Abstract
Introduction
Incisional negative pressure wound therapy (iNPWT) may reduce surgical site infections (SSI), which can have devastating consequences after incisional hernia repair. Few comparative studies investigate the effectiveness of this wound management strategy in this population. The objective of this study is to determine the effect of iNPWT on the incidence of SSI after complex incisional hernia repair.
Methods
All adult patients undergoing open incisional hernia repair at a single center from 2016 to 2019 were reviewed. A commercial iNPWT dressing was used at the discretion of the surgeon. Patients were grouped by type of dressing; iNPWT and standard sterile dressings (SSD). Coarsened exact matching was used to create balanced cohorts for comparison using age, sex, American Society of Anesthesiologists classification, wound classification, and surgical urgency. The primary outcome was the composite incidence of superficial and deep SSI within 30 days. Secondary outcomes included non-infectious surgical site occurrences (SSO), overall complications, length of stay (LOS), emergency department visits, and readmission at 30 days.
Results
134 patients underwent complex hernia repair, with 114 patients included after matching (34 iNPWT, 51 SSD). Composite incidence of superficial and deep SSI was 19.3% (11.8% vs. 27.5%, p = 0.107), with significantly lower rates of deep SSI in patients receiving iNPWT (2.9% vs. 17.6%, p = 0.045). After accounting for residual differences between groups, iNPWT was associated with decreased incidence of composite SSI (RR 0.36, 95% CI [0.16, 0.87]). Median LOS was longer in patients with iNPWT (7 vs. 5 days, p = 0.001). There were no differences in SSO, overall complications, readmission, or emergency department visits.
Conclusion
In patients undergoing incisional hernia repair, the use of iNPWT was associated with a lower incidence of SSI at 30 days. Future studies should focus on cost effectiveness of iNPWT, its impact on long-term hernia recurrences, and the identification of patient selection criteria in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients undergoing complex incisional hernia repair (IHR) are at high risk for a range of wound complications [1]. In these procedures, the incidence of wound complications including surgical site infection (SSIs) ranges from 15 to 46% [2]. Together these complications are called surgical site occurrences (SSOs) and include SSI, hematoma, seroma, wound dehiscence, and enterocutaneous fistula. The consequences of these wound complications can be devastating, especially in the context of complex IHR with prosthetic mesh. SSI is associated with hernia recurrence, and mesh infection will often require reintervention, long-term antibiotic therapy, protracted wound care, and possible mesh excision [3, 4]. These outcomes are costly [5, 6] and associated with poor quality of life [7].
Negative pressure wound therapy (NPWT) was developed in the 1990s to assist in wound healing. It consists of a sealed foam dressing through which suction is applied via tubing to draw exudate and liquid material from the wound. NPWT has been widely used to treat open and chronic wounds [8]. Since the early 2010’s, the use of incisional negative pressure wound therapy (iNPWT) on closed surgical incisions has been proposed as a means to reduce surgical site occurrences, including SSI. At least two proprietary iNPWT systems are commercially available and in clinical use today. However, the effectiveness of iNPWT has yet to be established [9,10,11]. In complex IHR, evidence regarding the use of iNPWT is equivocal and of low quality overall. Despite the lack of evidence, iNPWT remains of particular interest as a wound management strategy given the high incidence of SSOs and their potentially devastating consequences. iNPWT may be most effective in patients at higher risk of wound complication, and may be cost-effective when the SSI incidence is as high as 16% [12]. Further evidence to guide selection criteria for this intervention is needed, especially in resource conscious settings. The objective of this study is to estimate the impact of iNPWT on the incidence of superficial and deep SSI in a matched cohort of adult patients undergoing complex IHR.
Methods
Study design
We performed a retrospective matched cohort analysis of patients undergoing complex IHR repair at a single university hospital from January 2016 to December 2019. The study was approved by the institution’s Research Ethics Committee and access to patient charts was obtained from the institution’s Director of Professional Services in lieu of individual informed consent of participants. No industry funding or outside sponsorship was provided for this study. We included adult patients undergoing open IHR involving component separation or mesh greater than 16 × 9 cm, and who met criteria for “complex abdominal hernia” as per a consensus-based definition by Slater et al. [13]. Both emergency and elective cases were included. Stoma and dirty cases were included as iNPWT was also employed at the surgeon’s discretion in these scenarios. Day surgeries, cases without primary wound closure at the time of surgery, and patients with post-operative follow-up less than 30 days were excluded.
