Abstract
Objectives/hypothesis
Intraoperative neuromonitoring (IONM) is a useful adjunct for recurrent laryngeal nerve (RLN) mapping and identification in transoral endoscopic thyroidectomy vestibular approach (TOETVA). This experimental study aimed to investigate the feasibility, safety, thresholds required of an endoscopic forceps that combine the function of surgical dissection and nerve stimulation.
Study design
Prospective experimental research.
Methods
TOETVA was performed in 12 piglets, i.e., 24 RLNs and 24 vagal nerves (VN). RLNs electromyography (EMG) was recorded via endotracheal surface electrodes. Baseline EMG of VN and RLN were recorded and compared by (a) percutaneously placed monopolar stimulator probe (Group I), (b) adapted Maryland endoscopic dissector applied on nerves at its tip-end (Group II) and (c) endoscopic dissector tip-lateral applied (Group III). EMG profiles, amplitude, latency, waveform, thresholds and supra-maximal stimulation (5 mA) were analyzed.
Results
Application of the endoscopic device was feasible in all TOETVA and did not result in any morbidity. 24 RLNs and VNs were detected, stimulated and monitored. With increase of stimulation current, the amplitude of EMG increased, showing a dose–response curve. Mean VN stimulation thresholds were: Group I 0.28 mA, Group II 0.56 mA, Group III 0.58 mA (P1 = 0.00, P2 = 0.00, P3 = 0.11). Minimal current to evoked a maximal VN response was: Group I 0.65 mA, Group II 1.07 mA and Group III 1.14 mA (P1 = 0.00, P2 = 0.00, P3 = 0.48). Minimal current to evoke a RLN maximal response was Group I 0.6 mA, Group II 0.95 mA and Group III 1.05 mA (P1 = 0.00, P2 = 0.00, P3 = 0.31). Latency values were similar to each group. Repetitive (> 10 min) supra-maximal (> 5 mA) electrical stimulation was safe.
Conclusions
The application of endoscopic stimulating dissector is simple, effective and safe way to monitor both VN and RLN function during a TOETVA animal model. It provides surgeons with real-time feedback of EMG response and can be applied as a tool for RLN monitoring. Endoscopic instrument required higher current to evoke EMG response compared to hand probe stimulation. Tip-end required less current to evoke EMG response compared to tip-lateral mode of stimulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
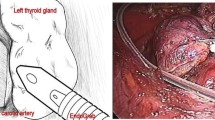
To apply intermitted intraoperative neuromonitoring (IONM) of the recurrent laryngeal nerve (RLN) in transoral endoscopic thyroidectomy vestibular approach (TOETVA), several varieties of electrodes have been proposed as (A) percutaneously placed hand-stimulating probe commonly used in open surgery, (B) long probes placed through the trocar, (C) adapting endoscopic Maryland dissecting instruments to the IONM system, and (D) flexible electrode wires [1,2,3,4], (Fig. 1).
Each mode of application has advantages and disadvantages (Table 1). Simultaneous IONM and thyroid gland dissection requires a combination of techniques and technology whose complex implementation is ill-suited to the conditions of endoscopic surgery. Furthermore, the frequent shifting between the uses of dissecting instruments and stimulating probe are time-consuming [5]. A combination of these two instruments is a worthwhile future direction for IONM.
The purpose of the current experimental study was to investigate the feasibility, safety, threshold application required of an adapted endoscopic Maryland stimulating dissecting instrument, which combines the function of surgical dissection, nerve stimulation and monitoring in TOETVA.
Materials and methods
Setting, regulations, policies and principles
The Institutional Animal Care and Use Program Committee in Research of Jilin University approved this prospective experimental study. The present study was supported by Jilin provincial special fund for healthcare (no. SCZSY201714 and SCZSY201504) and the Outstanding Young Talent Foundation Project of Science and Technology Department in Jilin Province (Grant no. 20170520018JH) in China. The conduct of experimentation on living animals was exclusively by and/or under the close supervision of qualified attending veterinarian personnel.
