Abstract
Introduction
The Endoluminal Functional Lumen Imaging Probe (Endoflip) can be used to provide objective measurements of the gastroesophageal junction during fundoplication, and recent publications have suggested that this device could improve surgical outcomes. However, the impact of operative variables has not been clearly reported. The aim of this study is to determine the effect of these variables on functional lumen imaging probe (FLIP) measurements.
Methods
Following implementation of a standardized operative FLIP protocol, all data were collected prospectively and entered into a quality database. This database was queried for patients undergoing hiatal hernia repair and fundoplication. The protocol utilized various balloon volumes (30 and 40 ml), patient positions (flat and reverse Trendelenburg) and amounts of insufflation (15 mmHg pneumoperitoneum and no pneumoperitoneum).
Results
Between August 2018 and February 2020, 97 fundoplications were performed by a single surgeon. Multivariable analysis without interactions demonstrated that a 40 ml volume fill resulted in significantly higher minimum diameter (Dmin), cross-sectional area (CSA), intra-balloon pressure (IBP) and distensibility index (DI) compared to a 30 ml volume fill (p < 0.001). While reverse Trendelenburg positioning resulted in a significantly higher Dmin, IBP and CSA compared to the flat position (all p < 0.05), there was little impact of positioning on DI. Lastly, pneumoperitoneum significantly increased IBP (p < 0.001) but did not affect Dmin (p = 0.697) or CSA (p = 0.757), which resulted in a significant decrease in DI (p < 0.001) when compared to measurements without pneumoperitoneum. Multivariable analysis allowing for interactions demonstrated significant two-way interactions between balloon volume and pneumoperitoneum (p = 0.047), as well as patient position and pneumoperitoneum (p < 0.001).
Conclusion
Surgeons should consider balloon volume and the presence or absence of pneumoperitoneum when interpreting distensibility during or after fundoplication. Additionally, we suggest a formal standardized protocol for FLIP measurements to utilize a 40 ml volume fill in reverse Trendelenburg without pneumoperitoneum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
For decades, surgeons have been striving to perfect the gold-standard operation to treat reflux: the laparoscopic fundoplication. Among these attempts is a general desire to use scientific, objective data to improve the artistry that is a large component of foregut surgery. The intra-operative collection of data, capable of influencing the procedure in real-time, has long been sought, and the Endoluminal Functional Lumen Imaging Probe (Endoflip) (Medtronic; Dublin, Ireland) has the potential to fill this void. While several studies have demonstrated the feasibility of using the functional lumen imaging probe (FLIP) intra-operatively during hiatal hernia repair and fundoplication, the lack of a standardized protocol has hindered generalization and interpretation of the data [1,2,3,4,5]. This is particularly detrimental as recent literature has suggested that the FLIP could be used to improve patient outcomes after fundoplication [1, 2, 4].
The FLIP is a balloon-based catheter that uses impedance planimetry technology to evaluate the geometry of any sphincter in response to volume-controlled distention. The balloon can be filled with various volumes of a specially formulated saline solution, and the minimum diameter (Dmin), cross-sectional area (CSA) and distensibility index (DI) of any sphincter in the gastrointestinal (GI) tract can be measured. While the FLIP has been widely used in the gastroenterology literature, there are several variables present in the operating room during laparoscopic surgery that complicates usage of FLIP technology. The effect of FLIP balloon volume, patient positioning and presence or absence of pneumoperitoneum has not been clearly defined, and this has prevented the development of a standardized intra-operative protocol. For example, while Ilczyszyn et al. found that pneumoperitoneum decreased DI, Nathanson et al. found that pneumoperitoneum increased DI, and both of these studies are limited by small sample sizes [5, 6].
This study aims to determine whether balloon volume fill, patient position and pneumoperitoneum affect FLIP measurements during laparoscopic surgery. By performing FLIP evaluation in a variety of different conditions, we aim to determine how FLIP measurements are impacted by these variables. This will aid interpretation of FLIP data and creation of a protocol in which collection and analysis of future data could be more scientifically sound. We hypothesize that balloon volume and pneumoperitoneum will affect FLIP measurements, specifically distensibility, while patient positioning will have no effect.