Surgical technique
Complex IHR were performed by a total of 7 surgeons with practice interest in hernia surgery. All patients received pre-operative antibiotics. The choice of repair was decided according to hernia characteristics and surgeon expertise, with a preference for retro-rectus repair when possible. Where tension-free approximation of the facia was not achieved, component separation was routinely performed to ensure facial closure with mesh reinforcement. Bridging repairs were avoided, with transversus abdominis release (TAR) being the preferred method of component separation if required. In the beginning of the study period, anterior component separation with external oblique release (EOR) was also performed. Mesh reinforcement was used in almost all cases, with a preference for extraperitoneal placement. In an extraperitoneal position, self-fixating Parietex™ ProGrip™ mesh (Medtronic, Mansfield, MA) was most common, while Parietex™ composite mesh was most commonly used in the intraperitoneal position. Where permanent synthetic mesh could not be used, slowly resorbing GORE®BIO-A® Tissue Reinforcement (Gore Medica, Flagstaff, AZ) or absorbable VICRYL® mesh (Ethicon, Cincinnati, OH) was used. Skin flaps were performed as needed, and concomitant musculocutaneous flaps or panniculectomy were performed by a plastic surgeon in selected cases. Surgical drains were routinely placed in sub-fascial and subcutaneous planes and removed on follow-up when drainage was minimal. Skin was most commonly closed with skin clips and abdominal dressings were placed immediately after closure.
Intervention
A proprietary negative pressure dressing (PREVENA™ incision management system, KCI San Antonio, TX) was available since October 2018 and was used at the discretion of the surgeon. Non-proprietary negative pressure dressings were also constructed with available NPWT supplies. Patients with these ‘home-made’ dressings were included in the iNPWT group and recorded as ‘home-made.’ All iNPWT dressings were placed directly to closed skin (without any penetrating inter-digitations), and were removed 5 to 7 days post-operatively. Standard sterile dressings (SSD) consisted of non-adherent sterile gauze secured with an adhesive border or adhesive tape. SSD were placed in the operating room and removed on post-operative day 2. Post-operative antibiotics were not routinely given, except in the presence of active infection.
Outcomes and covariates
Conventional demographics, comorbidities as per the Charlson Comorbidity Index, and operative characteristics were collected. Risk factors for surgical site occurrences including obesity, smoking within 1 year, diabetes, COPD, immunosuppression, presence of stoma, prior hernia repairs, and history of wound infection were collected. Operative details including technique of component separation, and mesh use, material, and position were recorded. The creation of a new stoma, the use of closed suction drains, and the involvement of a plastic surgeon were also recorded. Cases were identified as including a ‘skin flap’ in all anterior component separations and in procedures where the development of a skin flap via subcutaneous undermining was mentioned specifically by the surgeon in the operative note. A ‘tissue flap’ denoted the use of a myocutaneous flap for tissue coverage performed by a plastic surgeon.
Risk of surgical site infection was assessed using the 3-level Modified Hernia Grading Scale (MHGS) scale [2] and Ventral Hernia Risk Scores (VHRS) for SSI and SSO [14]. The MHGS was adapted from the 4-level Ventral Hernia Working Group (VHWG) grading scale [1] and classifies open hernia repairs into three grades depending on the presence of patient-level risk factors and surgical contamination: grade 1 (low risk); grade 2 (co-morbid patients); and grade 3 (contaminated cases). The VHRS for SSI is a prospectively validated risk score that was found to more accurately predict SSO and SSI compared to the VHWG grade [2, 15]. The VHRS ranges from 0 to 16 points for SSI and 0 to 15 points for SSO. Points are used to create risk groups and are calculated based on the presence of 6 risk factors: use of a mesh implant; concomitant hernia repair; creation of skin flaps; American Society of Anesthesiologists (ASA) class 3 or greater; Body Mass Index (BMI) 40 or greater; and wound class 4.
