Abstract
Background
To determine the therapeutic and cosmetic outcomes of patients with breast cancer treated with endoscopic axillary lymphadenectomy (EAL) combined with laparoscopically harvested pedicled omentum (LHPO) for immediate breast reconstruction.
Methods
Forty patients with early breast cancer underwent EAL, followed by quadrantectomy and LHPO for immediate breast reconstruction. All patients were evaluated for operating time, blood loss, postoperative hospital stay, complications, etc. The cosmetic outcomes were evaluated 6 months after the surgery, according to the Harris criteria.
Results
The average operating time was 308 min, including 39 min for EAL, 63 min for quadrantectomy, and 58 min for LHPO. The average blood loss was 70 ml, and was mainly incurred during breast resection. On average, the patients were discharged 9.5 days after the surgery. Partial graft necrosis and omental fat liquefaction occurred in one patient each. No other complications occurred after the surgery. No local recurrence or distant metastasis was found during the follow-up. The cosmetic results were mostly satisfactory. No size reduction of the reconstructed breast occurred after radiation therapy. Esthetic evaluation of the reconstructed breast showed that the cosmetic outcome was “excellent” in 35 patients, “good” in 4 patients, and “fair” in 1 patient.
Conclusions
EAL combined with LHPO for breast reconstruction is a viable, safe procedure that causes minimal surgical trauma and results in a soft, shapely breast postoperatively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Breast-conserving surgery (BCS) has become one of the chief surgical modalities for treating early breast cancer. BCS can enhance the quality of life of the patient, but it does not influence long-term mortality [1]. The rate of BCS is considered an important index of the quality of breast cancer treatment [2]. However, if the resected portion of the breast is bigger than 70 cm3 or constitutes over 25 % of the total breast mass, breast reconstruction is required to prevent severe deformity [3]. Most breast reconstructions involve breast prostheses or autologous tissue grafts. The disadvantages of prosthetic breast reconstruction include capsular contracture, hardening, and displacement of the implant. Furthermore, the shape of the prosthesis does not change with body posture, which patients report feels strange. Breast reconstruction with autologous grafts such as latissimus dorsi myocutaneous flap and transverse rectus abdominis myocutaneous flap involves severe surgical trauma, donor-site deformities, and an “unreal” feeling in the breast [4, 5]. It has long been reported that the omentum can be used for breast reconstruction because it is soft, highly vascularized, has strong survivability, and is resistant to infection. Moreover, it can be shaped in any way. Kiricuta [6] first reported the use of an omental flap for breast reconstruction after mastectomy in 1963. However, due to the requirement of open surgery to harvest the omentum, the procedure involved massive surgical trauma, which limited its clinical application. Jimenez et al. [7] used laparoscopic techniques to harvest large omental flaps in 2002, and thus, the technique of breast reconstruction with omental flap was revitalized. Zaha and Inamine [8] and Zaha et al. [9, 10] have applied this technique to perform one-stage breast reconstructions in more than 100 patients with breast cancer, which is the largest series reported to date; the surgical results in these patients were very satisfactory. Nevertheless, the technique still required a large axillary incision for conventional axillary lymph node dissection (CALND), and this would affect the postoperative esthetic appearance and upper-extremity function.
In this paper, we report our experience with endoscopic axillary lymphadenectomy (EAL) combined with laparoscopically harvested pedicled omentum (LHPO) for breast reconstruction. The aim of this study is to determine the therapeutic effects and cosmetic outcomes of patients treated with our EAL–LHPO technique.
Patients and methods
The study was approved by the ethics committee of our hospital. Forty patients were informed about all available treatment methods, including sentinel lymph node biopsy, and were fully informed about the risk of each operation. All the patients chose to undergo the EAL–LHPO method and provided written informed consent.
