Abstract
Background
Laparoscopic total proctocolectomy (TPC) with or without ileoanal pouch is a major operation for which the traditional benefits of laparoscopy were not immediately apparent, in part due to the longer operating times. The use of energy devices has been shown to improve operative outcomes for patients who undergo laparoscopic segmental colectomies, but there are limited data for laparoscopic TPC (LTPC).
Methods
All patients who underwent LTPC between January 2002 and July 2011 were identified from a prospectively maintained institutional-review-board-approved database. Univariate and multiple linear regression analyses were performed to assess the impact of electrothermal bipolar vessel sealers (EBVS) for vessel ligation on operative time. Secondary outcomes included vessel ligation failures, estimated blood loss, and other intra- and postoperative outcomes.
Results
One hundred and forty-five patients underwent LTPC, including 126 restorative ileoanal pouch and diverting ileostomy operations and 19 TPC and end ileostomy procedures. Fifteen percent of LTPCs were totally laparoscopic, 45 % were laparoscopic-assisted, 32 % were hand-assisted, and 8 % were laparoscopic-converted cases. Laparoscopic vessel ligation was performed using EBVS (76 %), endoscopic staplers (12 %), or hybrid techniques (12 %). Vessel ligation groups were similar in demographics, body mass index, surgical indication, immunosuppression, and prior surgery. EBVS were associated with shorter median operative times (247 vs. 290 vs. 300 min, p = 0.018) and fewer vessel ligation failures (1 vs. 11 vs. 12 %, p = 0.027) compared with endoscopic staplers and hybrid techniques, respectively. There were no differences in estimated blood loss and intra-operative complications among the three groups. Length of stay, 30-day morbidity, and 30-day re-operation rates were also similar. On multiple linear regression analysis, EBVS were a significant predictor of operative time (p = 0.019).
Conclusions
Routine use of electrothermal bipolar vessel ligation for LTPC is associated with shorter operative time and fewer vessel ligation failures without higher risk of complications than other vessel control methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Laparoscopic total proctocolectomy (LTPC) has been shown to be both safe and feasible [1]. Unlike as was quickly noted with segmental colectomies, however, the benefits of laparoscopy were not immediately demonstrated for LTPC. Furthermore, the benefits of laparoscopy might be mitigated by the longer operative times of LTPC compared with open procedures [1–6], perhaps having deterred surgeons from adopting this potentially demanding approach.
One factor shown to impact operative time is vessel ligation technique. Various options exist for vessel ligation during laparoscopic colon resections, including electrothermal bipolar vessel sealers (EBVS), endoscopic staplers (ES), endoscopic clips (EC), and ultrasonic coagulating shears (UCS). The routine use of energy devices has been shown to improve operative outcomes for patients who undergo laparoscopic segmental colectomies. A meta-analysis that included six randomized controlled trials with 446 patients assessed the impact of energy sources for colon mobilization, dissection, and vessel ligation on operative outcomes of laparoscopic colectomies [7]. Although there was significant heterogeneity among the studies, the use of energy sources was associated with shorter operative time, improved hemostasis, and easier instrument handling.
To date, only three studies have specifically evaluated the impact of EBVS for vascular ligation on outcomes of LTPC: two retrospective studies and one prospective randomized study (Table 1) [8–10]. In these studies, the benefits of energy sources for vascular ligation included shorter operative times, fewer failures of vessel ligation, less estimated blood loss, and lower costs. However, these studies all featured small sample sizes.
The goal of our study was to evaluate the impact of EBVS for vessel ligation on operative time and other intra-operative and postoperative outcomes for LTPC in a large cohort.
Materials and methods
Patients
After institutional review board approval, all patients who underwent an LTPC between January 2002 and July 2011 at the Cleveland Clinic Florida were identified from a prospectively maintained database. All patients with concomitant colorectal cancer were excluded.