Materials and methods
Data collection
Once appropriate institutional review board approval was obtained, we performed a query on a prospectively maintained quality gastroesophageal (GE) database for all patients undergoing fundoplication after institution of a standardized FLIP protocol. Due to the quality nature of the database, consent for participation is not required at our institution; HIPAA compliance was maintained throughout the study. Any patient presenting with a GE chief complaint is added to the database. Utilizing the electronic medical record, research associates collect pre-operative (e.g., age, BMI, presenting symptoms) and intra-operative (e.g., operative length, mesh use) data, and any missing information is clarified with the attending surgeon in a timely manner.
Endoluminal functional lumen imaging probe (endoflip)
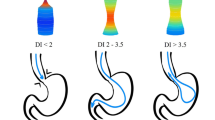
The FLIP catheter is 240 cm long, with an outer diameter of 3 mm. At the distal end is a balloon, and housed within the balloon are 17 impedance planimetry sensors along with excitation electrodes at either end of the balloon. The excitation electrodes emit a constant low current through an electrical field, created by a specially formulated saline solution of known conductivity. Using the voltage drop between sensors and leveraging Ohm’s law, the cross-sectional area between each pair of sensors can be measured. Additionally, a solid-state pressure transducer at the distal end of the catheter measures intra-bag pressure; dividing CSA by intra-bag pressure provides the distensibility index (DI).
Operative protocol
All operations were performed by a single surgeon (MBU) in a standardized fashion. After induction of general anesthesia and establishment of pneumoperitoneum (15 mmHg), the crus was dissected and if present, the hiatal hernia was reduced. Dissection was complete once there was at least 3 cm of intra-abdominal esophagus. A cruroplasty was then performed with three permanent, interrupted posterior sutures. The repair was buttressed with absorbable mesh in cases of paraesophageal hernia or very large crural defects. Fundoplications were then performed over a traditional bougie or the FLIP balloon at the discretion of the surgeon. Any patient with esophageal dysmotility underwent a partial fundoplication, as did patients with normal motility and normal FLIP measurements following hernia reduction. Any patient with normal esophageal motility and abnormal FLIP measurements following hernia reduction underwent a complete (Nissen) fundoplication.
Intra-operative FLIP protocol
A standardized intra-operative FLIP protocol was instituted in August 2018 using an Endoflip 1.0 unit and an 8 cm catheter (EF-325). FLIP evaluation was performed in the operating room by one of four research fellows (BS, MA, HW or ZC). For each case, the catheter pre-check process was complete and pressure was referenced to atmospheric pressure. FLIP evaluation was performed at the following timepoints throughout the operation:
-
1.
After intubation—“Initial” measurement
-
2.
After crural dissection and/or hernia reduction
-
3.
After cruroplasty
-
4.
After fundoplication and removal of bougie—“Final” measurement
For each measurement, the catheter was placed transorally and advanced into the stomach. The balloon was inflated to 20 ml, then slowly withdrawn until an hourglass shape was seen on the FLIP monitor. The balloon was then inflated to 30 ml and left in place for 30 s to allow for stabilization. Minimum diameter (Dmin), cross-sectional area (CSA), intra-balloon pressure (IBP) and distensibility index (DI) were then recorded with the following parameters:
-
1.
Insufflated, reverse Trendelenburg, 30 ml volume fill
-
2.
Insufflated, reverse Trendelenburg, 40 ml volume fill
-
3.
Insufflated, flat, 40 ml volume fill
-
4.
Insufflated, flat, 30 ml volume fill
-
5.
Desufflated, flat, 30 ml volume fill
-
6.
Desufflated, flat, 40 ml volume fill
-
7.
Desufflated, reverse Trendelenburg, 40 ml volume fill
-
8.
Desufflated, reverse Trendelenburg, 30 ml volume fill
If the FLIP catheter was unable to be passed due to a paraesophageal hernia, initial measurements were not obtained and the first set of FLIP measurements were obtained after the hernia was completely reduced.
Statistical analysis
Patient demographics, operative details and FLIP measurements were summarized using mean ± standard deviation or frequency with percentage. Multivariable mixed-effects linear regression with random intercepts was used to assess differences in FLIP measurements between intra-operative variables, while controlling for age, BMI, sex, timepoint in operation, hernia type, presence of mesh, fundoplication type and re-do operations. Main effects models (without interactions), in addition to models allowing for two- and three-way interactions between intra-operative variables, were analyzed. All statistical analysis was performed using SAS 9.3 (SAS Institute, Cary, NC) with two-tailed tests and a significance level of p < 0.05.