Patients
Between March 2011 and October 2013, 40 patients with breast cancer underwent EAL and quadrantectomy, immediately followed by breast reconstruction with an LHPO. The mean body mass index (BMI) was 22.8. The characteristics of the patients are listed in Table 1. All tumors were evaluated preoperatively using mammography, ultrasonography, and computed tomography or magnetic resonance imaging and diagnosed as breast cancer through pre- or intraoperative biopsy.
The inclusion criteria were as follows: (1) stage I or II breast cancer with a single tumor, (2) maximum tumor diameter ≤30 mm, (3) strong desire to maintain an esthetic appearance of the breast, and (4) no history of abdominal laparotomy or intraabdominal malignancy.
Surgical procedures
Preoperative preparation
This included marking the location of the tumor, the contour of the breast, and the range of resection.
Position
Under general anesthesia with tracheal intubation, the patient was placed in the supine straddle position with the affected side raised by 30°. The ipsilateral arm was abducted to 90°, and the forearm was hung along the frame of the bed.
EAL
A nanocarbon tracer was subcutaneously injected under the areola or around the tumor. Next, a 10-mm incision was made along the midaxillary line at the level of the lower margin of the breast (inspection incision), and lipolysis liquid (250 ml of physiological saline, 250 ml of sterile purified water, 30 ml of 5 % NaHCO3, 20 ml of 2 % lidocaine, and 1 ml of 0.1 % adrenaline mixed to form a total volume of 551 ml) was injected into the axilla at several points. Then, the axillary fat was aspirated through the inspection incision, and a 10-mm trocar was inserted into the liposuction hole. Through this trocar, CO2 was infused into the axilla to about 11 mm Hg of pressure to establish the working space. Finally, two additional 5-mm trocars were placed in the anterior axillary line at the nipple level (first operating incision) and the posterior axillary line at the nipple level (second operating incision), respectively. The cobweb-like lymphatic, remaining fat, and lymph nodes attached to the blood vessels were severed and peeled with ultrasound scissors. Axillary level I, II, and occasionally level III lymph nodes were dissected. The axilla was washed with distilled water and continuously drained using a rubber tube placed in the axilla through the inspection incision (Fig. 1).
Endoscopically assisted quadrantectomy
A 30-mm incision was made along the areola, and wide excision of the breast (>25 % of the breast tissue), including the tumor with margins of at least 20 mm, was performed. We used the endoscope for cutting along the edge of the breast when the areolar incision was too small or when the volume of the breast was so large that the view was unclear. Samples of the lateral and superior margins, the fascia of the pectoralis major under the tumor, and the tissue under the nipple were sent for frozen-section analysis to confirm negative margins (Fig. 2).
LHPO
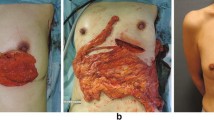
The patient was placed in the supine position, and the surgeon stood at the patient’s crotch. The laparoscopic instruments were replaced with clean ones to prevent the spread of tumor cells. A 10-mm incision was made 50 mm below the umbilicus in the midline (inspection incision). Two 5-mm incisions for the surgical instruments were made on each side of the lower abdomen at the edge of the rectus muscle. One additional 30-mm incision was made below the arch of the ribs on the affected side. The omentum was first evaluated for size and adhesions, and then moved in the cephalad direction. The dissection was then started at the middle of the transverse colon. After gaining entry into the lesser sac, the dissection was slowly advanced, to both the right and left, between the omentum and the transverse colon. We selected the gastroepiploic artery and vein (GEAV) on the affected side as a pedicle, and the branches of the GEAV were divided at a site as close to the stomach wall as possible. After dissection of the omentum, a subcutaneous tunnel approximately two fingers wide was prepared from the 30-mm incision below the arch of the ribs toward the residual cavity of the breast. Oval forceps were inserted into the abdominal cavity, and the pedicled omental flap was carefully taken out and transferred to the residual cavity to reconstruct the breast, while avoiding any twisting. The 30-mm incision was closed after the flap was pulled out to prevent postoperative incisional hernia. Finally, the abdominal cavity was flushed, and all the incisions were closed (Fig. 3).
