Abstract
Background
In digestive cancers, it is mandatory to diagnose peritoneal metastasis prior to selecting therapy. Therefore, exploratory laparoscopy has gained wider clinical acceptance. In laparoscopy, the peritoneal metastasis is pathologically confirmed by excisional biopsy; however, there remain technical difficulties in performing precise diagnosis and adequate biopsy on small peritoneal lesions without damaging organs. We have focused on “optical biopsy” using probe-based confocal laser endomicroscopy (pCLE). The aims of this study were (1) to optimize current CLE system for real-time observation of peritoneal metastases and (2) to assess its potential usefulness as diagnostic modality in preclinical settings.
Methods
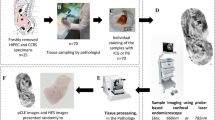
To optimize condition and evaluate feasibility, we prepared peritoneal metastasis mice model with gastric cancer cell line (MKN-45). On Day 10 after seeding, the mice were laparotomized and performed pCLE observations with CellvizioLAB (LSU-F 400/488 nm, Mauna Kea Technologies, Paris, France). We evaluated two different CLE probes, three different dyes, and optimal interval time. The detected sites were excised and pathologically evaluated on its morphology. Next, the feasibility and safety were validated in porcine model for clinical usage. After injection of fluorescein, pCLE was applied for the observation of intra-abdominal organs.
Result
A miniature probe-type pCLE system with 60 μm focal depth (UltraMini O) and 1 % fluorescein dye was chosen for good visualization in mice model. The irregular microarchitecture images suspected to malignancy were obtained from the metastases. In the porcine model, observation of abdominal organs was feasible without any organ injury in the laparoscopic procedures. The dosage of 1 % fluorescein (3 ml/body) was appropriate in observing intra-abdominal organs, and each intra-abdominal organ was clearly observed with the same imaging quality we obtained in mice model.
Conclusion
The pCLE was feasible and safe and potentially useful for the diagnosis of the peritoneal metastasis in in vivo animal models.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Peritoneal metastasis is a common metastatic condition in digestive cancers, particularly gastric and pancreatic cancers. It occurs by the dissemination of cancer cells from primary lesions. The prognosis of patients with these cancers has been reported to depend on the presence of it [1–3]. Therefore, it is mandatory to evaluate peritoneal metastasis prior to selecting an appropriate therapy. However, despite the development of diagnostic imaging methods such as computed tomographic scanning and magnetic resonance imaging, their results are not satisfactory. Therefore, an exploratory laparoscopy is typically performed to determine whether microperitoneal metastases are involved [4–6]. In laparoscopy, we first examine the abdominal cavity to investigate peritoneal nodules; then, we perform an excisional biopsy to confirm the findings histopathologically. An accurate diagnosis requires the combination of imaging evaluations, biopsy techniques, and diagnostic maneuvers.
The detection of small lesions has been facilitated by the development of laparoscopic imaging systems (e.g., high-resolution, high-quality image-processing techniques) and additional new imaging modalities, such as photodynamic diagnosis (PDD), mediated with 5-aminolevulinic acid (5-ALA) [7, 8]. However, it is still difficult to make a diagnosis based only on pathological findings from a microlesion, because uncertainty is inherent in biopsy technique in case that the target lesions are difficult to be visualized in the procedure because of its smallness and anatomical reasons. Moreover, several technical problems remain to be resolved; for example, it is difficult to obtain sufficient biopsy tissue without damaging an organ, including the intestinal tract, mesentery, and the great vessel surface; also, the decision must be delayed, due to the time involved in sample transportation, processing, and analysis, which are required for making the diagnosis.