Results
Patient demographics
Between August 2018 and February 2020, 97 patients underwent laparoscopic fundoplication and intra-operative FLIP evaluation following a standardized protocol. Patient demographics and intra-operative details are shown in Table 1.
FLIP measurements change between individual operative steps
A summary of the FLIP measurements obtained during surgery are shown in Table 2. A visual representation of the changes in FLIP measurements are displayed in Fig. 1. All differences from one timepoint to the next were statistically significant for Dmin, IBP, CSA and DI (all p < 0.05). In general, Dmin, CSA and DI all increase after hernia reduction, then decrease with crural closure. The opposite is seen with IBP, in which the pressure drops following hernia reduction, then increases after crural closure. Following fundoplication, Dmin, CSA, IBP and DI all increase slightly.
In 15 out of 58 (25.9%) paraesophageal hernia cases, initial measurements were unable to be performed due to inability to pass the FLIP catheter. Other situations in which initial measurements could not be obtained were as follows: Hemodynamic instability following induction and intubation, FLIP unit in use in another procedure, prolonged delay to case start or surgeon preference.
Distensibility is significantly affected by balloon volume and pneumoperitoneum
Multivariable analysis to compare the effect of intra-operative variables without interactions demonstrated that patient positioning had a significant effect on Dmin, IBP and CSA, but no overall effect on DI (Table 3). On the other hand, a 40 ml volume fill increased all FLIP measurements, including DI, compared to a 30 ml volume fill. Lastly, the presence of pneumoperitoneum did not affect Dmin or CSA, but significantly increased IBP, resulting in an overall decrease of DI.
FLIP measurements are more consistent when obtained without pneumoperitoneum
When allowing for interactions, multivariable analysis indicated that there was no significant interaction between patient position and balloon volume and no significant three-way interaction. However, the effects of both patient position (p < 0.001) and balloon volume (p = 0.047) on DI differed by the presence or absence of pneumoperitoneum. Measurements taken with pneumoperitoneum showed that DI was higher with flat positioning compared to RT (b = 0.28 ± 0.10 mm2/mmHg, p = 0.004) and 40 ml volume fill compared to 30 ml (b = 0.40 ± 0.09 mm2/mmHg, p < 0.001). Without pneumoperitoneum, DI was slightly higher with RT positioning compared to flat (b = 0.21 ± 0.10 mm2/mmHg, p = 0.039) and DI was similar for 40 ml volume fill compared to 30 ml (b = 0.16 ± 0.11 mm2/mmHg, p = 0.123). Figure 2 visualizes these findings by summarizing all measurements obtained after cruroplasty. Insufflated measurements are systematically higher for flat positioning and 40 ml volume fill, however, desufflated measurements are fairly similar regardless of position and fill.
Summary of mean distensibility measurements obtained after cruroplasty. Mean insufflated measurements range from 1.49 to 2.20 mm2/mmHg. All pairwise comparisons are significantly different (all p < 0.001), except 30 ml flat vs. 40 ml reverse Trendelenburg (RT) (p = 0.998). Mean desufflated measurements range from 2.48 to 2.85 mm2/mmHg. All pairwise comparisons are statistically different (all p < 0.001), except 30 ml flat vs. 40 ml flat (p = 0.400) and 30 ml RT vs. 40 ml RT (p = 0.225)
Discussion
This is the first study to look specifically at intra-operative variables that affect FLIP measurements and to evaluate their potential interactions. The evidence provided in this study shows that while balloon volume and pneumoperitoneum have a significant effect on FLIP distensibility, patient positioning had little overall effect. Additionally, FLIP measurements obtained without pneumoperitoneum seem to be more consistent than those obtained with pneumoperitoneum.
While patient position did not appear to have a significant overall effect on distensibility (Table 3), measurements recorded in RT resulted in higher Dmin, IBP and CSA. Since distensibility is calculated by dividing CSA by IBP, it is likely that the proportional increase in these parameters resulted in minimal change of the DI. Alternatively, when examining interactions between patient position and pneumoperitoneum, we demonstrated that when desufflated, RT positioning resulted in higher DI than flat, but when insufflated, RT positioning resulted in lower DI than flat. These opposing effects may also explain why there is no overall significant difference in DI with positional changes (Fig. 3).