The primary outcome was a composite measure of superficial and deep SSI within 30 days, following the Center for Disease Control definitions [16, 17]. The composite measure included wound infection involving only skin and subcutaneous tissue (superficial) or the muscle and fascia layers of the abdominal wall (deep). Intraabdominal infection were classified as “organ/space SSI” as per CDC definitions, and were not included in the composite outcome. Secondary outcomes included non-infectious SSOs, overall complications, length of stay (LOS), emergency department visits, and readmission within 30 days. Late SSIs and hernia recurrence beyond 30 days were also collected, with follow-up until 180 days post-operatively.
Coarsened exact matching and statistical analysis
Statistical analysis was performed in R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria) and coarsened exact matching (CEM) was performed using the CEM package [18]. Similar to other matching methods, CEM is used to control for confounding introduced by imbalances in baseline patient-level characteristics between treatment and control groups [19, 20]. Compared to propensity score matching, CEM may produce less variance and bias in estimates of causal effect [21]. Matching also served to account for selection and confounding bias introduced by the use of iNPWT at the surgeon’s discretion. Patients were grouped by type of dressing used: iNPWT vs. standard sterile dressings (SSD). Selecting among patients operated prior to the availability of iNPWT, we used CEM to create balanced cohorts for comparison using age, sex, ASA class, wound contamination, and surgical urgency.
Chi-squared or Fisher’s Exact tests and Student’s T tests or Kruskal–Wallis tests were performed for comparison of categorical and continuous variables, respectively. For 30-day outcomes, multiple logistic regression was performed to account for additional differences between groups that may have introduced confounding. Clinically relevant variables were tested in a step-wise approach to select a model using a Bayesian information criterion. The final model included iNPWT treatment, technique of component separation, VHRS for SSI, and smoking exposure. Risk ratios were estimated using marginal standardization from logistic regression. For outcomes beyond 30 days, Kaplan–Meier curves were used to describe SSI occurrence and log-rank tests were used to compare cumulative probabilities of SSI between groups. A cox proportional hazard model was used to evaluate iNPWT as a predictor of SSI after adjusting for technique of component separation, smoking exposure, and BMI.
Sensitivity analysis for loss to follow-up was performed. Survival analysis was repeated after including cases with less than 30 days of follow-up. To evaluate the consequence of missed SSIs among cases lost to follow-up, multiple logistic regression was repeated after reclassifying iNPWT cases lost to follow-up as having developed deep or superficial SSI within 30 days. Regression was used to adjust for VHRS for SSI, procedure duration, and smoking exposure in this analysis.
Results
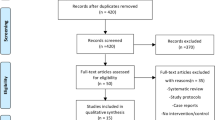
Among 245 ventral hernia repairs performed during the study period, 134 met the criteria for complex IHR. An additional 9 cases were excluded due to failure to achieve abdominal wall or skin closure at the time of surgery, and 11 cases were excluded due to inadequate follow-up. A total 114 cases were included prior to matching (Fig. 1). Overall, the incidence of composite SSI in the cohort was 19.3% at 30 days. Median follow-up was 164 days, with 19% follow-up beyond 1 year, and 6% beyond 2 years.
Flow diagram. aCases were excluded if they did not meet at least one criteria for “complex” abdominal wall hernia as per consensus definition by Slater et al. [13] (defect size and location, patient history and risk factors, contamination and soft tissue condition, and clinical scenario)
After CEM, a total of 85 patients were retained in the matched cohort, with 34 patients receiving iNPWT, and 51 matched controls receiving SSD. The groups were similar with respect to age, sex, BMI, and ASA scores, and comorbidities (Table 1). Certain individual risk factors for wound complications were somewhat more prevalent in the iNPWT group. These included diabetes and smoking in the year of surgery, although these did not reach statistical significance. Prior wound infection was more common in the iNPWT group, as was the frequency of multiple prior hernia repairs. All matched cases were performed on an elective basis (Table 2). Contamination class was similar, the use of closed suction drains, tissue flaps was similar between groups.
