Abstract
Background
Robotic system may have potential advantages to facilitate the technically challenging splenic hilar lymphadenectomy during gastrectomy for gastric cancer. However, robotic spleen-preserving splenic hilar lymphadenectomy is performed infrequently not only because of the limited availability of the robot but also because of its technical difficulty. In this study, we describe our technique of performing robotic spleen-preserving splenic hilar lymphadenectomy in detail to facilitate wider application and present operative outcomes and the follow-up results of the procedure.
Methods
From 2005 to 2015, 93 patients underwent robotic total gastrectomy with D2 lymphadenectomy. One patient with obvious lymph node (LN) metastasis received splenectomy and was excluded from the analysis. Intraoperative complications, operation and console time, estimated blood loss, postoperative morbidity and mortality, the number of harvested LNs in total and at the splenic hilum, and 5-year overall survival were analyzed, retrospectively.
Results
Among the 92 patients, robotic spleen-preserving splenic hilar lymphadenectomy was successfully performed in 91 patients except one who experienced intraoperative splenic artery injury which demanded splenectomy to be performed simultaneously. The overall mean operation time and console time were 287.2 ± 66.0 and 180.2 ± 47.2 min, respectively. Mean estimated blood loss was 141.1 ± 227.0 ml. The mortality was 1.1% (1/92). The overall postoperative morbidity rate was 16.3% (15/92). There was no case of pancreatic fistula, whole splenic infarction, or the delayed aneurysm of splenic artery. The mean numbers of harvested LNs in total and at the splenic hilum were 50.8 ± 18.1 and 1.9 ± 2.6. The 5-year overall survival was 86.3% and 5-year recurrence-free survival was 87.4%.
Conclusion
This study suggests that robotic application for spleen-preserving splenic hilar lymphadenectomy could be a feasible and safe method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Following a trend towards proximal migration, the incidence of gastric cancer in the upper third of the stomach has gradually increased over the years [1,2,3,4]. The standard treatment for proximal gastric cancer at an advanced stage is total gastrectomy with D2 lymphadenectomy, which includes the dissection of lymph nodes (LNs) at the splenic hilum (No.10 LNs) according to the Japanese guidelines [5]. Traditionally, to complete No.10 LNs dissection, splenectomy was performed. However, simultaneous splenectomy has not been found to be superior to No.10 LNs dissection with spleen preservation with regard to survival. Moreover, studies have shown that preservation of the spleen decreases morbidity compared to splenectomy in patients who underwent total gastrectomy [6,7,8]. Accordingly, spleen-preserving splenic hilar lymphadenectomy has been increasingly applied [8], although it is a technically challenging procedure, even for experienced surgeons and especially when performed by minimally invasive approaches [9,10,11].
Compared with conventional laparoscopic surgery, robotic surgery has been reported to offer more precise dissection around vessels for lymphadenectomy with the help of various technical advantages, such as three-dimensional imaging, motion scaling, tremor filtering, coaxial alignment, and articulating wristed instruments [11,12,13]. Robotic application could make the performance of splenic hilar lymphadenectomy easier, compared to laparoscopy [13]. However, robotic spleen-preserving splenic hilar lymphadenectomy is not popular, not only because of the limited availability of the robot for gastrectomy but also because of its difficulty.
In this study, along with a video demonstration, we describe our surgical technique for performing robotic spleen-preserving splenic hilar lymphadenectomy in detail, to facilitate wider application of this procedure. Additionally, we present operative outcomes and follow-up results of the procedure.
Materials and methods
Patients and indications
From July 2005 to October 2015, 1142 patients with gastric cancer underwent robotic gastrectomy at Severance Hospital, Yonsei University Health System, Korea. There were 228 patients who underwent robotic total gastrectomies. Among them, 93 patients with gastric cancer underwent robotic total gastrectomy with D2 lymphadenectomy. Among these patients, one underwent simultaneous splenectomy because of strong suspicion of No.10 LNs metastasis. The other 92 patients underwent robotic total gastrectomy with spleen-preserving D2 lymphadenectomy. All of these 92 robotic total gastrectomies with D2 lymphadenectomy were performed by two surgeons, Hyung WJ and Kim HI, who individually perform over 200 gastrectomies for gastric cancer in a year. Clinicopathologic features and surgical outcomes, including demographic data, intraoperative findings, pathology reports, postoperative recovery data, and follow-up results were extracted from a prospective database. This retrospective study was approved by the Institutional Review Board (4-2016-0718). The need for signed informed consent from each patient was waived by the institutional review board because of the retrospective design of the study.