Animal breeds
Twelve species of Duroc–Landrace piglets provided by the Laboratory Animal Center of Jilin University, were tested.
Sedation, analgesia, and anesthesia
Piglets received induction anesthesia via i.v. thiopental 15 mg/kg administration. Muscle relaxants were avoided during all procedures. General anesthesia was maintained using isoflurane (2.0–3.0%) and oxygen (2.0 L/min). Heart rate was recorded continuously via pulse oximeter photoplethysmography, as electrical stimulation induces artifacts in electrocardiogram recordings. In addition, recorded continuously throughout surgery were arterial oxygen saturation (SaO2), blood pressure, and respiratory rate.
Equipment setting
Intraoperative neuromonitoring system
IONM system (NIM-Response 3.0 System, Medtronic, Jacksonville, Florida, USA) was set with a reduced response threshold to identified small response at 100 µV, stimulation rejection artifact at 2.6 ms, stimulus at 100 µs duration at 4 Hz. Endotracheal tube-based surface electrodes system was applied (Trivantage EMG tube, Medtronic, Jacksonville, Florida, USA). Size 6 to 8 internal diameter (ID) endotracheal EMG tubes were used. Proper tube position was verified by direct visualization after pig neck extension before operation and intraoperatively obtaining first vagal nerve (V1) stimulation value > 500mcV.
Neuromonitoring stimulation probe
A ball tip (1.0 mm) monopolar stimulating probe (incrementing stimulating probe, Medtronic, Jacksonville, Florida, USA) 10-cm handle and 9-cm shaft, was used for percutaneous nerve stimulation. Skin was pierced with an 18-gauge syringe needle (diameter, 1.2 mm), then the stimulation probe was introduced (Fig. 2).
Endoscopic stimulating dissecting instruments
We developed a prototype of endoscopic stimulating dissecting instruments, which combines the function of endoscopic dissection and nerve stimulation. Conventional dissecting instruments (forceps) was connected to the monitor by a stimulation wire. In detail, an endoscopic Maryland dissecting instrument (code DJ-FL05, Kangji Medical Equipment co, Ltd. China), 330 mm length and 5 mm diameter was tested for the current study. Device tips length are 18 mm long, with an opening angle ≥ 50°. The instrument offers 360° rotation (Fig. 3A, C). 2-mm tips of the forceps are exposed, and can be applied at tip-end and/or tip-lateral (Fig. 3B). Tips material are made by steel, usable. The device is a commercially available dissecting and needle holder instrument that complies with the Chinese standards. The clinical engineer adapted the instrument to the IONM connective box through a cable. The one end of the cable is connected to the connective box as to the traditional intermitted nerve monitoring probe (Fig. 3C), the other end connected to the dissecting instrument (Fig. 3D). IONM system set-up is the same as intermittent monitoring probe mode of application, i.e., achieving tissue separation and nerve monitoring at the same time (with intermission of the coagulation function).
Experimental set-up, operation, evaluations and endpoints
TOETVA procedure, VN and RLN exposure
TOETVA procedure have been previously described in both human and animal series [6, 7]. Figure 4 details surgery. The cavity that was created had the subcutaneous tissue and platysma as the roof and the trachea, the sternohyoid and sternothyroid muscles on the floor. The muscles were then separated in the midline and the thyroid gland exposed. The thyroid gland was freed from the trachea. Hemostasis was then confirmed. The VNs and RLNs were identified, exposed and monitored.
Quantitative EMG thresholds
Primary objective of this study was to assess the reliability of the endoscopic stimulating instrument for VN and RLN monitoring by comparing EMG signals recorded with the monopolar stimulating probe. VN and RLN were stimulated by 0.1 to 1.0 mA (stepwise by 0.1-mA increments), then 1.5, 2.0, 2.5, 3.0, 3.5 and 5.0 mA current intensity. EMG baseline amplitude, latency, and waveform morphologies were recorded (Supplement Video). In detail, both the VN (Fig. 5) and RLN (Fig. 6) were stimulated by the intermitted monopolar atraumatic ball tip stimulator probe (Group I) (Figs. 5A, 6A), the tip-end (Group II) (Figs. 5B, 6B) and tip-lateral (Group III) (Figs. 5C, 6C) of the adapted endoscopic dissecting instruments. RLN and VN recording locations were the same per each device. The front end of the instrument was not rotated during stimulation to avoid affecting the monitoring value.