Definition of patient and operative characteristics
The LTPC cases were performed by seven board-certified colorectal surgeons with advanced experience in laparoscopic colorectal surgery. In addition, colorectal surgery residents and/or fellowship-accredited fellows assisted in all cases. Procedures were classified as LTPC with ileal pouch–anal anastomosis (IPAA) and a diverting ileostomy or as LTPC with an end ileostomy. Furthermore, procedures were categorized as being performed by high-LTPC volume (>50 cases/surgeon during the study period) or low-LTPC volume surgeons (<10 cases/surgeon during the study period). There were two high-LTPC volume and five low-LTPC volume surgeons in this study. Procedures were also categorized as being performed in high- or low- LTPC volume years (2008–2011: >30 cases per year vs. 2002–2007: <15 cases per year). Normal and overweight body mass indices were defined by criteria of the National Institutes of Health as a BMI of 18.5–25 kg/m2 and ≥25 kg/m2, respectively [11].
The laparoscopic approach was categorized as total laparoscopic, laparoscopic-assisted, hand-assisted, or laparoscopic-converted. Total laparoscopic (TL) was defined as a completely laparoscopic operation with extraction of the specimen through the perineum or the stoma site, without any additional incisions. Laparoscopic-assisted (LA) was defined as a totally laparoscopic procedure, with an incision for specimen extraction, including a Pfannenstiel or umbilical incision. Hand-assisted (HA) was defined as a planned Pfannenstiel or lower midline incision used to insert a hand-assisted working port to perform the operation laparoscopically. Laparoscopic-converted (LC) was defined as any unplanned incision—due to failure to progress or technical difficulties—that was used to complete the dissection, mobilization, vessel ligation, or transection of the bowel or any incision >5 cm.
Immunosuppression was defined as any of the following: steroid equivalent of ≥5 mg of prednisone daily for longer than 3 weeks, chemotherapy within 6 weeks of the operation, methotrexate or other disease-modifying rheumatoid agents, transplant medications, or biologics.
Vessel ligation
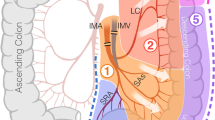
The ileocolic arcade was preserved or divided according to surgeon preference. The middle colic and the inferior mesenteric or superior rectal vessels were divided with a low ligation. The remainder of the mesentery was distally divided, close to the colon wall.
The vessel ligation technique was at the discretion of the surgeon. Methods of vessel ligation were grouped into one of three categories: (1) electrothermal bipolar vessel sealers (EBVS), using 5- or 10-mm instruments with three lines of overlapping sealing applications; (2) endoscopic staplers (ES), using 45- or 60-mm endoscopic linear cutters with a vascular 1.0-mm staple height cartridge; or (3) hybrid techniques (HT), using more than one vascular ligation method primarily (not due to management of a device failure) including EBVS, ES, EC, and/or UCS. Vessel ligation failure was defined as bleeding due to a failure to adequately ligate a vascular pedicle, which necessitated additional vessel ligation maneuvers.
Outcomes
The primary outcome was operative time and was defined as the time from skin incision to skin closure. The secondary outcomes included failure of vessel ligation, estimated blood loss, intra-operative transfusions, intra-operative complications, length of incision, and postoperative outcomes. The estimated blood loss was prospectively collected by measuring irrigation fluid and weighing surgical sponges. A chart review was conducted to ascertain vessel ligation technique and management of failed vascular pedicle ligations. Length of incision was measured at the end of the operation and prospectively recorded. Postoperative outcomes were collected by chart review and included length of stay, prolonged ileus (defined by placement of a nasogastric tube or need for parenteral nutrition), readmission (within 30 days postoperatively), reoperation (within 30 days postoperatively), and overall 30-day postoperative morbidity. The following specific postoperative complications were evaluated: wound infections, anastomotic leaks, intra-abdominal or pelvic abscesses, anastomotic bleeds, intra-abdominal bleeds, and medical complications including pneumonia, deep vein thrombosis, urinary tract infection, pulmonary embolism, and myocardial infarction.
Statistical analysis
Data are presented as means with standard deviations, medians with interquartile ranges, or percentages as appropriate. LTPC cases performed with EBVS, ES, or HT were compared using the Chi-square trend, one-way ANOVA, and Kruskal–Wallis tests where appropriate. A multiple linear regression model was constructed, including any variable thought to be clinically relevant a priori and those that were significant on univariate analysis at a p value <0.10. As our main interest was the effect of EBVS, for the purpose of the multiple linear regression, we grouped ES and HT together, comparing EBVS to all other techniques. Statistical analyses were performed using JMP® 11 (SAS Institute Inc., Cary, NC, 1989–2007).