The preoperative diagnosis of gastric cancer was confirmed by upper endoscopy and biopsy. For clinical staging, all patients underwent preoperative endoscopic ultrasound and abdominopelvic computed tomography. In our institution, patients with serosal involvement or suspicion of extraperigastric LN metastasis are excluded from undergoing minimally invasive surgery, except in clinical trials. Accordingly, patients with a primary tumor of proper muscle invasion regardless of circumferential tumor location or invasion deeper than the proper muscle located on the lesser curvature of the stomach and without obvious metastatic LNs along the splenic hilum on preoperative examination were considered candidates for robotic total gastrectomy with spleen-preserving D2 lymphadenectomy.
Surgical technique (supplementary videos)
After positioning, securing, and preparing the patient in the supine position under general anesthesia, a 12-mm trocar was placed at the midline just below the umbilicus for inserting a dual lens laparoscope. After pneumoperitoneum of 12 mmHg was achieved, the operating table was placed in a 15° reverse Trendelenburg position. After determining the optimal location of the port sites, four additional ports were inserted under camera visualization. Specifically, two 8-mm ports for the first and third arms were placed 1 cm below the costal angle bilaterally, as far laterally as possible; the ports should be at least 1 cm above the level of the bowel when viewed internally. The last 8-mm port for the second arm was inserted 2 to 4 cm above an imaginary line intersecting the middle of the camera port and the right subcostal port. This step granted easier access to the pancreatic head and duodenum and facilitated a proper angle for working with the ultrasonic shears. Next, a 12-mm assistant port was placed 1 to 2 cm below an imaginary line drawn from the insertion site of the first arm to the umbilical port. After completing the insertion of the trocars, the surgical cart was docked on the patient. The instrumentation and settings on the cart consisted of a 30-degree down endoscope: Maryland bipolar forceps in the first arm, ultrasonic shears in the second arm, and Cadiere forceps in the third arm.
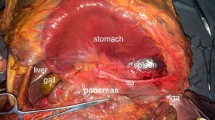
The surgery began with liver retraction utilizing a suture and a gauze pad as a “sling” as reported previously [14]. Thereafter, partial omentectomy was performed, about 4 cm away from the gastroepiploic arcade, toward the lower pole of the spleen. Continuing the dissection, the roots of the left gastroepiploic artery and vein were divided after the branch to the splenic lower pole to dissect the No.4sb LNs. Dissecting adhesions between the lower pole of the spleen and the omentum was performed in advance to prevent potential bleeding due to tearing of the splenic capsule. Next, after dividing the left gastroepiploic vessels, Cadiere forceps in the third robotic arm were used to grasp the soft tissues along the greater curvature of the fundus medio-cranially, tensing the gastrosplenic ligament. Upon doing so, the distal portions of the splenic artery and splenic hilum were well exposed. When performing dissection of the No.10 LNs, we did not mobilize the posterior attachment of the spleen to facilitate spontaneous exposure of the spleen and splenic hilar area by gravitational countertraction of the spleen into the splenic fossa.
Continuing on, the soft tissues covering the splenic vascular trunks were opened, and the distal splenic artery was skeletonized at the upper border of the pancreas (No.11d LNs). LNs bearing fatty tissue were then dissected along the splenic artery up to the branching point of the splenic lobar arteries which was used as the starting point for the splenic hilar lymphadenectomy which was performed in the order of the lower polar, hilar, and upper polar areas of the spleen (Fig. 1). First, lymphatic fatty tissues were dissected from the branching point toward the lower pole of the spleen along the inferior splenic lobar artery; the inferior splenic lobar artery should be exposed to the origin of the left gastroepiploic vessel.