Safety of stimulation
VN and RLN were continuously stimulated with the tip-end of the endoscopic dissecting instruments for > 10 min by 5.0 mA, 4 Hz, width, 100 ls. Changes in EMG amplitude, latency, and threshold and electrocardiography (EKG) monitoring were recorded.
Statistical investigation
All data are reported as mean ± standard deviation (SD). Statistical analyses were performed using the software package SPSS® v. 22 for Windows® (IBM, Armonk, New York, USA). Group comparisons were analyzed with one-way analysis of variance. Group comparisons were performed using Student’s t test. P < 0.05 was considered statistically significant. Sample size was not calculated.
Results
Animal breeds and model
There were 12 male pigs, mean weight 19.7 ± 1.3 (range 18–21 kg), mean age 54.8 ± 1.6 (range 52–57 days), providing 24 RLNs (12 left, 12 right) and 24 VNs (12 left, 12 right). TOETVAs were successfully performed with no occurrence of complications. Laryngeal and vagus nerves were exposed and monitored. Mean operating time 43.4 ± 6.05 (range 35–53 min).
Quantitative EMG thresholds
VN stimulation
Mean VN stimulation thresholds were: group I 0.28 mA (range 0.2–0.3 mA), Group II 0.56 mA (0.4–0.6 mA) and Group III 0.58 mA (0.4–0.6 mA) (P1 = 0.00, P2 = 0.00, P3 = 0.11) (Table 2). EMG amplitudes signals at different nerve stimulation locations are shown in Table 3. There was a positive correlation between the stimulus current and the resultant laryngeal EMG amplitude (Fig. 7A) (Video S1). With the increase of stimulation current, the EMG amplitude in each group reached a platform. The minimal stimulus current to evoked maximal response was for Group I 0.65 mA, Group II 1.07 mA and Group III 1.14 mA (Table 2 and Fig. 7A) (P1 = 0.00, P2 = 0.00, P3 = 0.48). VN latencies were: left 9.89 ms and right were 6.93 ms.
RLN monitoring
Mean RLN stimulation thresholds were: Group I 0.27 mA (range 0.2–0.3 mA), Group II 0.54 mA (0.3–0.6 mA) and Group III 0.55 mA (0.3–0.6 mA) (P1 = 0.00, P2 = 0.00, P3 = 0.75) (Table 2). EMG amplitudes at different nerve stimulation sites are shown in Table 4. There was a positive correlation between the stimulus current and the resultant laryngeal EMG amplitude (Fig. 7B). With the increase of stimulation current, the amplitude of EMG in each group reached a platform. The minimal stimulus current that could evoke a maximal response was Group I 0.6 mA, Group II 0.95 mA and Group III 1.05 mA (Table 2 and Fig. 7B) (P1 = 0.00, P2 = 0.00, P3 = 0.31). The EMG signal was elicited by shunt stimulation when the tips of endoscopic device ran closer to the RLN (Video S2). With this method, we are able to map and recognize the exact RLN position. RLN latencies of the left and the right RLNs were 4.12 and 4.17 ms, respectively.
Repetitive VN and RLN stimulation
After continuous, supra-maximal (> 5 mA) VN stimulation by the tip-end of the endoscopic dissecting instruments for > 10 min, the VN EMG amplitudes recorded were 1.073 ± 166 uV, i.e. 94.4% baseline amplitudes (Table 3) (P > 0.05; Fig. 8). After repetitive supra-maximal (> 5 mA) RLN stimulation by the tip-end of the endoscopic dissecting instruments for > 10 min, the RLN EMG amplitudes recorded were 1.237 ± 225 uV, i.e. 94.7% baseline amplitudes (Table 4) (P > 0.05; Fig. 8). EMG latency were constant throughout the entire period of monitoring.