All patients were treated with systemic therapy and radiation therapy according to standard institutional protocols (National Comprehensive Cancer Network [NCCN] Breast Cancer Practice Guideline). In addition, patients with Her-2 (+++) were treated with Herceptin. No case of breast cancer recurrence was found during the follow-up, which lasted for 6–36 months after the operation.
Therapeutic effects and cosmetic outcomes evaluation
All patients were evaluated for operating time, blood loss, postoperative hospital stay, complications, etc. Cosmetic outcomes were evaluated 6 months after the surgery, according to the Harris criteria [11]. (1) Excellent: size and shape of the reconstructed breast are almost the same as those of the original breast; (2) good: deformity of the reconstructed breast involves less than 1/4 of the original breast; (3) fair: deformity of the reconstructed breast involves 1/4–1/2 of the original breast; and (4) poor: breast deformity involves more than 1/2 of the original breast.
Results
Operative results
The operating time ranged from 210 to 420 min, with an average of 308 min, including an average time of 39 min for EAL, 63 min for breast quadrantectomy, and 58 min for LHPO. The average blood loss during the operation was 70 ml, and was mainly incurred during breast quadrantectomy. Intraoperative conversion to open surgery due to uncontrollable bleeding was not required in any patient. On average, patients were discharged 9.5 days (6–18 days) after the operation and were followed up for 15.6 months (6–36 months). Partial graft necrosis and omentum fat liquefaction occurred in one patient each. One patient had numbness in the inner side of the ipsilateral upper arm and recovered after 3 months. The other patients recovered quickly, and no other complications occurred after the surgery. No local recurrence or distant metastasis was found during the follow-up period (Tables 2, 3, 4).
Cosmetic results
The cosmetic results were mostly satisfactory at 6 months after the surgery. The scars on the breast, chest wall, and abdomen were not obvious. No deformity was found in the axilla in any patient. All patients were satisfied with the reconstructed breast, except for the patient who suffered partial graft necrosis. No size reduction of the reconstructed breast was found after radiation therapy (Table 5; Figs. 4, 5).
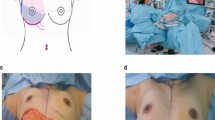
Clinical example of EAL combined with LHPO for one-stage breast reconstruction. A Marking the location of the tumor, the contour of the breast, and the range of breast resection before the surgery. B The size and shape of the reconstructed breast are almost the same as those of the original breast. The scars on the breast and abdomen are not obvious. No axillary deformity or limitation occurred in the affected upper limb 6 months after the surgery. C Thirty-six months after the surgery, the appearance of the reconstructed breast is the same as the original breast
Discussion
With the development of laparoscopic techniques, the aim of cancer surgery is not only the complete removal of the tumor but also the minimization of surgical trauma and improvement of the patient’s quality of life. EAL, which has been now been carried out worldwide, does not affect upper-limb function since it leaves only three small scars on the lateral chest wall. In fact, no significant differences in overall survival have been observed between EAL and CALND [12], and thus, EAL is preferred by both doctors and patients. LHPO, instead of open surgery, for breast reconstruction results in a soft, shapely breast [13]. Therefore, we combined EAL with LHPO for breast reconstruction in order to achieve improved therapeutic and cosmetic outcomes.