Confocal laser endomicroscopy (CLE) is based on the principle of tissue reflectance or tissue fluorescence, with the application of fluorescence agents (e.g., fluorescein sodium) [9, 10]. CLE generates images that elucidate cellular architecture and microvasculature at a level comparable to that achieved with traditional histology [11, 12]. In particular, several studies have shown that this technology is useful for determining pathology in a variety of gastrointestinal tissue sites, including Barrett’s esophagus [13–15], and lesions in the duodenum [16], colonic mucosa [17, 18], and pancreatic biliary tract [19–21]. Moreover, it has been reported that CLE, incorporated into gastrointestinal endoscopy, is a useful approach for diagnosing various malignancies, including gastric [22, 23], esophageal [24, 25], colorectal [26, 27], and pancreatic cancers [28]. In addition, the device could be used to acquire optical biopsies, an alternative method for making a definitive diagnosis. Thus, we reasoned that the combination of exploratory laparoscopy and probe-based CLE (pCLE) could be used for screening and might replace a conventional targeted biopsy. This approach might be a practical replacement for the current procedures involved in making a diagnosis. However, there is no report about the optimal condition for discrimination between peritoneal metastasis and normal organ.
The aims of this study were (1) to optimize current CLE system for real-time observation of peritoneal metastases and (2) to assess its potential usefulness as diagnostic modality in preclinical settings.
Materials and methods
All animal experiments were conducted in accordance with the Institutional Guidelines for Ethics in our institutions. We obtained Institute of Cancer Research (ICR) nu/nu mice from Charles River, Japan (Yokohama, Japan).
Experiment 1: optimization of pCLE system in murine model
We selected the probe-based CLE system (CellvizioLAB; LSU-F 400/488 nm, Mauna Kea Technologies, Paris, France), principally because it was acquired for clinical use subsequent to this study. The CLE system consisted of a reusable imaging probe connected to a laser scanning unit. The scanning wavelength was 488 nm. These systems were directory connected personal computer and its liquid crystal display (LCD). We could watch and process pCLE images in real time, and after the fact, the images or movies were editable with Image cell version 3.8 (Mauna Kea Technologies, Paris, France). We established peritoneal metastases of gastric cancer in mice model by implanting gastric cancer cells, as previously described [29]. Mice were injected intra-abdominally (in the right flank) with 100 μl of 3.0 × 106 MKN-45 cells (JCRB0254) suspended in PBS (1:1 v/v).
The pCLE was applied to anesthetized mice after injecting dye (100 μl/mouse) into the tail vein. The pCLE was used under direct vision via an open laparotomy wound. The pCLE probes were manipulated manually and placed directly in contact with each organ and tumor lesion. In all procedures, the pCLE images were recorded and stored digitally for later evaluation. The sites of observation under various conditions were dissected for histological investigation. The images were compared to each other and to the histopathological findings. The sites observed with pCLE were dissected, fixed in 10 % neutral buffered formalin, and embedded in paraffin blocks. The blocks were thin-sliced into 2.5-μm sections. In particular, the gastric wall and subphrenic peritoneum were sliced in sections horizontal to the surface of peritoneum at 5-μm intervals, proceeding from a depth of 40 μm and ending at a depth of 70 μm. All the slides were stained with hematoxylin and eosin (HE) and evaluated histologically. Findings from HE-stained sections evaluated by microscopic visualization of the morphology were compared to pCLE images.
We evaluated two different types of probes, the Proflex UltraMini O and the Proflex Z (Mauna Kea Technologies, Paris, France), to determine which was optimal for intra-abdominal observation, because few reports had evaluated the different probe models for diagnosing peritoneal nodules. The two probes we selected for observing cell morphology were proposed in the manufacture’s specifications. Five minutes (min) after an intravenous (IV) injection of 1 % fluorescein sodium (100 μl/mouse), each probe was placed in contact with liver and peritoneal nodules.
-
The Proflex UltraMini O (LP-0013, 2.6 mm diameter, 60 μm focal depth) was suitable for imaging vessels, angiogenesis, cell fate, and cell morphology; its utility depended on cell layer thickness and cell invasiveness in tissues.
-
The Proflex Z (LP-0012, 1.8 mm diameter, 100 μm focal depth) was suitable for imaging blood flow through a vessel (without penetration) and imaging deeper cell layers of a tumor, organ, or tissue.