Unexpected outcomes
No adverse or non-anticipated experimental potential outcomes affecting the animals were recorded.
Discussion
This work evaluates the feasibility, reliability, safety and effectiveness of an endoscopic forceps for standardized functional electrical stimulation of the RLN and VN in TOETVA.
To our knowledge, this study provides the first experimental IONM test using an adapted endoscopic dissecting instrument.
The electrode is a Maryland dissector, 330 mm length, 5 mm diameter, with 18 mm tips long and > 50° opening angle with integrated 360° rotation (Fig. 1A, C). The device is commonly applied as a dissecting, coagulation and needle holder instrument in endoscopic thyroidectomy [7]. Active stimulation electrode is located on its tip, in a 2 mm exposed area.
The goals of the study were to (i) demonstrate that stimulation using the device results in EMG signal waveform; and (ii) compare the performance of the device with that of a traditional hand probe electrode.
Direct stimulation was applied and evaluated at two sites, one at the 24 RLNs and the other at the 24 VNs, and allowed IONM of these two structures with no electrical artifacts. De facto, the EMG signals obtained for the endoscopic instrument were stable and reliable at both VN and RLN sites.
To ensure safety, the stimulation intensity of the endoscopic electrical impulses were increased to > 5 mA with repetitive impulses (> 10 min). No electrocardiographic arteiacts occur, neither any change in blood pressure values. Both RLN and VN EMG signals remained stable.
Interestingly, overall low-level electrical currents (~ 1.5 mA) were sufficient to elicit supra-maximal responses at the RLN and VN in both groups. In this experimental study, the endoscopic device (both tip-end and lateral-end) required a higher stimulating current than the hand probe accessory to achieve and EMG signal. The electrical field generated by the endoscopic instrument is concentrated on its tip and low-intensity currents are needed in comparison with lateral stimulation. The amount of energy delivered by the hand stimulation was less than the endoscopic electrode.
Furthermore, the EMG signal was elicited by shunt stimulation when the tips of endoscopic device ran closer to the RLN [8]. With this method, we are able to map and recognize the exact RLN position during TOETVA [8].
Advantages of using the endoscopic device in TOETVA are listed below: (a) the device is valuable when pre-dissectioning the thyroid testing the VN; (b) the device takes up less hindrance in the operating field and no additional accessories are needed; (c) no risk of inadvertent needle-electrode displacement; (d) RLN EMG responses can be monitored continuously throughout the surgical procedure, particularly during the crucial thyroid gland dissection phases; (e) no percutaneous electrode insertion, the device is inserted by trocars; (f) there may be a need for high stimulation intensities, but have no adverse physiological effects; (g) the instrument provide the surgeon with a simple and convenient way to dissect the structure and, in the meantime, to confirm RLN or conversely to exclude the possibility that the structure contains RLN; (h) allows concomitant dissecting and monitoring both of the RLN and VN [9].
The most common drawbacks of this study are indicated below: (a) the endoscopic instrument only provided intermittent stimulation; (b) a study is needed to evaluate if the endoscopic instrument is able to detect the adverse EMG change as a warning criterion of RLN stress; (c) the instrument used in this study is a prototype; (d) extremely careful in translating these results from an experimental animal model to clinical situation. The clinical situation in thyroid surgery may be different for the number of dissecting, stimulating and coagulation applications of the instrument. (e) There was no follow-up on pigs, neither by laryngoscopy or nerves histological evaluation.
With the development of endoscopic dissecting and stimulating device, we would expect that in the near future, the use of a hand-held or long stimulating probe may be no longer necessary, and the inconvenience that results from shifting of the dissecting forceps and stimulating probe can also be completely avoided [5, 10].