Schematic illustration of the strategy for performing robotic spleen-preserving splenic hilar lymph node dissection. Lymph nodes bearing fatty tissue were dissected along the splenic artery (SA) up to the branching point of the splenic lobar arteries. The branching point was used as the starting point for performing splenic hilar lymphadenectomy, which was performed in the lower polar ①, hilar ②, and upper polar ③ areas of the spleen
Dissection of the middle hilar area from the branching point toward the splenic hilum was then continued. The area between the superior splenic lobar artery and the inferior splenic lobar artery was dissected thoroughly. During the dissection, LNs bearing soft tissues at the surface of the terminal branches of the splenic vessels were gently lifted ventrally using Maryland bipolar forceps, while the ultrasonic shears were used to dissect the tissues around the terminal branches of the splenic vessels until skeletonized. When dissecting the lymphatic tissues behind the splenic vessels, Maryland bipolar forceps were first utilized to create proper angles in front of the Gerota’s fascia prior to dissecting with the ultrasonic shears. After partial mobilization, the lymphatic fatty tissues behind the splenic vessels were pulled upward, gently rotated from the dorsal side to the lateral side of the vessels, and then dissected. Sometimes, nerve fibers around the arteries could be gently grasped and retracted to rotate and expose the dorsal side of the vessels, or vessel loops were used. Also, the surgical plane anterior to the Gerota’s fascia was kept intact to prevent iatrogenic injury to retroperitoneal organs.
Next, restarting from the branching point, lymphatic fatty tissues were dissected toward the upper pole of the spleen along the superior splenic lobar artery using a similar technique as described above. During the dissection, the origins of the short gastric vessels were carefully exposed and divided at the root (No.4sa LNs dissection). Great care was taken when approaching the upper pole of the spleen since the last branch of the short gastric vessels in the upper pole is usually very short and easily injured. Therefore, before dividing this branch, countertraction of the stomach was performed by moving the Cadiere forceps to the right upper side of the abdominal cavity, toward the left lobe of the liver to tense and expose the branch adequately. The branch was then divided at the root. Thus, No.11d, No.10, and No.4sa LNs were removed en bloc.
The procedures for the dissection of other LNs and digestive tract reconstruction have been described in our previous publications [11, 12, 15]. After the operation, a standardized postoperative care protocol was applied [16].
Statistical analysis
The IBM SPSS statistics (Version 23 for Mac; IBM Corp., NY, USA) software package was used to conduct all statistical analyses. Continuous data were expressed as mean ± standard deviation. Patients were followed from the date of operation until September 30, 2015 or their death. Overall and recurrence-free survival curves were calculated by the Kaplan–Meier method.
Results
The present study included 45 males and 47 females, with a mean age of 51.9 ± 13.1 years and a mean body mass index of 22.8 ± 3.7 kg/m2. The patient characteristics are summarized in Table 1. During robotic spleen-preserving No.10 LNs dissection, there was one splenic artery injury during the dissection of No.11d, which forced us to perform a simultaneous splenectomy. All other cases were successfully performed by the robot. The overall mean operation time and console time were 287.2 ± 66.0 min and 180.2 ± 47.2 min, respectively, and the mean estimated blood loss was 141.1 ± 227.0 mL.
The mean postoperative hospital stay, time to first flatus, and time to first liquid diet intake were 12.8 ± 30.2, 3.1 ± 0.8, and 4.2 ± 5.1 days, respectively. The overall postoperative morbidity rate was 16.3% (15/92), consisting of one intraperitoneal bleeding, three instances of intraluminal bleeding, four occurrences of anastomotic leakage of the esophagojejunostomy, one anastomotic leakage of the esophagojejunostomy and pleural effusion, three wound infections, two intraperitoneal abscesses, and one subcutaneous emphysema. There were no instances of pancreatic fistula, whole splenic infarction, or delayed aneurysm of the splenic artery. Among the 92 patients, there was one mortality (1.1%), which resulted from a massive small bowel infarction occurred by accidental superior mesenteric artery thrombosis during the angiographic intervention to control bleeding at an esophagojejunal anastomosis site.
A mean of 50.8 ± 18.1 LNs was retrieved for all patients. The mean number of retrieved No.10 and No.11d LNs were 1.9 ± 2.6 and 2.0 ± 2.0. LN metastases were noted in 30 patients (32.6%): four patients (4.3%) exhibited No.10 LNs metastasis and two patients (2.2%) had No.11d LNs metastasis. The mean number of overall metastatic LNs among patients with LN metastasis was 6.8 ± 6.5.