Procedure durations were longer in the iNPWT group, with more patients undergoing posterior component separation and extraperitoneal mesh placement. The creation of skin flaps was also more common in the iNPWT group. The distribution of MHGS was similar between groups, although VHRS for SSO differed between groups with a larger proportion of patients receiving iNPWT in higher risk groups (Table 3).
At 30 days post-operatively, the crude incidence of the composite SSI outcome was 21.1% with no statistically significant difference between the iNPWT and SSD groups in the matched cohort (Table 4). The incidence of deep SSI, however, was significantly lower in the iNPWT group (2.9% vs. 17.6%, p = 0.045). Overall the incidence of non-infectious SSOs were similar between groups, as were interventions for wound complications. Median length of stay was longer among patients receiving iNPWT (7 vs 5 days, p = 0.001). There was no difference in all complications, mortality, hospital readmissions, or emergency department visits. Results of multivariate logistic regression are shown in Table 3. After adjusting for the technique of component separation, VHRS for SSI, and smoking exposure, iNPWT predicted a lower incidence of composite SSI (RR 0.37 95% CI [0.15–0.87]).
The incidence of SSI beyond 30 days was 11.8% in the matched cohort (Table 4). There were no differences in late SSOs or hernia recurrence at 180 days. On Kaplan–Meier analysis, SSI did not significantly differ between groups in either the matched or unmatched cohorts with follow-up up to 180 days (Fig. 2). In multiple Cox proportional hazards regression, after adjusting for possible confounders, iNPWT was not significantly associated with SSI when late cases were included (Table 5). Among 18 patients with SSI at 30 days, 1 patient (4.5%) had a sterile seroma drained prior to developing infection. Among those with SSI beyond 30 days, 2 patients (16%) had a prior sterile intervention for seroma.
Kaplan–Meier curves for late surgical site infection. Kaplan–Meier curves showing cumulative probability of follow-up without surgical site infections (SSI) vs time in days in the full cohort (FC) and in the matched cohort (MC). Follow-up is limited to 180 days. Cumulative probabilities are compared using the Log-rank test and p values are reported below the curves. A Composite SSI in FC. B Composite SSI in MC. C Superficial SSI in FC. D Superficial SSI in MC. E Deep SSI in FC. F Deep SSI in MC
Among 11 cases excluded due to less than 30 days of follow-up, 9 received SSD and 2 received iNPWT. After the inclusion of these cases in matching, survival analysis supported the association between iNPWT and decreased incidence of deep SSI at 30 days (Fig. 3). Sensitivity analysis for the impact of missed SSIs in cases lost to follow-up (Table 6) suggested a preserved association between iNPWT and decreased SSI incidence, even when assuming the maximal effect of this potential bias (RR 0.45, 95% CI [0.20–1.01]).
Kaplan–Meier curves for deep SSI outcomes after inclusion of cases lost to follow-up. Kaplan–Meier curves showing cumulative probability of follow-up without surgical site infections (SSI) vs time in days. Includes cases excluded from primary analysis due to less than 30 days of follow-up. Cumulative probabilities are compared using the Log-rank test and p values are reported below the curves. A Deep SSI up to 30 days of follow-up. B Deep SSI up to 180 days of follow-up. SSD standard sterile dressing, iNPWT incisional negative pressure wound therapy
Discussion
iNPWT is a novel wound management strategy that has been used in complex IHR and other abdominal operations [10, 22, 23]. However, evidence regarding its effectiveness in IHR remains equivocal and of low quality. This study evaluated the effectiveness iNPWT among patients undergoing complex IHR at a university hospital, where iNPWT was used at the discretion of the operating surgeon. In this retrospective matched cohort analysis, we found a significantly decreased incidence of deep SSI among patients receiving iNPWT compared to SSD in matched historical controls at 30 days.