Based on prior publications and the manufacturer’s instructions, fluorescein sodium (FS) is considered as an appropriate dye for in vivo studies that implemented pCLE. In addition, FS is convenient and readily available to a great majority of clinicians for clinical use. However, no studies had reported optimal kinds for observing the intra-abdominal lining. We selected three dyes, FS, indocyanine green (ICG), and patent blue (PB), which were previously proven to be safe and bioavailable in humans. After each dye was injected intravenously, the liver and peritoneal nodules were observed for 30 min. Each dye was prepared as follows:
-
1 % FS: clinical grade 10 % fluorescein sodium (FLUORESCITE® for Intravenous Injection, Alcon Japan Ltd, Tokyo, Japan) was diluted in sterile normal saline (0.9 % NaCl) to a final concentration of 1 % FS.
-
2.5 % ICG: DIAGNOGREEN® for injection (Daiichi Sankyo Company, Ltd, Tokyo, Japan) was diluted in sterile normal distilled water to a final concentration of 2.5 % ICG.
2 % PB: Patent Blue VF (Wako Pure Chemical Industries, Ltd, Osaka, Japan) was diluted in sterile normal distilled water to a final concentration of 2 % PB.
In pCLE observations, the dye is used as a contrast agent. Therefore, the timing of dye extravasation is critical, because it makes it difficult to interpret the images. It is necessary to determine an optimal interval time after dye injection for the observation. After the intravenous injection of 1 % FS (0.1 ml/mouse), consecutive image sequences from the identical site (liver or peritoneal nodules) were recorded at every 1, 3, 5, 10, and 15 min.
Experiment 2: feasibility study of intra-abdominal tumor imaging in mice
First, we aimed to demonstrate the usefulness and feasibility of a pCLE-based diagnosis. Mice were placed under deep general anesthesia, and laparotomy was performed. We observed both normal organs (diaphragm, gastric serosa, greater omentum, and liver) and peritoneal nodules (one on the mesentery and another at the hilar of the spleen), under conditions established previously.
Experiment 3: exploratory laparoscopy in the porcine model
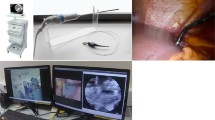
We performed exploratory laparoscopy in a porcine model to evaluate the feasibility and safety of pCLE in the abdominal cavity. A 35-kg female, crossbred pig was fed ad libitum preoperatively. The pig fasted overnight before laparoscopic inspection. The pig was placed under general anesthesia, and one 12-mm trocar and two 5-mm trocars were inserted. The 12-mm trocar was placed at the umbilicus to form the laparoscope port; the other two trocars (5-mm) were placed at the bilateral midclavicular lines to create a working cavity. In this procedure, pCLE probe was inserted from 5-mm trocar and normal organs (liver, subphrenic peritoneum, omental fat, and gastric serosa) were observed by laparoscopically manipulating pCLE probes with standard laparoscopic forceps at 3–10 min after the IV administration of 3 ml of 1 % FS, based on the conditions determined in the murine model.
Results
Experiment 1
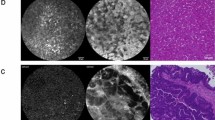
The pCLE images of cancer cells (Fig. 1A) and hepatocytes (Fig. 1B) were acquired with two kinds of probe. Images of cell morphology that were acquired with the Proflex UltraMini O probe showed more distinct external cell margins than those acquired with the Proflex Z probe. In particular, in cancer cells, it was easier to diagnose an irregularity in cell shape with the Proflex UltraMini O than with the Proflex Z probe.
Optimization of probes. All images were obtained after an injection of 1 % FS and an interval of 3–10 min. A, left Peritoneal nodule acquired with the Proflex UltraMini O (scale bar 20 μm). A, middle The same peritoneal nodule imaged with the Proflex Z (scale bar 50 μm). B, left Liver image acquired with the Proflex UltraMini O (scale bar 20 μm). B, middle The same liver site imaged with the Proflex Z (scale bar 50 μm). A, B, right HE staining images (scale bar 20 μm)
We evaluated 2 % PB, 2.5 % ICG, and 1 % FS dyes. In pCLE images, after the 2 % PB injection, no details could be discerned. After the ICG injection, cell microarchitecture was not discriminated. However, after the 1 % FS injection, distinct morphologies of hepatocytes and tumor cells were obtained (Fig. 2A–C).