Over a median follow-up period of 53.8 months, 12 patients (13.0%) died. The 5-year overall survival rate was 86.3% (Fig. 2A). The median survival time was 53.5 months. There were eight recurrences in the breast (n = 2), splenic hilum (n = 1), ovary (n = 2), peritoneum (n = 2), and bone (n = 1). The splenic hilar recurrence was treated by curative splenectomy. The 5-year recurrence-free survival rate was 87.4% (Fig. 2B). The median recurrence-free survival time was 49.0 months.
Discussion
In the present study, we demonstrated acceptable early postoperative outcomes and satisfactory long-term results of robotic spleen-preserving No.10 LNs dissection in patients with proximal gastric cancer. The rates of intraoperative robotic procedure-related complications, and postoperative mortality and morbidity were comparable to those of open or laparoscopic surgery reported in previous studies [9,10,11, 17, 18]. Moreover, long-term survival and recurrence after the robotic procedure were similar to those reported in the literature for open or laparoscopic D2 LN dissection [11, 17, 18].
Since preoperative staging modalities are not accurate enough to predict metastasis to the No.10 LNs and therefore allow dissection of the nodes to be omitted [9], No.10 LNs dissection remains essential. If splenic hilar lymphadenectomy were not performed, the possibility of residual disease at the No.10 LNs would increase. Studies have reported a high frequency of No.10 LNs metastases, up to 26% in advanced proximal tumors [19]. Indeed, D2 lymphadenectomy has been found to improve the survival of patients with positive No.10 LNs [20,21,22]. Patients undergoing total gastrectomy with standard D2 lymphadenectomy for advanced tumors exhibited higher 5-year survival rates than patients treated with D2 minus No.10 LNs dissection [17]. Accordingly, splenic hilar lymphadenectomy is necessary for curative total gastrectomy for gastric cancer, especially for advanced tumors.
Nevertheless, complete LN dissection at the splenic hilum without splenectomy is regarded as a technical challenge, especially during minimally invasive surgery. Therefore, only a few studies have described the procedure via a minimally invasive approach. Compared to open or laparoscopic surgery, the robotic procedure allows the surgeon to approach the deep and narrow splenic hilar area easily, without requiring the mobilization of the spleen. In laparoscopic surgery, surgeons face difficulties with retrieving LNs bearing tissue at the dorsal part of the pancreas and behind the splenic vessels with unwristed laparoscopic instruments. In contrast, features such as three-dimensional visualization, tremor filtration, scaled movement, and wrist articulation during robotic surgery enable precise LN dissection within a restricted space surrounded by intricate vascular anatomy. These advantageous features not only help minimize injury to the parenchyma of the pancreas, spleen, and splenic vessels, but they also facilitate a more stable and complete dissection [11,12,13]. Although the mean operation time of robotic total gastrectomy was longer than that of laparoscopic total gastrectomy, the incidence of overall complications (11.9%–18.8% vs. 10.3%–24.5%) and estimated blood loss (50-163 ml vs. 60–210.7 ml) following the robotic procedure were similar or less than the laparoscopic procedure [11, 23, 24]. Furthermore, we found that robotic procedure could harvest more LNs along the splenic artery (2.3 vs. 1.0, P = 0.013), as well as the sum of LNs at the splenic hilum and artery (3.6 vs. 1.9, P = 0.014), compared with the laparoscopic procedure in our previous study [11]. And in the present study, the mean number of retrieved splenic hilar and No.11d LNs were 1.9 and 2.0, respectively, which were comparable with our previous study. Additionally, robotic gastrectomy exhibits a shorter learning curve than that for laparoscopic gastrectomy [23]. Thus, we suggest that robotic splenic hilar lymphadenectomy may be a preferable approach to open or laparoscopic surgery.
Surgeons may find our method helpful using the following technical tips: First, preoperative assessment of the splenic vascular anatomy via three-dimensional reconstructed images of computed tomography scans might be useful for a safe dissection [9]. Second, we utilize Cadiere forceps to provide sufficient and steady countertraction and Maryland bipolar forceps to create proper angles during dissection of soft tissues at the splenic hilum, especially for tissue located behind the vessels. Third, if the routes of the splenic vessels are tortuous and embedded behind the parenchyma of the pancreas, it may be necessary to compress and retract the pancreas using a laparoscopic instrument padded with gauze for better exposure. Sometimes vessel loops can also be useful to provide better exposure. Fourth, if a good angle cannot be achieved to dissect the splenic hilar area with non-endo-wristed ultrasonic shears, use of other endo-wristed devices (e.g., Hook or monopolar scissors) may be helpful. Finally, care should be given to preserving the branches of the splenic vessels during dissection; however, a few tiny branches of the splenic vessels can be safely sacrificed.