NPWT has been widely adopted in the management of open wounds [8]. More recently, iNPWT has been proposed as an effective strategy in the prophylaxis of SSIs and wound complications for closed surgical wounds. The application of negative pressure is thought to stimulate wound healing through several mechanisms. Negative pressure may improve capillary circulation and oxygen delivery at the wound site [24] while removing excess exudate and debris from the wound. The iNPWT’s barrier may also promote sterility and a favorable environment for healing. Mechanical offloading of tension at the wound site may also promote apposition of the wound edges, and is of particular relevance in large abdominal incisions associated with complex IHR [25]. A role for iNPWT in preventing intraabdominal infection is not supported by these mechanisms. However, the abdominal wall fascia and the potential spaces created during IHR are continuous with more superficial layers of the wound. An effect of iNPWT at the level of the deep soft tissues of an incision is plausible. The reduction in deep SSI observed in the study is consistent with these proposed mechanisms of action and supports a role for iNPWT in the prevention of SSI after complex IHR.
Our results were consistent with other published retrospective studies in this population. A recent meta-analysis of 11 studies evaluating iNPWT on wound complications in complex IHR reported a 50% reduction in SSI and wound separation in a pooled analysis [22]. The review included a predominance of small retrospective studies (9 retrospective studies) and two RCTs involving oncologic resections. The results of these two RCTs may not be applicable to complex IHR, as wounds associated with IHR differ importantly from midline laparotomy incisions used in oncologic resection, especially with regards to undermining and use of mesh. We identified eight studies that have specifically investigated iNPWT in the context of IHR since 2012. Four studies supported an effect of iNPWT in reducing SSI [26,27,28,29]. With the exception of one case series of 199 cases, these were small retrospective studies that compared iNPWT to historical controls. All but one reported consecutive cases by a single surgeon, which strengthened their internal validity. Only two provided estimates of effect size and measures of confidence. Four studies found no difference in SSI with iNPWT [30,31,32,33]. These include two studies with more than 100 cases. All four used iNPWT at the surgeon’s discretion, and most included cases performed by different surgeons and using different approaches. This variability may have introduced bias but may also lend to the external validity of their results.
Together, these studies had several limitations, including a heterogeneity in surgical approach, patient characteristics, and iNPWT design and duration. However, these limitations reflect real-world challenges in the implementation and evaluation of surgical interventions. Confounding and selection bias are limitations in several of these studies. In a resource conscious setting, iNPWT may be targeted to patients who are thought to benefit most—that is, patients with risk factors for SSI. In this context, the use of iNPWT at the discretion of the surgeon precludes a direct before-and-after comparison. Several studies have avoided this source of bias by reporting consecutive series performed by a single surgeon [28, 29, 34, 35]. Others, however, make no explicit attempt to account for this.
In this study, we attempt to mitigate the effect of selection and confounding bias by employing a matched cohort analysis, selecting controls from a cohort whose surgery was performed prior to the availability of a proprietary iNPWT device at our institution. Residual difference between the iNPWT and SSD groups after matching appeared to favor a lower risk of SSI among the controls, thereby underestimating the effect of iNPWT. In other words, patients in whom iNPWT was selected were at higher risk of SSI compared to patients who received SSD. Based on clinical judgement and salient differences between groups, we performed regression analysis adjusting for technique of component separation, VHRS for SSI and smoking exposure. Even after accounting for these variables, iNPWT was associated with an 11.4% absolute reduction in SSI incidence compared to SSD, corresponding to a number needed to treat of 7.
Our regression analysis suggests that iNPWT may be effective across all MHGS grades, however, we were limited by sample size and number of events to meaningfully estimate effects across strata of hernia grade. Other studies, including a case comparative study of 199 consecutive cases by Soares et al., found that iNPWT reduced SSI only in higher grade hernias (MHGS Grade 2 and 3) [29]. This group has subsequently published two case series demonstrating dramatic reductions in SSI (5.2%) and SSO (12.9%) using their HVAC system in high-risk patients [34, 35]. Large comparative studies are needed to evaluate the effect of iNPWT across different risk profiles in order to identify patients who benefit the most from this intervention.