Keeping in mind that DI is calculated by dividing CSA by IBP, the interaction seen between insufflation and positioning is not surprising. As demonstrated by the multivariable analysis, the presence of pneumoperitoneum results in a significant increase of IBP but no change in the CSA. The external pressure of pneumoperitoneum is high enough to transfer into the balloon to increase IBP, but not strong enough to force an increase of Dmin or CSA, and the result is a significant decrease in DI. Because the FLIP balloon is infinitely compliant, in RT, more of the balloon’s fluid is displaced distally and subject to the pressure of pneumoperitoneum, resulting in a lower DI. Conversely, without pneumoperitoneum, there is no external pressure on the intra-abdominal portion of the FLIP balloon, resulting in a higher DI due to the smaller denominator.
The interaction between insufflation and balloon volume can be similarly explained by the external pressure of pneumoperitoneum on the FLIP balloon. When desufflated, the increased balloon volume results in a proportional increase in CSA and IBP since there is no external pressure created by pneumoperitoneum. This minimizes the change in DI between 30 and 40 ml volume fill. However, when insufflated, the increased balloon volume results in a proportionally larger increase in CSA compared to IBP, resulting in a larger DI at 40 ml versus 30 ml volume fill.
At first glance, our findings seem to contradict those of Nathanson et al., who completed FLIP evaluation on 50 healthy patients undergoing laparoscopic surgery for a non-gastroesophageal complaint [6]. They found that pneumoperitoneum alone resulted in an increase in DI and attributed this to the upward displacement of the diaphragm exerting traction on the hiatal crura and gastroesophageal junction (GEJ). Interestingly, when looking at only our initial measurements (prior to dissection), pneumoperitoneum also appears to generally result in an increase in DI; however, at all subsequent time points, pneumoperitoneum results in a decrease in DI (Fig. 1). This suggests that it is feasible that upward traction of the diaphragm as a result of pneumoperitoneum causes an increase in DI, however, once the hiatus is dissected and the crura freed from the attachments to the esophagus, this effect no longer exists and the impact of pneumoperitoneum is the opposite. A study by Teitelbaum et al. supports this hypothesis, as they saw a significant increase in DI after release of pneumoperitoneum following laparoscopic Heller myotomy [7]. That being said, Ilczyszyn et al. performed FLIP evaluation during laparoscopic Nissen fundoplication and found a significant decrease in initial DI with pneumoperitoneum alone, however, their sample size was small with only 17 patients [5].
As a result of our findings, we would recommend a standardized intra-operative FLIP protocol during hiatal hernia repair and fundoplication to consist of measurements recorded in reverse Trendelenburg, 40 ml volume fill and without pneumoperitoneum. While positioning did not appear to have a large effect on DI, obtaining measurements in RT would minimize the need for position change during the operation and decrease time required to obtain measurements. And although 40 ml volume fill results in an increase in all FLIP measurements as compared to a 30 ml volume, GEJ distention only occurs when the more compliant ends of the balloon are first filled to capacity, allowing IBP to increase with added volume [8]. Therefore, we feel a 40 ml volume fill will provide more consistently reliable measurements. Lastly, measurements obtained without pneumoperitoneum are more consistent and less affected by patient position or balloon volume compared to those obtained with pneumoperitoneum. Additionally, this also allows for intra-operative FLIP evaluation to be comparable to FLIP data reported in the gastroenterology literature.
There are several limitations to our study. As demonstrated in Table 2, not every patient had a FLIP measurement recorded during every step of the operation. This may have been due to catheter malfunction, case duration, research fellow availability, surgeon preference or anesthesiologist preference. This is particularly relevant to the “Initial Measurements” in which the sample sizes are small. We have found that the initial measurements, especially in patients with large paraesophageal hernias, are not always reliable and do not affect our intra-operative decision making. For this reason, the initial measurements are not always collected. Despite this, our sample size remains relatively large in most cases and mixed-effects modeling with random intercepts accounts for missing data. Additionally, we did not specifically examine the effect of anesthesia on FLIP measurements, however, prior studies have suggested that there is no effect on general anesthesia and distensibility measurements [6, 9].