Optimal dye selection for pCLE observations in the abdominal cavity. Images show the same regions of tissue, but different dyes: left FS, middle ICG, and right PB. A Images of cancer cells observed in the peritoneal nodule. B Images of hepatocytes observed in the liver. All images are shown at the same image resolution (scale bar 20 μm)
To evaluate the time interval for pCLE imaging, we used the Proflex UltraMini O probe and 1 % FS (100 μl/mouse). At 1, 3, 5, 10, and 15 min after dye injection, we evaluated the observational resolution at the same sites on the liver and peritoneal nodules. The pCLE images obtained at 1 min after injection were too immature to determine the cell morphology. Images acquired at 3–10 min after injection provided sufficient details. On the other hand, the images acquired at 15 min after injection displayed poorly demarcated borders; in this case, the background noise had increased due to dye extravasation (Fig. 3A, B).
Changes in image quality over time. Mice were injected with FS, and images were acquired (left to right) at 1, 3, 5, 10, and 15 min after injection. At 1 min, the tissue was not fully stained, and cell shapes were difficult to recognize. On the other hand, after 15 min, the outer membranes of the cells appeared blurry, due to dye leakage. As a result, the optimal time period for pCLE observation of the intra-abdominal cavity was 3–10 min after dye injection. A Cancer cells in the peritoneal nodule, B hepatocytes (scale bar 20 μm)
Experiment 2
To study the normal state of intra-abdominal organs, the peritoneum, greater omentum, liver, spleen, and diaphragm were observed under the optimized conditions determined above. These areas were compared in the HE-stained sections and pCLE images (Fig. 4A–D). Then, 10 days after the cancer cells were injected, peritoneal metastases were established in the mice. Observations of peritoneal metastases with pCLE showed irregular cell arrangements and morphology, consistent with the HE findings. Moreover, in the cancerous nodules, the dye had leaked out of the blood vessels into the intercellular spaces; in contrast, no extravasation was observed in the healthy organs (Fig. 5A, B).
Experiment 3
The pCLE images were as clearly visualized in the pig as in the mouse model (Fig. 6A–D). Moreover, during the procedure of placing the pCLE probe in contact with the observation sites, no other organs were injured along the probe pathway.
Discussion
It is still difficult to diagnose accurately for peritoneal metastasis of digestive cancers, although various advanced imaging modalities have been developed. Due to the limited sensitivity of existing imaging modalities, moreover the lower invasiveness for the patients, the exploratory laparoscopy procedure has been widely used for detecting peritoneal metastasis. Currently, when nodules are detected in the peritoneum, an excisional biopsy is performed to determine whether the nodules are malignant. The sole means of making a definitive diagnosis of peritoneal metastasis is based on pathological histology. However, in some cases, the peritoneal nodules are minuscule, or due to the anatomical factors, it is not certain that the conventional biopsy specimens include the suspected nodules, as intended. In other cases, no cancer cells appear on the histology section, and it is not possible to diagnose malignancy. Furthermore, the target biopsy injures intra-abdominal organs and often leads to bleeding. In addition, when surgeons need to select a treatment intra-operatively, a diagnosis takes too much time with the existing procedures, due to the many steps involved. Under these circumstances, we need a real-time diagnostic modality for peritoneal metastasis without resecting tissues. The pCLE as a diagnostic tool for optical biopsy can be a possible candidate.