Results
One hundred forty-five patients underwent a LTPC between 2002 and 2011, of whom 126 had an IPAA with a diverting ileostomy and 19 had an end ileostomy. The mean age was 42 (±16.6) years, and 50 % were male. The indications for LTPC were mucosal ulcerative colitis (79 %), familial adenomatous polyposis (11 %), Crohn’s disease (7 %), polyps (2 %), and collagenous colitis (1 %). The distribution of surgical approaches was as follows: total laparoscopic (15 %), laparoscopic-assisted (45 %), hand-assisted (32 %), and laparoscopic-converted (8 %). Patients were divided into three groups based on laparoscopic vessel ligation technique: EBVS (n = 110, 76 %), ES (n = 18, 12 %), and HT (n = 17, 12 %).
All three groups were similar in age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) class, immunosuppression at the time of colectomy or within 6 weeks prior to colectomy, and history of prior abdominal surgery (Table 2). The use of EBVS or ES for vessel ligation was more likely to be performed by a high-LTPC volume surgeon compared to HT (87 vs. 83 vs. 59 %, respectively; p = 0.030), while EBVS were more commonly employed in the high-LTPC volume years than ES or HT (86 vs. 22 vs. 18 %, respectively; p < 0.0001). Accordingly, the use of EBVS increased throughout the study period (Fig. 1).
On univariate analysis, EBVS was associated with significantly shorter median operative times (247 vs. 290 vs. 300 min, p = 0.018) and fewer vessel ligation failures (1 vs. 11 vs. 12 %, p = 0.027) compared with ES and HT, respectively. Vessel ligation failure was managed with an endoscopic stapler in the sole case of EBVS failure, and by endoscopic clips or conversion to open ligature technique in the two cases of ES failure and in the two cases of HT failure, respectively. EBVS were also associated with a shorter length of incision (7.0 vs. 9.0 vs. 13.0 cm, p = 0.0025) compared with the ES and HT approach. There were no differences in estimated blood loss and intra-operative complications between the three groups (Table 3). Postoperative outcomes, including length of stay, 30-day morbidity, and 30-day re-operation rates, were also similar (Table 4). Only 30-day re-admission rates were fewer in the EBVS group (42 vs. 61 vs. 71 %, p = 0.039). When performing the same univariate analysis with respect to operative time as the dependent variable, BMI > 25 kg/m2 (280 vs. 240 min, p = 0.008) and low-LTPC volume surgeon (280 vs. 250 p = 0.05) were predictive of significantly longer operative times. Laparoscopic approach—namely a TL, LA, or HA approach—had no significant impact (257 vs. 244 vs. 260 min, p = 0.60). Similarly, high-LTPC volume years (2008–2011) were not predictive of shorter operative time (248 vs. 270, p = 0.086), as can be observed in Fig. 2.
In order to control for the confounding effect of LTPC volume on operative time, a multiple linear regression model to predict operative time was constructed. After accounting for age, BMI, type of laparoscopic approach, high-LTPC volume surgeons, and years, the use of EBVS remained a significant predictor of shorter operative times (p = 0.019). In addition, BMI (p = 0.0002) was also a significant predictor of shorter operative times (Table 5).
Discussion
LTPC involves the division and ligation of multiple large vessels and a lengthy mesentery, the approaches which can vary by surgeon. Currently, the multiple options for vessel ligation during laparoscopic colectomies include EBVS, ES, EC, and UCS. Furthermore, some surgeons opt to mobilize the colon laparoscopically and complete part or all of the vessel ligation with open vascular ligature. In an attempt to standardize instrumentation and identify the optimal technique to improve operative outcomes, studies have investigated the benefits of different methods, but few have reported on this subject specifically in proctocolectomies. To our knowledge, this is the largest study to date to address the impact of vessel ligation technique on LTPC outcomes. We addressed this question in a large cohort of LTPC cases performed in one institution and found the use of EBVS for vessel ligation to be a predictor of shorter operative time.