It is difficult to deal with the bleeding in case of vascular injury of the splenic hilum. To deal with bleeding by vascular injury of the splenic hilum, various surgical techniques are required. With the help of various technical advantages, such as three-dimensional imaging, motion scaling, tremor filtering, coaxial alignment, and articulating wristed instruments, robotic procedure allows the surgeon to approach the deep and narrow splenic hilar area easily without requiring mobilization of the spleen, and facilitates manipulation of robotic instruments within a restricted space surrounded by intricate vascular anatomy. These advantageous features not only help minimize injury to the splenic vessels, but also facilitate relatively easier hemostasis when intraoperative bleeding occurs due to the vascular injury of the splenic hilum.
This study has a few limitations that warrant consideration: First, almost half of the included patients were postoperatively diagnosed as having early cancer, which somewhat compromises the generalizability of our results to advanced cancer. Second, robotic surgery has been reported to be more expensive than laparoscopic and open approaches [24]. Despite these limitations, the purpose of this study was to describe robotic spleen-preserving No.10 LNs dissection in detail and to report data that suggest the safety and feasibility of robotic No.10 LNs dissection. Although this study was only a single-arm analysis, we presented our results comparing robotic and laparoscopic spleen-preserving splenic hilar lymphadenectomy in a previous publication [11]. To our knowledge, this is the first study to describe a detailed procedure for performing robotic spleen-preserving splenic hilar lymphadenectomy during total gastrectomy for gastric cancer.
Our initial results suggest that the procedure is feasible and safe, facilitating spleen-preserving No.10 lymphadenectomy for advanced and complicated gastric cancer surgery.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108
Blot WJ, Devesa SS, Kneller RW, Fraumeni JF Jr (1991) Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 265(10):1287–1289
Hasegawa S, Yoshikawa T (2010) Adenocarcinoma of the esophagogastric junction: incidence, characteristics, and treatment strategies. Gastric Cancer 13(2):63–73
Liu K, Yang K, Zhang W, Chen X, Chen X, Zhang B, Chen Z, Chen J, Zhao Y, Zhou Z, Chen L, Hu J (2016) Changes of esophagogastric junctional adenocarcinoma and gastroesophageal reflux disease among surgical patients during 1988–2012: a single-institution. High-volume experience in China. Ann Surg 263(1):88–95
Japanese gastric cancer association (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14(2):113–123
Yang K, Chen XZ, Hu JK, Zhang B, Chen ZX, Chen JP (2009) Effectiveness and safety of splenectomy for gastric carcinoma: a meta-analysis. World J Gastroenterol 15(42):5352–5359
Yu W, Choi GS, Chung HY (2006) Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg 93(5):559–563
Li C, Kim S, Lai JF, Oh SJ, Hyung WJ, Choi WH, Choi SH, Zhu ZG, Noh SH (2009) Lymph node dissection around the splenic artery and hilum in advanced middle third gastric carcinoma. Eur J Surg Oncol 35(7):709–714
Hyung WJ, Lim JS, Song J, Choi SH, Noh SH (2008) Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J Am Coll Surg 207(2):e6–11
Huang CM, Chen QY, Lin JX, Zheng CH, Li P, Xie JW, Wang JB, Lu J (2014) Laparoscopic spleen-preserving splenic hilar lymphadenectomy performed by following the perigastric fascias and the intrafascial space for advanced upper-third gastric cancer. PLoS ONE 9(3):e90345
Son T, Lee JH, Kim YM, Kim HI, Noh SH, Hyung WJ (2014) Robotic spleen-preserving total gastrectomy for gastric cancer: comparison with conventional laparoscopic procedure. Surg Endosc 28(9):2606–2615
Song J, Oh SJ, Kang WH, Hyung WJ, Choi SH, Noh SH (2009) Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg 249(6):927–932