Several reports have described pCLE examinations of the GI tract combined with endoscopy [14, 26]. In those studies, diagnoses of Barrett’s esophagus, Crohn’s colitis, and biliary tract strictures have indicated that pCLE is a promising tool for in vivo histological examinations. Consequently, we speculated that this system could be applied to intra-operative diagnoses of peritoneal metastasis. However, only a few reports have used the pCLE approach for examining intra-abdominal organs [30, 31]. Therefore, no optimal settings have been established for observing the intra-abdominal cavity and discriminate between normal organs and metastatic lesions. In this study, we have successfully optimized this system for intra-abdominal observations. Currently, in humans, this system is employed at an excitation wavelength of 488 nm; thus, in our evaluation, we selected the Cellvizio LAB (LSU-F 400/488 nm), which will facilitate the progression of this technique to clinical trials [32, 33]. Our results have also shown that FS is the best dye for observations in the abdominal cavity. It seemed logical that no efficient images were obtained by ICG and PB, because they needed to be excited at a longer wavelength than that of the experimental devices. If an improved system which can emit longer excitation (e.g., 660 nm) is developed and used in clinical practice, these agents will be a new option for dyes of optical biopsy. And, the drawback of this approach is that it only described cell morphology in detail for about 7 min (3–10 min), and the examination is only possible one time. In clinical use, few nodules will be encountered in a single surgery. Therefore, it would be useful in the future studies to develop new dyes that will facilitate imaging for longer durations.
In the next stage, we evaluated two different probes for detecting cell morphology, which were recommended by the manufacturer. The nodules of peritoneal metastasis are formed on the surface of the intra-abdominal organs. Thus, high resolution, rather than deep focal depth, was considered necessary for a differential diagnosis of the peritoneal nodules. On the other hand, in future evaluations of invasions into the serosa or other organs, both deep focal depth and high resolution will be required. For those applications, a probe combination designed for deep investigations would facilitate the detection of microstructural alterations.
To diagnose malignancy, it is necessary to examine the morphology of the cell nucleus. We have confirmed that it is possible to examine cell nuclei with acryflavin (data not shown). However, the concern with this methodology is that nuclear staining may be toxic and damage normal cells; the safety of the approach has not been demonstrated. Therefore, until the safety of a nuclear staining dye has been shown, this technique cannot be applied in the clinical practice.
Disseminated cancer cells tend to form micronodules, which are difficult to detect in a gross examination. To overcome this problem, we have applied 5-ALA-mediated photodynamic diagnosis (PDD) method for the detection of micronodules that are difficult to be detected with current imaging technology. In this method, we can find so tiny lesion that it is difficult to perform biopsy accurately. We speculate that the combination of PDD and pCLE may be synergistic for creating an optical biopsy with greater accuracy for detecting and diagnosing malignancy.
Finally, we evaluated the optimized pCLE in a porcine laparoscopy setting, as a preclinical test. The probe had been operated manually in the mice model, but was manipulated with laparoscopic forceps in the porcine model. In this model, the stability and pressure of contact at the observational sites are uncertain, due to respiratory movements and the difficulty in stabilizing the probe tips in the pig anatomy. Any damage onto the observation sites was therefore our technical concerns. As a result, the image quality with pCLE was similar to that achieved with HE staining of histopathological sections, without any gross injuries onto the observation sites or other intra-abdominal organs. This showed that pCLE might be efficient and safe for performing an exploratory laparoscopy to examine peritoneal metastasis. The FS dye has been used clinically, particularly in ophthalmological examinations, and its safety has been established for human use. Thus, pCLE may be introduced for clinical use in exploratory laparoscopy. In addition, since the UltraMini O probe is 2.6 mm in diameter, which can be inserted through miniature catheter, it may be possible that we bring a probe to a target by echo-guided manipulation and examine various abdominal organs using this system. Thus, this system may be applicable not only to exploratory laparoscopy but also to abdominal examination with local anesthesia.