Akari et al. [8] were the first group to evaluate vessel ligation technique for LTPC. They compared 18 cases using EBVS to 15 using UCS—all of which were performed for ulcerative colitis—and observed a shorter operative time (approximately 80 min), less estimated blood loss, and less postoperative bleeding in the EBVS group. Marcello et al. [10] prospectively evaluated the impact of energy devices on outcomes of 100 laparoscopic colectomies, randomizing 52 patients to EBVS and 48 to ES or EC. They reported their results both as a whole cohort of 100 patients and by type of colectomy. A subgroup of 23 patients underwent total colectomy, including 16 LTPC, and investigators observed a reduced operative time of around 30 min with EBVS, though statistical significance was not achieved. They also reported fewer ligation failures (1.2 vs. 12 %) and a significant mean cost saving of 248$ per total colectomy in the EBVS group. However, this study was not powered to detect significant differences within each subgroup, and furthermore, the LTPC cases could not be isolated from the laparoscopic total abdominal colectomies. Finally, in 2010, Nakajima et al. [9] included 66 cases of LTPC in their retrospective study, comparing 37 performed with EBVS to 29 with UCS. They demonstrated a shorter operative time of almost 90 min in the EBVS group, as well as less estimated blood loss.
To our knowledge, the present study of 145 patients is the largest to date to address the impact of vessel ligation techniques on the outcomes of LTPC. We found that the use of EBVS for vessel ligation shortened operative time, with a reduction of 40–50 min when compared to ES or HT. This effect persisted on multiple linear regression analysis, indicating that EBVS had a statistically significant independent impact on operative time. This observed reduction in operative time can be explained, namely by reduced instrument changes and fewer ligation failures (and subsequent irrigation, suction, and re-ligation). Additionally, vessel ligation by ES necessitates isolation of the vascular pedicle, creation of a mesenteric window on either side of the blood vessel, followed by ligation and division, which can contribute to a significant duration of time in a TPC where there are multiple vessels to ligate. In contrast, vessel ligation by EBVS may be by mass ligation or by mere identification of the vascular pedicle prior to ligation and division.
However, there are many factors other than vessel ligation technique that may impact total operative time, and these include surgeon experience with the case, disease factors such as inflammation, adhesions from prior abdominal surgeries, and patient factors such as obesity. Our three groups of patients were similar with respect to all patient and operative characteristics. The HT group had a lower percentage of cases performed by the two high-LTPC volume surgeons, but these same surgeons’ use of EBVS and ES were similar (87 vs. 83 %, p = 0.66). Therefore, surgeon experience cannot account for the difference in operative time observed in the EBVS and ES groups, specifically. There was also a significant difference in the percentage of cases performed in high-LTPC volume years, with EBVS being increasingly employed throughout the study period. However, we have been performing LTPC at our institution since 1992 [12], and the period of this study was specifically chosen to exclude the LTPC learning curve. BMI certainly is another important predictor of operative outcomes and has been well documented in previous studies to increase both operative times (as in the current study) and postoperative morbidity following TPC [13, 14]. Unfortunately, this is most often not a modifiable factor and thus emphasizes the importance of identifying those factors within the surgeon’s control that may reduce operative times. In our multiple linear regression model, after accounting for all these variables, EBVS still had a significant impact on operative time.
There are several other benefits to the use of EBVS for vessel ligation. We observed fewer vessel ligation failures with the use of EBVS compared to ES and HT, which is consistent with other studies [10, 15]. EBVS utilize a high current with a low voltage for ligation and division of vessels up to 7 mm in diameter. When the forceps are closed onto tissues, the energy delivered quickly denatures the collagen and elastin in the vessel wall, allowing protein to form a strong seal [16, 17]. Accordingly, this can also lead to a reduction in intra-operative estimated blood loss [8, 9], though we did not observe this trend.
In conclusion, the use of EBVS for vessel ligation during LTPC is safe and highly successful. We have demonstrated in a large cohort of 145 patients who underwent LTPC that EBVS shorten operative time and are associated with fewer vessel ligation failures. This is of particular use for TPC cases, where the overall operative time is longer than for segmental colectomies. However, due to the retrospective nature of this study, a clear limitation is the possibility of a selection bias, and although we addressed the impact of specific surgeons and time within the study period as possible confounding factors in our analysis, this bias cannot be completely eliminated. In the quest for continued technological advancements to improve operative efficiency and outcomes, a large prospective comparison of vascular pedicle ligation methods in LTPC is needed to confirm these promising results.