Duration of follow-up ranges from 30 days [26, 28, 30, 34] to a median of 190 days [33] among studies evaluating iNPWT in complex IHR. Although follow-up does not discernibly influence the distribution of outcomes across studies, few discuss the timing of SSI and SSO occurrence. Soares et al. noted that 90-day follow-up was a particular strength of their study [29], and Vargo et al. noted that all wound complications requiring intervention in their series occurred more than 4 weeks post-operatively [26]. In our study, 35% of all SSIs occurred beyond 30 days, and the differences in SSI incidence between groups were no longer significant on Kaplan–Meier and regression analysis after extending follow-up from 30 to 180 days. Non-infectious SSOs, wound-related interventions, and readmissions were similar between groups in both analyses. These results may suggest that iNPWT improves short-term SSI incidence, but may not translate into better long-term outcomes. Ensuring follow-up beyond 30 days should be considered in subsequent evaluations of iNPWT in IHR.
Our study was limited by its retrospective and observational design. Despite the promising results of prior retrospective studies of iNPWT in other abdominal operations, subsequent large RCTs have failed to demonstrate that benefit [36,37,38]. Another important limitation of our study included the change in surgical technique over time. Similar to Pauli et al. [30], we observed a shift in surgical technique over the course of the study period. Early in the study period, EOR and TAR each accounted for 50% of component separations performed, whereas the proportion of TAR increased to 80% of all component separations by 2019. EOR, which requires extensive skin flaps, has been excluded from some studies of iNPWT [30], while being the primary focus of others [29, 35]. Our study was neither designed nor powered to detect differences between component separation techniques. However, EOR was equivalent between groups, while TAR was performed significantly more often in the iNPWT group. This distribution of component separations between the matched groups would likely favor a higher incidence of SSI in the iNPWT group, thus negatively biasing the effect of iNPWT. Indeed, adjusting for component separation in regression analysis only strengthened the association between iNPWT and lower SSI incidence. Loss to follow-up is another potential limitation of this study. While most patients with a wound complication are likely to seek care, it is possible that certain patients from distant referral sites were treated for SSI at another institution. The impact of this potential bias was unlikely to influence the conclusions of this study however, as a sensitivity analysis supported the association between iNPWT and the decreased incidence of SSI at 30 days. Cost remains an important limitation to the use of iNPWT and evidence from cost evaluation studies of this intervention is limited [10]. Chopra et al. estimated that iNPWT may be cost-effective and potentially cost-saving when SSI incidence is greater than 16% in the context of IHR [12]. Furthermore, cost analyses may only be relevant to the specific healthcare system or institution (both based on purchase cost of the device as well as the baseline incidence of SSIs), and therefore may not be widely generalizable.
Conclusion
In patients undergoing complex IHR, the use of iNPWT was associated with a lower incidence of deep SSI at 30 days. After adjusting for residual differences between groups, a significant association between iNPWT and a composite outcome of deep and superficial SSI was observed. Our results support the pursuit of further prospective evaluations of this intervention, including randomized trials where feasible. The incidence of late SSI beyond 30 days in this cohort underscores the need to include long-term follow-up in subsequent studies. Future studies should focus on the cost effectiveness of iNPWT in this population, and the identification of patient selection criteria for its use.