Our study has several limitations. First, to gain approval for clinical use, we must validate the efficacy and safety of pCLE in a human clinical trial. In parallel, we must build a consensus to establish criteria for diagnosing peritoneal metastasis based on pCLE imaging. Currently, a consensus is in the process of forming about the pattern of pCLE images needed to comprise an optical biopsy, particularly in Barrett’s esophagus and inflammatory bowel disease. The consensus board must include surgeons, who actually manipulate the system, and pathologists. With the use of telepathology or similar systems, the accuracy and reliability of the diagnosis can be improved. We have shown that the pCLE can provide a means to visualize cell morphology in the abdominal cavity as accurately as a histopathological section analysis.
In conclusion, we have successfully optimized the current CLE system for observation of peritoneal metastases. The pCLE system is potentially useful as real-time cancer diagnosis.
References
Nakao A, Fujii T, Sugimoto H, Kanazumi N, Nomoto S, Kodera Y, Inoue S, Takeda S (2006) Oncological problems in pancreatic cancer surgery. World J Gastroenterol 12:4466–4472
Marrelli D, Pedrazzani C, Morgagni P, de Manzoni G, Pacelli F, Coniglio A, Marchet A, Saragoni L, Giacopuzzi S, Roviello F, on behalf of the Italian Research Group for Gastric Cancer (IRGGC) (2011) Changing clinical and pathological features of gastric cancer over time. Br J Surg 98:1273–1283. doi:10.1002/bjs.7528
Imano M, Okuno K (2014) Treatment strategies for gastric cancer patients with peritoneal metastasis. Surg Today 44:399–404. doi:10.1007/s00595-013-0603-8
Ishigami S, Uenosono Y, Arigami T, Yanagita S, Okumura H, Uchikado Y, Kita Y, Kurahara H, Kijima Y, Nakajo A et al (2014) Clinical utility of perioperative staging laparoscopy for advanced gastric cancer. World J Surg Oncol 12:350
Yoon H (2014) New approaches to gastric cancer staging: beyond endoscopic ultrasound, computed tomography and positron emission tomography. World J Gastroenterol 20:13783. doi:10.3748/wjg.v20.i38.13783
Kikuchi H, Kamiya K, Hiramatsu Y, Miyazaki S, Yamamoto M, Ohta M, Baba S, Konno H (2014) Laparoscopic narrow-band imaging for the diagnosis of peritoneal metastasis in gastric cancer. Ann Surg Oncol 21:3954–3962. doi:10.1245/s10434-014-3781-8
Kishi K, Fujiwara Y, Yano M, Inoue M, Miyashiro I, Motoori M, Shingai T, Gotoh K, Takahashi H, Noura S, Yamada T, Ohue M, Ohigashi H, Ishikawa O (2012) Staging laparoscopy using ALA-mediated photodynamic diagnosis improves the detection of peritoneal metastases in advanced gastric cancer. J Surg Oncol 106:294–298. doi:10.1002/jso.23075
Kishi K, Fujiwara Y, Yano M, Motoori M, Sugimura K, Ohue M, Noura S, Marubashi S, Takahashi H, Sakon M (2014) Diagnostic laparoscopy with 5-aminolevulinic-acid-mediated photodynamic diagnosis enhances the detection of peritoneal micrometastases in advanced gastric cancer. Oncology 87:257–265. doi:10.1159/000365356
Wallace MB, Meining A, Canto MI, Fockens P, Miehlke S, Roesch T, Lightdale CJ, Pohl H, Carr-Locke D, LöHr M, Coron E, Filoche B, Giovannini M, Moreau J, Schmidt C, Kiesslich R (2010) The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment Pharmacol Ther 31:548–552. doi:10.1111/j.1365-2036.2009.04207.x