In CALND, an incision of 60–80 mm is usually made on the breast or axilla, and this leads to a bad postoperative appearance of the breast, edema, numbness, and limited mobility in the upper limb. In contrast, EAL, which can be successfully completed through three 5- to 10-mm incisions in the lateral chest wall, meets the requirements of conventional surgery and does not affect upper-limb function. Due to the amplification and special perspective of endoscopic vision, the veins, arteries, lymphatic vessels, and nerves in the axilla, and especially, the level II and III lymph nodes and Rotter’s lymph nodes were clearly identified. The target tissues were removed accurately and easily, while largely avoiding injury to the vessels and nerves, which is not possible with CALND. The small veins and lymphatic vessels that drain blood and lymph from the upper limb were preserved. Therefore, the possibility of arm lymphedema was reduced, and no case of postoperative arm lymphedema occurred in this study. In our study, EAL was performed before tumor removal in order to prevent the spread of cancer cells, via blood and lymphatic vessels, which would have otherwise occurred during tumor removal. This conforms to the principles of tumor surgery. Similar results have been reported by other studies on EAL [12, 14]. This technique not only ensures good therapeutic outcomes and reduces complications but also provides good cosmetic results, and thus, is greatly superior to CALND [15]. According to the 2014 NCCN breast cancer guidelines, sentinel lymph node biopsy is recommended in patients with early-stage breast cancer. However, due to the 8 % false-negative rate of sentinel lymph node biopsy, and the fact that EAL is less invasive and causes less functional limitation of the upper limb, our patients chose to undergo EAL.
We have optimized the endoscopic approach by summarizing the previous 60 cases of EAL (lateral thoracic wall approach; Fig. 1A). Through the preoperative injection of a lymphatic tracer and the stratified injection of an improved lipolysis solution into the axilla (mixed with 30 ml of 5 % sodium bicarbonate solution to obtain 551 ml lipolysis liquid), liposuction can be carried out immediately. Compared with the mastoscopic axillary lymph node dissection reported by Chengyu et al. [16], our technique reduced the operating time by about 10 min. In addition, the axillary fat was dissolved completely, and the intercostobrachial nerve innervating the skin was preserved well, which largely reduced the incidence of postoperative sensory disturbances (Fig. 1B). Only one of our patients developed ipsilateral upper-limb numbness, but without axillary pain, malformation, dyskinesia, or lymphatic edema of the upper extremity.
In each of the 40 patients in our study, EAL was performed first and then 1/4–1/2 of the breast was resected, and finally LHPO for one-stage breast reconstruction was performed. Bowel function usually recovered 1 day after the surgery. Normal activity was recovered 4–5 days after the operation. All patients underwent the surgery successfully without any instances of uncontrollable bleeding and conversion to open surgery. No cases of stomach pain, indigestion, intestinal obstruction, and incisional hernia occurred in our study.
Slight swelling and hardening of the omentum in the reconstructed breast occurred by 1–3 months postoperatively. However, the texture of the omentum gradually recovered by 3–6 months after the surgery. This is consistent with the findings reported in the literature [8, 17]. The reason for this change is not very clear, but it may be related to local bleeding and transient ischemia in the omental flap. One of our patients developed partial graft necrosis, and required negative drainage for about 2 weeks, after which the incision healed. This patient had a history of gastric ulcer, and adhesions were found between the gastrocolic ligament and gastric wall during the operation. This may have led to injury of the gastroepiploic artery and eventually to partial graft necrosis. The cosmetic outcome in this patient was “fair”. Another patient in our study had a recurrent fat-like liquid exudate from the breast incision, resulting in a non-healing wound. Despite repeated debridement, the incision had failed to heal by 1 month later. Finally, the incision was sealed, and negative drainage was placed for about 3 weeks to promote the growth of granulation tissue. With these measures, the incision finally healed. The cosmetic result in this patient was “good”. Since the greater omentum has a secretory function, a small amount of liquid may have been secreted in the reconstruction site, leading to non-healing of the surgical wound. This condition can be treated by opening the incision and providing continuous suction until there is growth of granulation tissue, leading to wound healing. In one patient, a radial incision was used to resect the outer lower quadrant of the breast followed by omental reconstruction, and the postoperative incision scar caused mild ipsilateral breast deformation, sagging breasts, and contralateral asymmetry. In this patient also, the cosmetic outcome was evaluated as “good”. Such a deformity did not occur in the patients who received an areolar incision, which was thus a better choice. The other patients recovered quickly, and had no complications. In order to reduce recurrence and abdominal metastasis, we used a preoperative puncture biopsy, whenever possible, to diagnose the disease. The whole breast quadrant (more than 25 % of the breast tissue) was resected, including the tumor with safe margins of at least 20 mm. Thus, the tumor was not exposed, and metastasis was prevented. The laparoscopic instruments were replaced with clean ones to harvest the omentum. After the LHPO, the abdominal cavity was flushed, and the incision was closed immediately. These steps were taken to prevent the abdominal spread of tumor cells. In addition, the reconstructed area was treated with electron beam irradiation to reduce the risk of local recurrence after conventional chemotherapy and radiotherapy. All patients were followed up for 6–36 months, and no local recurrence and distant metastasis were found. Similar results have been reported in the literature [10, 13].