References
Ventral Hernia Working G, Breuing K, Butler CE, Ferzoco S, Franz M, Hultman CS, Kilbridge JF, Rosen M, Silverman RP, Vargo D (2010) Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery 148:544–558
Kanters AE, Krpata DM, Blatnik JA, Novitsky YM, Rosen MJ (2012) Modified hernia grading scale to stratify surgical site occurrence after open ventral hernia repairs. J Am Coll Surg 215:787–793
Iqbal CW, Pham TH, Joseph A, Mai J, Thompson GB, Sarr MG (2007) Long-term outcome of 254 complex incisional hernia repairs using the modified Rives-Stoppa technique. World J Surg 31:2398–2404
Lauren Paton B, Novitsky YW, Zerey M, Sing RF, Kercher KW, Todd Heniford B (2007) Management of infections of polytetrafluoroethylene-based mesh. Surg Infect 8:337–342
Cox TC, Blair LJ, Huntington CR, Colavita PD, Prasad T, Lincourt AE, Heniford BT, Augenstein VA (2016) The cost of preventable comorbidities on wound complications in open ventral hernia repair. J Surg Res 206:214–222
Plymale MA, Ragulojan R, Davenport DL, Roth JS (2017) Ventral and incisional hernia: the cost of comorbidities and complications. Surg Endosc 31:341–351
Rosen MJ, Bauer JJ, Harmaty M, Carbonell AM, Cobb WS, Matthews B, Goldblatt MI, Selzer DJ, Poulose BK, Hansson BME, Rosman C, Chao JJ, Jacobsen GR (2017) Multicenter, prospective, longitudinal study of the recurrence, surgical site infection, and quality of life after contaminated ventral hernia repair using biosynthetic absorbable mesh: the COBRA study. Ann Surg 265:205–211
Dumville JC, Owens GL, Crosbie EJ, Peinemann F, Liu Z (2015) Negative pressure wound therapy for treating surgical wounds healing by secondary intention. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD011278.pub2
Hyldig N, Birke-Sorensen H, Kruse M, Vinter C, Joergensen JS, Sorensen JA, Mogensen O, Lamont RF, Bille C (2016) Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg 103:477–486
Webster J, Liu Z, Norman G, Dumville JC, Chiverton L, Scuffham P, Stankiewicz M, Chaboyer WP (2019) Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD009261.pub3
World Health Organization (2018) Global guidelines for the prevention of surgical site infections. World Health Organization, Geneva
Chopra K, Gowda AU, Morrow C, Holton L 3rd, Singh DP (2016) The economic impact of closed-incision negative-pressure therapy in high-risk abdominal incisions: a cost-utility analysis. Plast Reconstr Surg 137:1284–1289
Slater NJ, Montgomery A, Berrevoet F, Carbonell AM, Chang A, Franklin M, Kercher KW, Lammers BJ, Parra-Davilla E, Roll S, Towfigh S, van Geffen E, Conze J, van Goor H (2014) Criteria for definition of a complex abdominal wall hernia. Hernia 18:7–17
Berger RL, Li LT, Hicks SC, Davila JA, Kao LS, Liang MK (2013) Development and validation of a risk-stratification score for surgical site occurrence and surgical site infection after open ventral hernia repair. J Am Coll Surg 217:974–982
Liang MK, Goodenough CJ, Martindale RG, Roth JS, Kao LS (2015) External validation of the ventral hernia risk score for prediction of surgical site infections. Surg Infect 16:36–40
Centers for Disease C (2019) National Healthcare Safety Network Surveillance (NHSN) Patient Safety Component Manual 2019
Centers for Disease C (2016) 2014 National and State Healthcare associated infections progress report. Centers for Disease Control
Iacus, M S, King, Gary, Porro, Giuseppe (2018) cem: coarsened exact matching
Stevens GA, King G, Shibuya K (2010) Deaths from heart failure: using coarsened exact matching to correct cause-of-death statistics. Popul Health Metr 8:6
Wells AR, Hamar B, Bradley C, Gandy WM, Harrison PL, Sidney JA, Coberley CR, Rula EY, Pope JE (2013) Exploring robust methods for evaluating treatment and comparison groups in chronic care management programs. Popul Health Manag 16:35–45
Blackwell M, Iacus S, King G, Porro G (2009) Cem: coarsened exact matching in stata. Stata J 9:524–546
Tran BNN, Johnson AR, Shen C, Lee BT, Lee ES (2019) Closed-incision negative-pressure therapy efficacy in abdominal wall reconstruction in high-risk patients: a meta-analysis. J Surg Res 241:63–71
Sahebally SM, McKevitt K, Stephens I, Fitzpatrick F, Deasy J, Burke JP, McNamara D (2018) Negative pressure wound therapy for closed laparotomy incisions in general and colorectal surgery: a systematic review and meta-analysis. JAMA Surg 153:e183467