Conclusion
There is potential for the FLIP to enhance anti-reflux surgery and improve patient outcomes, however, its adoption is hampered by a lack of standardization regarding its use in the operation room as well as a poor understanding regarding the effect of intra-operative variables on FLIP measurements. Our study demonstrates that balloon volume and pneumoperitoneum have significant effects on distensibility and that we should consider a standardized intra-operative FLIP protocol for obtaining measurements with a 40 ml volume balloon with patients in reverse Trendelenburg and no pneumoperitoneum. Utilizing this standardized protocol will enhance future research by allowing data to be pooled and to allow for meaningful comparisons across studies.
References
Su B, Novak S, Callahan ZM, Kuchta K, Carbray J, Ujiki MB (2020) Using impedance planimetry (EndoFLIPTM) in the operating room to assess gastroesophageal junction distensibility and predict patient outcomes following fundoplication. Surg Endosc 34:1761–1768. https://doi.org/10.1007/s00464-019-06925-5
Turner B, Helm M, Hetzel E, Gould JC (2019) Is that “floppy” fundoplication tight enough? Surg Endosc. https://doi.org/10.1007/s00464-019-06947-z
DeHaan RK, Davila D, Frelich MJ, Gould JC (2017) Esophagogastric junction distensibility is greater following Toupet compared to Nissen fundoplication. Surg Endosc 31:193–198. https://doi.org/10.1007/s00464-016-4956-0
Kim MP, Meisenbach LM, Chan EY (2018) Tailored fundoplication with endoluminal functional lumen imaging probe allows for successful minimally invasive hiatal hernia repair. Surg Laparosc Endosc Percutan Tech 28:178–182. https://doi.org/10.1097/SLE.0000000000000527
Ilczyszyn A, Botha AJ (2014) Feasibility of esophagogastric junction distensibility measurement during Nissen fundoplication. Dis Esophagus 27:637–644. https://doi.org/10.1111/dote.12130
Nathanson LK, Brunott N, Cavallucci D (2012) Adult esophagogastric junction distensibility during general anesthesia assessed with an endoscopic functional luminal imaging probe (EndoFLIP®). Surg Endosc 26:1051–1055. https://doi.org/10.1007/s00464-011-1996-3
Teitelbaum EN, Boris L, Arafat FO, Nicodème F, Lin Z, Kahrilas PJ, Pandolfino JE, Soper NJ, Hungness ES (2013) Comparison of esophagogastric junction distensibility changes during POEM and Heller myotomy using intraoperative FLIP. Surg Endosc 27:4547–4555. https://doi.org/10.1007/s00464-013-3121-2
Kwiatek MA, Kahrilas K, Soper NJ, Bulsiewicz WJ, McMahon BP, Gregersen H, Pandolfino JE (2010) Esophagogastric junction distensibility after fundoplication assessed with a novel functional luminal imaging probe. J Gastrointest Surg 14:268–276. https://doi.org/10.1007/s11605-009-1086-1
Campagna RAJ, Carlson DA, Hungness ES, Holmstrom AL, Pandolfino JE, Soper NJ, Teitelbaum EN (2019) Intraoperative assessment of esophageal motility using FLIP during myotomy for achalasia. Surg Endosc. https://doi.org/10.1007/s00464-019-07028-x
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr Ujiki received speaker payments from Medtronic for instructional courses on the use of Endoflip. Disclosures outside the scope of this work: Drs. Linn, Haggerty, and Ujiki receive payment for lectures from Gore. Dr. Ujiki is a board member for Boston Scientific, is a paid consultant to Olympus and Apollo, and receives payment for lectures from Apollo and Erbe. Dr. Haggerty received consultant and speaker fees from the renal division of Medtronic for work with peritoneal dialysis catheters and insertion techniques, development of educational materials, and serving as a lecturer and proctor for hands-on courses. Drs. Bailey Su, Mikhail Attaar, Harry Wong, Zachary Callahan, Woody Denham, Ms. Kristine Kuchta and Mr. Stephen Stearns have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Su, B., Attaar, M., Wong, H. et al. Using a standardized intra-operative endoflip protocol during fundoplication to identify factors that affect distensibility. Surg Endosc 35, 5717–5723 (2021). https://doi.org/10.1007/s00464-020-08034-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-08034-0