Compared with other reconstruction techniques, LHPO for breast reconstruction has the following advantages: (1) Omental breast reconstruction has more advantages in terms of flexibility, symmetry, and “realness”. The omentum is soft and can be easily shaped as needed, unlike other filling materials such as prosthesis and muscle. Thus, the omentum can be conveniently used to fill in any part of the breast. (2) Omental tissue has a rich blood supply, good viability, and strong resistance to infection. In addition, the shape and size of the omentum remains stable after radiotherapy, and it is suitable for one-stage breast reconstruction after BCS. (3) The omentum can be harvested laparoscopically using three 0.5- to l-cm trocars inserted into the abdominal wall. Thus, the abdominal organs are minimally affected, and normal oral feeding can be restored on the day after the surgery. The surgery is relatively simple and usually lasts less than 1 h [8]. (4) Since the omentum is intraabdominal, LHPO does not result in donor-site deformities. It has been reported that the incidence rate of incisional hernia after open surgery to obtain the omentum is 20–26 % [18]. However, no case of incisional hernia occurred in our study, in which we used laparoscopic techniques to harvest the omentum.
The disadvantage of LHPO for breast reconstruction is that it is difficult to accurately assess the omental condition before the surgery, as no appropriate preoperative imaging methods can accurately reveal the omental condition. In our study, the BMI of the patients ranged from 16.5 to 32.3. We believe that our technique can be used in obese patients. Generally, omental tissue can meet the needs of breast reconstruction when no more than 1/3 of the breast has been resected. Therefore, LHPO is more suitable for breast reconstruction after BCS. In two of our patients with relatively large tumors, 1/2 of the breast was resected, and the omental tissue was insufficient to fill in the breast defect. Thus, the affected breast was smaller than the contralateral breast. In both these patients, the cosmetic results were evaluated as “good”. Patients with a history of abdominal surgery may have omental adhesions, which render the harvesting of an omental flap difficult. Therefore, a history of abdominal surgery or peritonitis should be taken into account when planning this type of surgery, in order to prevent surgical failure because of intraoperative difficulties in developing the omentum. However, a history of laparoscopic appendectomy or laparoscopic fallectomy does not affect the outcomes of this breast reconstruction surgery. Our study included one patient with a history of laparoscopic appendectomy and one with a history of laparoscopic fallectomy. Both patients were found to have mild omental adhesions intraoperatively, but the omentum could be easily separated.
Conclusion
EAL combined with LHPO for breast reconstruction is a viable, safe procedure that causes minimal surgical trauma and results in a soft, shapely breast postoperatively.