Xia C-Y, Yu A-X, Qi B, Zhou M, Li Z-H, Wang W-Y (2014) Analysis of blood flow and local expression of angiogenesis-associated growth factors in infected wounds treated with negative pressure wound therapy. Mol Med Rep 9:1749–1754
Wilkes RP, Kilpad DV, Zhao Y, Kazala R, McNulty A (2012) Closed incision management with negative pressure wound therapy (CIM): biomechanics. Surg Innov 19:67–75
Vargo D (2012) Negative pressure wound therapy in the prevention of wound infection in high risk abdominal wound closures. Am J Surg 204:1021–1023
Gassman A, Mehta A, Bucholdz E, Abthani A, Guerra O, Maclin MM Jr, Esposito T, Thomas C (2015) Positive outcomes with negative pressure therapy over primarily closed large abdominal wall reconstruction reduces surgical site infection rates. Hernia 19:273–278
de Vries FEE, Atema JJ, Lapid O, Obdeijn MC, Boermeester MA (2017) Closed incision prophylactic negative pressure wound therapy in patients undergoing major complex abdominal wall repair. Hernia 21:583–589
Soares KC, Baltodano PA, Hicks CW, Cooney CM, Olorundare IO, Cornell P, Burce K, Eckhauser FE (2015) Novel wound management system reduction of surgical site morbidity after ventral hernia repairs: a critical analysis. Am J Surg 209:324–332
Pauli EM, Krpata DM, Novitsky YW, Rosen MJ (2013) Negative pressure therapy for high-risk abdominal wall reconstruction incisions. Surg Infect 14:270–274
Condé-Green A, Chung TL, Holton LH 3rd, Hui-Chou HG, Zhu Y, Wang H, Zahiri H, Singh DP (2013) Incisional negative-pressure wound therapy versus conventional dressings following abdominal wall reconstruction: a comparative study. Ann Plast Surg 71:394–397
Mehdorn M, Niebisch S, Scheuermann U, Gockel I, Jansen-Winkeln B (2019) Incisional negative pressure wound therapy does not reduce surgical site infections in abdominal midline incisions: a case control study. Acta Chir Belg. https://doi.org/10.1080/00015458.2019.1599180
Diaconu SC, McNichols CHL, Ngaage LM, Liang Y, Ikheloa E, Bai J, Grant MP, Nam AJ, Rasko YM (2018) Closed-incision negative-pressure therapy decreases complications in ventral hernia repair with concurrent panniculectomy. Hernia 24(1):49–55
Hicks CW, Poruk KE, Baltodano PA, Soares KC, Azoury SC, Cooney CM, Cornell P, Eckhauser FE (2016) Long-term outcomes of sandwich ventral hernia repair paired with hybrid vacuum-assisted closure. J Surg Res 204:282–287
Rodriguez-Unda N, Soares KC, Azoury SC, Baltodano PA, Hicks CW, Burce KK, Cornell P, Cooney CM, Eckhauser FE (2015) Negative-pressure wound therapy in the management of high-grade ventral hernia repairs. J Gastrointest Surg 19:2054–2061
Blackham AU, Farrah JP, McCoy TP, Schmidt BS, Shen P (2013) Prevention of surgical site infections in high-risk patients with laparotomy incisions using negative-pressure therapy. Am J Surg 205:647–654
Murphy P, Kuper T, Ott M (2019) Negative pressure wound therapy for surgical site infection prevention requires further study before widespread adoption. JAMA Surg. https://doi.org/10.1001/jamasurg.2019.0428
Shen P, Blackham AU, Lewis S, Clark CJ, Howerton R, Mogal HD, Dodson RM, Russell GB, Levine EA (2017) Phase II randomized trial of negative-pressure wound therapy to decrease surgical site infection in patients undergoing laparotomy for gastrointestinal, pancreatic, and peritoneal surface malignancies. J Am Coll Surg 224:726–737
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Feldman has received educational grants from Theator and Merck. Dr. Vassiliou has attended an educational course funded by KCI. Dr. Lee has received an investigator-initiated research grant from Johnson & Johnson. Drs. Hopkins, Eustaches, Fried, Khwaja, and Fata have no conflicts of interest or financial ties to disclose. Ms. Ganescu, Cipolla, and Kaneva have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hopkins, B., Eustache, J., Ganescu, O. et al. S116: Impact of incisional negative pressure wound therapy on surgical site infection after complex incisional hernia repair: a retrospective matched cohort study. Surg Endosc 35, 3949–3960 (2021). https://doi.org/10.1007/s00464-020-07857-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-07857-1