Becker V, van den Broek FJ, Buchner AM, Dekker E, Wallace MB, von Delius S, Schneider A, Schmid RM, Meining A (2011) Optimal fluorescein dose for intravenous application in miniprobe-based confocal laser scanning microscopy in pigs. J Biophotonics 4:108–113. doi:10.1002/jbio.201000028
Neumann H, Kiesslich R, Wallace MB, Neurath MF (2010) Confocal laser endomicroscopy: technical advances and clinical applications. Gastroenterology 139(388–392):e2. doi:10.1053/j.gastro.2010.06.029
Wallace MB, Fockens P (2009) Probe-based confocal laser endomicroscopy. Gastroenterology 136:1509–1513. doi:10.1053/j.gastro.2009.03.034
Neumann H, Langner C, Neurath MF, Vieth M (2012) Confocal laser endomicroscopy for diagnosis of Barrett’s esophagus. Front Oncol. doi:10.3389/fonc.2012.00042
Bertani H, Pigò F, Dabizzi E, Frazzoni M, Mirante VG, Manno M, Manta R, Conigliaro R (2012) Advances in endoscopic visualization of Barrett’s esophagus: the role of confocal laser endomicroscopy. Gastroenterol Res Pract 2012:1–5. doi:10.1155/2012/493961
Berzosa M, Wallace MB (2014) Surveillance of Barrett’s esophagus: why biopsy if you can endomicroscopy. Gastrointest Endosc 79:222–223. doi:10.1016/j.gie.2013.11.019
Bakhru MR, Sethi A, Jamidar PA, Singh SK, Kwon RS, Siddiqui UD, Sawhney M, Talreja JP, Kline P, Malik U, Gaidhane M, Sauer BG, Kahaleh M (2013) Interobserver agreement for confocal imaging of ampullary lesions: a multicenter single-blinded study. J Clin Gastroenterol 47(5):440–442. doi:10.1097/MCG.0b013e3182745f2b
Kuiper T, van den Broek F, van Eeden S, Wallace M, Buchner A, Meining A, van Hee K, Fockens P, Dekker E (2011) New classification for probe-based confocal laser endomicroscopy in the colon. Endoscopy 43:1076–1081. doi:10.1055/s-0030-1256767
Ussui VM, Wallace MB (2012) Confocal endomicroscopy of colorectal polyps. Gastroenterol Res Pract 2012:1–6. doi:10.1155/2012/545679
Meining A, Shah R, Slivka A, Pleskow D, Chuttani R, Stevens P, Becker V, Chen Y (2012) Classification of probe-based confocal laser endomicroscopy findings in pancreaticobiliary strictures. Endoscopy 44:251–257. doi:10.1055/s-0031-1291545
Smith I, Kline PE, Gaidhane M, Kahaleh M (2012) A review on the use of confocal laser endomicroscopy in the bile duct. Gastroenterol Res Pract 2012:1–5. doi:10.1155/2012/454717
Caillol F, Bories E, Autret A, Poizat F, Pesenti C, Ewald J, Turrini O, Delpero JR, Monges G, Giovannini M (2014) Evaluation of pCLE in the bile duct: final results of EMID study: pCLE: impact in the management of bile duct strictures. Surg Endosc. doi:10.1007/s00464-014-3986-8
Bok GH, Jeon SR, Cho JY, Cho J-H, Lee WC, Jin SY, Choi IH, Kim HG, Lee TH, Park EJ (2013) The accuracy of probe-based confocal endomicroscopy versus conventional endoscopic biopsies for the diagnosis of superficial gastric neoplasia (with videos). Gastrointest Endosc 77:899–908. doi:10.1016/j.gie.2013.01.018
Jeon SR, Cho WY, Jin SY, Cheon YK, Choi SR, Cho JY (2011) Optical biopsies by confocal endomicroscopy prevent additive endoscopic biopsies before endoscopic submucosal dissection in gastric epithelial neoplasias: a prospective, comparative study. Gastrointest Endosc 74:772–780. doi:10.1016/j.gie.2011.05.005
Guo J, Li C-Q, Li M, Zuo X-L, Yu T, Liu J-W, Liu J, Kou G-J, Li Y-Q (2015) Diagnostic value of probe-based confocal laser endomicroscopy and high-definition virtual chromoendoscopy in early esophageal squamous neoplasia. Gastrointest Endosc. doi:10.1016/j.gie.2014.10.041