References
Dionigi G, Wu CW, Tufano RP, Rizzo AG, Anuwong A, Sun H, Carcoforo P, Antonino C, Portinari M, Kim HY (2018) Monitored transoral endoscopic thyroidectomy via long monopolar stimulation probe. J Vis Surg. 26(4):24. https://doi.org/10.21037/jovs.2017.12.25(eCollection 2018)
Inabnet WB 3rd, Suh H, Fernandez-Ranvier G (2017) Transoral endoscopic thyroidectomy vestibular approach with intraoperative nerve monitoring. Surg Endosc 31(7):3030. https://doi.org/10.1007/s00464-016-5322-y(Epub 2016 Nov 10)
Wang Y, Yu X, Wang P, Miao C, Xie Q, Yan H, Zhao Q, Zhang M, Xiang C (2016) Implementation of intraoperative neuromonitoring for transoral endoscopic thyroid surgery: a preliminary report. J Laparoendosc Adv Surg Tech A. 26(12):965–971 (Epub 2016 Sep 1)
Witzel K, Benhidjeb T (2009) Monitoring of the recurrent laryngeal nerve in totally endoscopic thyroid surgery. Eur Surg Res 43(2):72–76. https://doi.org/10.1159/000220596(Epub 2009 May 27)
Chiang FY, Lu IC, Chang PY, Sun H, Wang P, Lu XB, Chen HC, Chen HY, Kim HY, Dionigi G, Wu CW (2015) Stimulating dissecting instruments during neuromonitoring of RLN in thyroid surgery. Laryngoscope. 125(12):2832–2837. https://doi.org/10.1002/lary.25251(Epub 2015 Mar 26)
Zhang D, Li S, Dionigi G, Wang T, Zhang J, Xue G, Sun H (2018) Feasibility of continuous intraoperative neural monitoring during transoral endoscopic thyroidectomy vestibular approach in a porcine model. J Laparoendosc Adv Surg Tech A. https://doi.org/10.1089/lap.2018.0054(Epub ahead of print)
Anuwong A, Sasanakietkul T, Jitpratoom P, Ketwong K, Kim HY, Dionigi G, Richmon JD (2018) Transoral endoscopic thyroidectomy vestibular approach (TOETVA): indications, techniques and results. Surg Endosc 32(1):456–465. https://doi.org/10.1007/s00464-017-5705-8Epub 2017 Jul 17
Chiang FY, Wang LF, Huang YF, Lee KW, Kuo WR (2005) Recurrent laryngeal nerve palsy after thyroidectomy with routine identification of the recurrent laryngeal nerve. Surgery 137:342–347
Randolph GW, Dralle H, Abdullah H et al (2011) Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery. Laryngoscope 121(suppl 1):S1–S16
Wu CW, Dionigi G, Sun H et al (2014) Intraoperative neuromonitoring for the early detection and prevention of RLN traction injury in thyroid surgery a porcine model. Surgery 155:329–339
Acknowledgements
The present study was financially supported by Jilin provincial special fund for health care (no. SCZSY201714 and SCZSY201504) and the Outstanding Young Talent Foundation Project of Science and Technology Department in Jilin Province (Grant no. 20170520018JH), China.
Author information
Authors and Affiliations
Contributions
(I) HS, GD: Conception and design; (II) HS: Administrative support; (III) HS, GD, DZ: Collection and assembly of data; (IV) HS, GD, DZ: Data analysis and interpretation; (V) All authors: Manuscript writing; (VI) All authors: Final approval of manuscript
Corresponding authors
Ethics declarations
Disclosure
The authors Daqi Zhang, Shijie Li, Gianlorenzo Dionigi, Jiao Zhang, Tie Wang, Yishen Zhao, Gaofeng Xue and Hui Sun have no conflict of interest to disclose, and no other funding or financial relationship with the surgical industry.
Ethical approval
This article does not contain any studies with human participants performed by any of the Authors.
Informed consent
Informed consent not applicable in the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, D., Li, S., Dionigi, G. et al. Stimulating and dissecting instrument for transoral endoscopic thyroidectomy: proof of concept investigation. Surg Endosc 34, 996–1005 (2020). https://doi.org/10.1007/s00464-019-06936-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06936-2