock’s canal. We recognize that the robotic platform is not indispensable for this kind of surgery when compared to the traditional, laparoscopic approach that provides excellent outcomes [7, 15,16,17]. However, the robotic platform may provide an easier instrument manipulation in hard-to-reach areas, such as the deep pelvic space. Surgeons and future research will identify, for each specific surgical method, its right indication and use, based on technical feasibility, safety, results and cost-effectiveness, parallel to man-powered related issues that are specific to each medical centre.
A statistically significant improvement was evidenced in NPRS and PGIC during follow-up. In our series, the time elapsed from the onset of symptoms to diagnosis and treatment was the only variable that correlated with the efficacy of surgery (p = 0.01). Perioperative results were satisfactory, with only one patient (3%) requiring analgesics and a median hospital stay of 1 day.
However, this study is not without limitations. It is a retrospective study with relatively small sample size, short follow-up, and no control arm. No evaluation of the impact of the surgery on erectile, bowel and lower urinary tract symptoms was performed.
Conclusion
RPNR is a safe and effective approach for the pain resolution caused by PNE. Timely nerve decompression is suggested to enhance outcomes.
References
Luesma MJ, Galé I, Fernando J (2021) Diagnostic and therapeutic algorithm for pudendal nerve entrapment syndrome. Med Clin 157(2):71–78
Pereira A, Pérez-Medina T, Rodríguez-Tapia A, Chiverto Y, Lizarraga S (2018) Correlation between anatomical segments of the pudendal nerve and clinical findings of the patient with pudendal neuralgia. Gynecol Obstet Invest 83:593–599
Engeler D, Baranowski AP, Berghmans B et al (2022) EAU guidelines on chronic pelvic pain
Hibner M, Castellanos ME, Drachman D, Balducci J (2012) Repeat operation for treatment of persistent pudendal nerve entrapment after pudendal neurolysis. J Minim Invasive Gynecol 19(3):325–330
Klifto K, Dellon AL (2020) Persistent genital arousal disorder: treatment by neurolysis of dorsal branch of pudendal nerve. Microsurgery 40(2):160–166
Beco J, Climov D, Bex M (2004) Pudendal nerve decompression in perineology: a case series. BMC Surg 4:15
Erdogru T, Avci E, Akand M (2014) Laparoscopic pudendal nerve decompression and transposition combined with omental flap protection of the nerve (Istanbul technique): technical description and feasibility analysis. Surg Endosc 28(3):925–932
Rey D, Oderda M (2015) The first case of robotic pudendal nerve decompression in pudendal nerve entrapment syndrome. J Laparoendosc Adv Surg Tech A 25(4):319–322. https://doi.org/10.1089/lap.2014.0013
Labat JJ, Riant T, Robert R, Amarenco G, Lefaucheur JP, Rigaud J (2008) Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria). Neurol Urodyn 27:306–310
Clavien PA, Barkun J, de Oliveira ML et al (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250(2):187–196
Aoun F, Alkassis M, Tayeh GA, Chebel JA, Semaan A, Sarkis J, Mansour R, Mjaess G, Albisinni S, Absil F, Bollens R, Roumeguère T (2021) Sexual dysfunction due to pudendal neuralgia: a systematic review. Transl Androl Urol 10(6):2500–2511
Dellon AL, Coady D, Harris D (2015) Pelvic pain of pudendal nerve origin: surgical outcomes and learning curve lessons. J Reconstr Microsurg 31(4):283–290
Peng PWH, Tumber PS (2008) Ultrasound-guided interventional procedures for patients with chronic pelvic pain—a description of techniques and review of literature. Pain Physician 11(2):215–224
Robert R, Labat JJ, Bensignor M, Glemain P, Deschamps C, Raoul S, Hamel O (2005) Decompression and transposition of the pudendal nerve in pudendal neuralgia: a randomized controlled trial and long-term evaluation. Eur Urol 47(3):403–8
Possover M (2009) Laparoscopic management of endopelvic etiologies of pudendal pain in 134 consecutive patients. J Urol 181(4):1732–1736
Jottard K, Bruyninx L, Bonnet P, Wachter S (2020) A minimally invasive endoscopic transgluteal procedure for pudendal nerve and inferior cluneal nerve neurolysis in case of entrapment: 3-and 6-month results. The ENTRAMI technique for neurolysis. Int J Colorectal Dis 35(2):361–364
Bollens R, Mjaess G, Sarkis J, Chemaly AK, Nemr E, Daher K, Semaan A, Chebel JA, Absil F, Aoun F (2021) Laparoscopic transperitoneal pudendal nerve and artery release for pudendal entrapment syndrome. Surg Endosc 35(11):6031–6038
Author information
Authors and Affiliations
Contributions
All authors have made a significant contribution to the findings and methods in the paper. All authors have read and approved the final draft and consider the manuscript to be honest work.
Corresponding author
Ethics declarations
Disclosures
Carlo Giulioni, Anastasios D. Asimakopoulos, Filippo Annino, Giulia Garelli, Julien Riviere, Julie Piechaud-Kressmann, Nam-Son Vuong, Laurent Hugo Lopez, Jean-Baptiste Roche, Jean Rouffilange, Jean-Luc Hoepffner, Andrea Benedetto Galosi, Richard Pierre Gaston, Thierry Piechaud, Grégory Pierquet have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 172975 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Giulioni, C., Asimakopoulos, A.D., Annino, F. et al. First case-series of robot-assisted pudendal nerve release: technique and outcomes. Surg Endosc 37, 5708–5713 (2023). https://doi.org/10.1007/s00464-023-10096-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10096-9