References
Ahmed Ali U, Keus F, Heikens JT, Bemelman WA, Berdah SV, Gooszen HG, van Laarhoven CJ (2009) Open versus laparoscopic (assisted) ileo pouch anal anastomosis for ulcerative colitis and familial adenomatous polyposis. Cochrane Database Syst Rev 1:CD006267
Kelly K, Condon ET, Redmond HP, Kirwan WO (2010) The benefits of a laparoscopic approach in ileal pouch anal anastomosis formation: a single institutional retrospective case-matched experience. Ir J Med Sci 179:197–200
Gu J, Stocchi L, Geisler DP, Kiran RP (2011) Staged restorative proctocolectomy: laparoscopic or open completion proctectomy after laparoscopic subtotal colectomy? Surg Endosc 25:3294–3299
Fajardo AD, Dharmarajan S, George V, Hunt SR, Birnbaum EH, Fleshman JW, Mutch MG (2010) Laparoscopic versus open 2-stage ileal pouch: laparoscopic approach allows for faster restoration of intestinal continuity. J Am Coll Surg 211:377–383
Tileny HS, Lovegrove RE, Heriot AG, Purkayastha S, Constantinides V, Nicholls RJ, Tekkis PP (2007) Comparison of short-term outcomes of laparoscopic vs open approaches to ileal pouch surgery. Int J Colorectal Dis 22:531–542
El-Gazzaz GS, Kiran RP, Remzi FH, Hull TL, Geisler DP (2009) Outcomes for case-matched laparoscopically assisted versus open restorative proctocolectomy. Br J Surg 96:522–526
Tou S, Malik AI, Wexner SD, Nelson RL (2011) Energy source instruments for laparoscopic colectomy. Cochrane Database Syst Rev 5:CD007886
Akari Y, Noake T, Kanazawa M, Yamada K, Momosaki K, Nozoe Y, Inoue A, Ishibashi N, Ogata Y, Shirouzu K (2004) Clipless hand-assisted laparoscopic total colectomy using Ligasure Atlas. Kurume Med J 51:105–108
Nakajima K, Nezu R, Ito T, Nishida T (2010) Hand-assisted laparoscopic restorative proctocolectomy for ulcerative colitis: the optimization of instrumentation toward standardization. Surg Today 40:840–844
Marcello PW, Roberts PL, Rusin LC, Holubkov R, Schoetz DJ (2006) Vascular pedicle ligation techniques during laparoscopic colectomy. A prospective randomized trial. Surg Endosc 20:263–269
NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults (1998) Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res 6:51S–209S
Reissman P, Salky BA, Pfeifer J, Edye M, Jagelman DG, Wexner SD (1996) Laparoscopic surgery in the management of inflammatory bowel disease. Am J Surg 171(1):47–50
Efron JE, Uriburu JP, Wexner SD, Pikarsky A, Hamel C, Weiss GG, Nogueras JJ (2001) Restorative proctocolectomy with ileal anal pouch anastomosis in obese patients. Obes Surg 11:246–251
Canedo JA, Pinto RA, McLemore EC, Rosen L, Wexner SD (2010) Restorative proctectomy with ileal pouch-anal anastomosis in obese patients. Dis Colon Rectum 53:1030–1034
Heniford BT, Matthews BD, Sing RF, Backus C, Pratt B, Greene FL (2001) Initial results with an electrothermal bipolar vessel sealer. Surg Endosc 15:799–801
Campbell PA, Cresswell AB, Frank TG, Cuschieri A (2003) Real time thermography during energized vessel sealing and dissection. Surg Endosc 17:1640–1645
Harold KL, Pollinger H, Matthews BD, Kercher KW, Sing RF, Heniford BT (2003) Comparison of ultrasonic energy, bipolar thermal energy, and vascular clips for the hemostasis of small-, medium-, and large-sized arteries. Surg Endosc 17:1228–1230
Acknowledgments
The authors thank Elektra McDermott for her editorial assistance and preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Steven D. Wexner receives an inventor’s income from Medtronic/Covidien for intellectual property license and consulting fees from Medtronic for consulting.
Rights and permissions
About this article
Cite this article
Garfinkle, R., Boutros, M., Hippalgaonkar, N. et al. Electrothermal bipolar vessel ligation improves operative time during laparoscopic total proctocolectomy: a large single-center experience. Surg Endosc 30, 2840–2847 (2016). https://doi.org/10.1007/s00464-015-4565-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4565-3