References
Veronesi U, Salvadori B, Luini A, Greco M, Saccozzi R, Del VM, Mariani L, Zurrida S, Rilke F (1995) Breast conservation is a safe method in patients with small cancer of the breast. Long-term results of three randomised trials on 1,973 patients. Eur J Cancer 31A:1574–1579
Caffo O, Amichetti M, Ferro A, Lucenti A, Valduga F, Galligioni E (2003) Pain and quality of life after surgery for breast cancer. Breast Cancer Res Treat 80:39–48
Krekel N, Zonderhuis B, Muller S, Bril H, van Slooten HJ, de Lange DKE, van den Tol P, Meijer S (2011) Excessive resections in breast-conserving surgery: a retrospective multicentre study. Breast J 17:602–609
Cyriac C, Sharma RK, Singh G (2010) Assessment of the abdominal wall function after pedicled TRAM flap surgery for breast reconstruction: use of modified mesh repair for the donor defect. Indian J Plast Surg 43:166–172
Rezai M, Darsow M, Kummel S, Kramer S (2008) Autologous and alloplastic breast reconstruction-overview of techniques, indications and results. Gynakol Geburtshilfliche Rundsch 48:68–75
Kiricuta I (1963) The use of the great omentum in the surgery of breast cancer. Presse Med 71:15–17
Jimenez AG, St GP, Sirois M, Hatheway M, Lethbridge R (2002) Free omental flap for skin-sparing breast reconstruction harvested laparoscopically. Plast Reconstr Surg 110:545–551
Zaha H, Inamine S (2010) Laparoscopically harvested omental flap: results for 96 patients. Surg Endosc 24:103–107
Zaha H, Onomura M, Nomura H, Umekawa K, Oki M, Asato H (2012) Free omental flap for partial breast reconstruction after breast-conserving surgery. Plast Reconstr Surg 129:583–587
Zaha H, Sunagawa H, Kawakami K, Touyama T, Yonaha T, Ohshiro N (2010) Partial breast reconstruction for an inferomedial breast carcinoma using an omental flap. World J Surg 34:1782–1787
Harris JR, Levene MB, Svensson G, Hellman S (1979) Analysis of cosmetic results following primary radiation therapy for stages I and II carcinoma of the breast. Int J Radiat Oncol Biol Phys 5:257–261
Luo C, Guo W, Yang J, Sun Q, Wei W, Wu S, Fang S, Zeng Q, Zhao Z, Meng F, Huang X, Zhang X, Li R, Ma X, Luo C, Yang Y (2012) Comparison of mastoscopic and conventional axillary lymph node dissection in breast cancer: long-term results from a randomized, multicenter trial. Mayo Clin Proc 87:1153–1161
Zaha H, Inamine S, Naito T, Nomura H (2006) Laparoscopically harvested omental flap for immediate breast reconstruction. Am J Surg 192:556–558
Gomatos IP, Filippakis G, Albanopoulos K, Zografos G, Leandros E, Bramis J, Konstadoulakis MM (2006) Complete endoscopic axillary lymph node dissection without liposuction for breast cancer: initial experience and mid-term outcome. Surg Laparosc Endosc Percutan Technol 16:232–236
de Wilde RL, Schmidt EH, Hesseling M, Mildner R, Frank V, Tenger M (2003) Comparison of classic and endoscopic lymphadenectomy for staging breast cancer. J Am Assoc Gynecol Laparosc 10:75–79
Chengyu L, Yongqiao Z, Hua L, Xiaoxin J, Chen G, Jing L, Jian Z (2005) A standardized surgical technique for mastoscopic axillary lymph node dissection. Surg Laparosc Endosc Percutan Technol 15:153–159
Cothier-Savey I, Tamtawi B, Dohnt F, Raulo Y, Baruch J (2001) Immediate breast reconstruction using a laparoscopically harvested omental flap. Plast Reconstr Surg 107(1156–1163):1164–1165
van Garderen JA, Wiggers T, van Geel AN (1991) Complications of the pedicled omentoplasty. Neth J Surg 43:171–174
Disclosures
Pusheng Zhang, Yunfeng Luo, Jianwen Deng, Guoli Shao, Shuai Han and Zonghai Huang have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Pusheng Zhang and Yunfeng Luo contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, P., Luo, Y., Deng, J. et al. Endoscopic axillary lymphadenectomy combined with laparoscopically harvested pedicled omentum for immediate breast reconstruction. Surg Endosc 29, 1376–1383 (2015). https://doi.org/10.1007/s00464-014-3808-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-014-3808-z