Canto MI, Anandasabapathy S, Brugge W, Falk GW, Dunbar KB, Zhang Z, Woods K, Almario JA, Schell U, Goldblum J et al (2014) In vivo endomicroscopy improves detection of Barrett’s esophagus-related neoplasia: a multicenter international randomized controlled trial (with video). Gastrointest Endosc 79:211–221
Gómez V, Buchner A, Dekker E, van den Broek F, Meining A, Shahid M, Ghabril M, Fockens P, Heckman M, Wallace M (2010) Interobserver agreement and accuracy among international experts with probe-based confocal laser endomicroscopy in predicting colorectal neoplasia. Endoscopy 42:286–291. doi:10.1055/s-0029-1243951
Shahid MW, Buchner AM, Coron E, Woodward TA, Raimondo M, Dekker E, Fockens P, Wallace MB (2012) Diagnostic accuracy of probe-based confocal laser endomicroscopy in detecting residual colorectal neoplasia after EMR: a prospective study. Gastrointest Endosc 75(525–533):e1. doi:10.1016/j.gie.2011.08.024
Meining A, Chen YK, Pleskow D, Stevens P, Shah RJ, Chuttani R, Michalek J, Slivka A (2011) Direct visualization of indeterminate pancreaticobiliary strictures with probe-based confocal laser endomicroscopy: a multicenter experience. Gastrointest Endosc 74:961–968. doi:10.1016/j.gie.2011.05.009
Emoto S, Yamaguchi H, Kamei T, Ishigami H, Suhara T, Suzuki Y, Ito T, Kitayama J, Watanabe T (2014) Intraperitoneal administration of cisplatin via an in situ cross-linkable hyaluronic acid-based hydrogel for peritoneal dissemination of gastric cancer. Surg Today 44:919–926. doi:10.1007/s00595-013-0674-6
Newton RC, Noonan DP, Vitiello V, Clark J, Payne CJ, Shang J, Sodergren M, Darzi A, Yang G-Z (2012) Robot-assisted transvaginal peritoneoscopy using confocal endomicroscopy: a feasibility study in a porcine model. Surg Endosc 26:2532–2540. doi:10.1007/s00464-012-2228-1
Delius S, Feussner H, Wilhelm D, Karagianni A, Henke J, Schmid RM, Meining A (2007) Transgastric in vivo histology in the peritoneal cavity using miniprobe-based confocal fluorescence microscopy in an acute porcine model. Endoscopy 39:407–411. doi:10.1055/s-2007-966439
Sharma P, Meining AR, Coron E, Lightdale CJ, Wolfsen HC, Bansal A, Bajbouj M, Galmiche J-P, Abrams JA, Rastogi A, Gupta N, Michalek JE, Lauwers GY, Wallace MB (2011) Real-time increased detection of neoplastic tissue in Barrett’s esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest Endosc 74:465–472. doi:10.1016/j.gie.2011.04.004
Dunbar KB, Okolo P, Montgomery E, Canto MI (2009) Confocal laser endomicroscopy in Barrett’s esophagus and endoscopically inapparent Barrett’s neoplasia: a prospective, randomized, double-blind, controlled, crossover trial. Gastrointest Endosc 70:645–654. doi:10.1016/j.gie.2009.02.009
Acknowledgments
We would like to thank M. Fujimoto and S. Serada for their instructions of basic experimental methods. This work was partially supported by Japan NOTES Research Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Hara H., Natatsuka R., Higashi S., Miyazaki Y., Makino T., Kurokawa Y., Yamasaki Y., Takiguchi S., Mori M., Dok Y., and Nakajima K. have no conflicts of interest or financial ties to disclose. Dr. Takahashi T. has received honoraria from SBI Pharmaceuticals Co, Ltd.
Rights and permissions
About this article
Cite this article
Hara, H., Takahashi, T., Nakatsuka, R. et al. A novel approach of optical biopsy using probe-based confocal laser endomicroscopy for peritoneal metastasis. Surg Endosc 30, 3437–3446 (2016). https://doi.org/10.1007/s00464-015-4626-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4626-7