Ballantyne GH (2007) Telerobotic gastrointestinal surgery: phase 2–safety and efficacy. Surg Endosc 21(7):1054–1062
Woo Y, Hyung WJ, Kim HI, Obama K, Son T, Noh SH (2011) Minimizing hepatic trauma with a novel liver retraction method: a simple liver suspension using gauze suture. Surg Endosc 25(12):3939–3945
Yang K, Bang HJ, Almadani ME, Dy-Abalajon DM, Kim YN, Roh KH, Lim SH, Son T, Kim HI, Noh SH, Hyung WJ (2016) Laparoscopic proximal gastrectomy with double-tract reconstruction by intracorporeal anastomosis with linear staplers. J Am Coll Surg 222(5):e39–45
Lee HW, Kim HI, An JY, Cheong JH, Lee KY, Hyung WJ, Noh SH (2011) Intracorporeal anastomosis using linear stapler in laparoscopic distal gastrectomy: comparison between gastroduodenostomy and gastrojejunostomy. J Gastric Cancer 11(4):212–218
Yang K, Zhang WH, Chen XZ, Chen XL, Zhang B, Chen ZX, Zhou ZG, Hu JK (2014) Survival benefit and safety of no. 10 lymphadenectomy for gastric cancer patients with total gastrectomy. Medicine 93(25):e158
Yang K, Lu ZH, Zhang WH, Liu K, Chen XZ, Chen XL, Guo DJ, Zhou ZG, Hu JK (2015) Comparisons between different procedures of No 10 lymphadenectomy for gastric cancer patients with total gastrectomy. Medicine (Baltimore) 94(33):e1305
Ishikawa S, Shimada S, Miyanari N, Hirota M, Takamori H, Baba H (2009) Pattern of lymph node involvement in proximal gastric cancer. World J Surg 33(8):1687–1692
Kosuga T, Ichikawa D, Okamoto K, Komatsu S, Shiozaki A, Fujiwara H, Otsuji E (2011) Survival benefits from splenic hilar lymph node dissection by splenectomy in gastric cancer patients: relative comparison of the benefits in subgroups of patients. Gastric Cancer 14(2):172–177
Hasegawa S, Yoshikawa T, Rino Y, Oshima T, Aoyama T, Hayashi T, Sato T, Yukawa N, Kameda Y, Sasaki T, Ono H, Tsuchida K, Cho H, Kunisaki C, Masuda M, Tsuburaya A (2013) Priority of lymph node dissection for Siewert type II/III adenocarcinoma of the esophagogastric junction. Ann Surg Oncol 20(13):4252–4259
Sasako M, McCulloch P, Kinoshita T, Maruyama K (1995) New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg 82(3):346–351
Park SS, Kim MC, Park MS, Hyung WJ (2012) Rapid adaptation of robotic gastrectomy for gastric cancer by experienced laparoscopic surgeons. Surg Endosc 26(1):60–67
Kim HI, Han SU, Yang HK, Kim YW, Lee HJ, Ryu KW, Park JM, An JY, Kim MC, Park S, Song KY, Oh SJ, Kong SH, Suh BJ, Yang DH, Ha TK, Kim YN, Hyung WJ (2016) Multicenter prospective comparative study of robotic versus laparoscopic gastrectomy for gastric adenocarcinoma. Ann Surg 263(1):103–109
Acknowledgements
This study was supported by a grant to Dr. Woo Jin Hyung from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (No. 1320270) and by grants to Dr. Kun Yang from the National Natural Science Foundation of China (No. 81772547) and the Sichuan University Scholarship Fund. We would like to thank Anthony Thomas Milliken, ELS (Editing Synthase, Seoul, Korea) for his assistance with editing the manuscript. We also acknowledge the assistance of BioScience Writers, LLC (Houston, TX, USA) with copyediting and correction of English language usage.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Woo Jin Hyung is a consultant for Ethicon and Verb Surgical, and has stock in Hutom. Drs. Kun Yang, Minah Cho, Chul Kyu Roh, Won Jun Seo, Seohee Choi, Taeil Son, and Hyoung-Il Kim have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MP4 114611 kb)
Supplementary material 2 (MP4 125410 kb)
Supplementary material 3 (MP4 178901 kb)
Rights and permissions
About this article
Cite this article
Yang, K., Cho, M., Roh, C.K. et al. Robotic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. Surg Endosc 33, 2357–2363 (2019). https://doi.org/10.1007/s00464-019-06772-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06772-4