Abstract
Background
The goal of this study was to evaluate the short-term outcomes of robotic-assisted lateral lymph node dissection for patients with advanced lower rectal cancer.
Methods
Between 2012 and 2013, 50 consecutive patients underwent robotic-assisted lateral lymph node dissection for rectal cancer in Shizuoka Cancer Center Hospital. Perioperative outcomes including operative time, operative blood loss, length of stay, postoperative complications, and histopathological data were collected prospectively.
Results
Median patient age was 62 years (range 36–74 years). Operative procedures included low anterior resections (n = 27), intersphincteric resections (n = 16), and abdominoperineal resections (n = 7). Bilateral lymph node dissection was performed in 44 patients. The median operative time was 476 min (range 320–683 min), and the median time required for lateral lymph node dissection was 165 min (range 85–257 min). The median blood loss was 27 mL (range 5–690 mL). There were no cases of open surgery or laparoscopic conversion. The median duration of postoperative hospital stay was 8 days (range 6–13 days). Clavien–Dindo classification Grade III–IV complications occurred in only one patient (2.0 %). There were no cases of anastomotic leak. There was no perioperative mortality. The median number of harvested lateral lymph nodes was 19 (range 5–47).
Conclusions
Robotic-assisted lateral lymph node dissection is a safe, feasible, and useful approach for patients with advanced lower rectal cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Several randomized trials have investigated the comparative oncological safety of laparoscopic surgery for colorectal cancer versus open surgery [1–4]. These studies have suggested that laparoscopic surgery is associated with less blood loss, faster recovery, and shorter hospital stays [5, 6].
However, when using laparoscopic surgery for rectal cancer, the circumferential resection margin positivity (16 %) and the conversion rate (34 %) was high in a subgroup analysis of the Medical Research Council Conventional versus Laparoscopic-Assisted Surgery In patients with Colorectal Cancer trial (MRC CLASICC trial) [7], which might be related to the high degree of technical difficulty when performing surgery in the narrow pelvic cavity.
Since the da Vinci surgical system (Intuitive Surgical, Sunnyvale, CA, USA) was approved by the food and drug administration (FDA) in 2000, use of robotic surgery has become widespread. Robotic total mesorectal excision for rectal cancer was first described in 2006 [8]. Robotic surgery requires high-quality three-dimensional imaging and utilizes sensitive, complicated manipulation of forceps to enable surgery in the narrow pelvic cavity [9, 10]. Baik et al. [11] rated the mesorectal specimen on three scales and reported that robotic surgery was superior to laparoscopic surgery in terms of accuracy of total mesorectum excision (TME). The safety and feasibility of rectal cancer surgery have been described when using laparoscopic surgery versus robotic surgery [11–14].
In Western countries, neoadjuvant chemoradiation therapy (CRT) has become the standard treatment for locally advanced lower rectal cancer. In Japan, lateral lymph node dissection (LLD) is the standard treatment for locally advanced lower rectal cancer. This is because the incidence of lateral lymph node metastasis from lower rectal cancer is 15.6–20.1 % [15, 16]. Further, 7.4 % of patients who are classified as preoperative lateral lymph node-negative by computed tomography (CT) or magnetic resonance imaging (MRI) were subsequently found to have lateral lymph node metastasis [17].
LLD for advanced lower rectal cancer seems to be a good indication for robotic surgery because of its much higher difficulty compared to laparoscopic TME for rectal cancer. There are few reports of patients undergoing robotic-assisted lateral lymph node dissection (RALLD), even among those undergoing laparoscopic LLD. Therefore, the goal of this study was to evaluate the short-term outcomes and safety of RALLD for patients with advanced lower rectal cancer.
Materials and methods
Between February 2012 and November 2013, we performed 159 consecutive robotic surgeries for rectal cancer at Shizuoka Cancer Center Hospital. Fifty of the 159 patients had undergone robotic surgery with LLD for advanced lower rectal cancer. Indications for LLD were lower rectal cancer with T3–4, or T1–2 rectal cancer with metastasis of lateral lymph node, as described by the Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines for the treatment of colorectal cancer [16]. Lower rectal cancer was defined as the lower border of the tumor located distal to the peritoneal reflection. Preoperative evaluation included digital rectal examination, histological confirmation of adenocarcinoma, colonoscopy, CT, and MRI and barium enema. Patients were staged using the tumor node metastasis (TNM) classification. Although the standard treatment was bilateral LLD, patients who were at high risk of complications due to comorbid conditions, such as uncontrolled diabetes or unstable angina, underwent only unilateral LLD. Neoadjuvant CRT was given when circumferential resection margin positivity was seen on preoperative CT or MRI.
Patients were followed up at every 3 months for 3 years after surgery and then every 6 months until 5 years. Male sexual function was assessed at 6 months and 12 months after surgery, using International Index of Erection Function (IIEF) and original questions regarding erection and ejaculation. Urinary function was also assessed. Residual urine measurement was performed after removal of urethral catheter on the fifth postoperative day. A residual urine volume of ≥50 mL was regarded as early urinary dysfunction. Patients performed self-catheterization until residual urine becoming <50 mL. All data were collected prospectively. Informed consent was obtained from all patients. This study was approved by the Institutional Review Board of Shizuoka Cancer Center Hospital.
Surgical technique
All operations were performed by three surgeons (Y.K., A.S., and T.Y.) with a long experience for laparoscopic colorectal surgery. They had no experience of laparoscopic LLD, but the experience of laparotomy LLD.
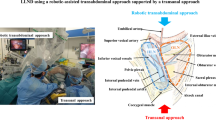
Patients were placed in the Trendelenburg position. In principle, one camera port, three 8 mm robotic ports, and two laparoscopic trocars were placed (Fig. 1). The docking method (e.g., dual docking, single docking, or hybrid method) was selected based on the individual case. All pelvic operations were performed using robotic surgery. Mainly, the surgeon operated arm 1 (scissors forceps) in the right hand and operated arm 2 (fenestrated forceps) in the left hand. Arm 3 (double fenestrated forceps) was also utilized to ensure the operative field. After mesorectal excision was done, LLD was performed. An electric scalpel was used for the incision rather than ultrasonic incision, coagulation system, or sealing device.
LLD were divided into three parts: the common iliac node, the internal iliac node, and the obturator node (Fig. 2). First, the ureter was identified and mobilized to cross the vas deferens. The hypogastric nerve was taped to preserve the pelvic plexus and pelvic splanchnic nerves. Next, the lateral surface of the pelvic plexus was separated so that the autonomic nerve system, including the hypogastric nerve, the pelvic splanchnic nerves, and the pelvic plexus, formed one plane. This plane was the medial border of the internal lymph node dissection (video file 1).
To dissect the common iliac lymph nodes (Fig. 3), the ventral and medial portions of the common iliac artery and vein were exteriorized from the aortic bifurcation, and dissection was performed along the blood vessel wall to the bifurcation of the internal and external iliac artery.
For the obturator lymph nodes, the border between internal iliac vessels and the obturator lymph nodes was separated. This border extended to the border of the adipose tissues surrounding the urinary bladder and led to the stratum disjuncture extending from the pelvic wall. The lateral surface of the internal pudendal artery was exfoliated to Alcock’s canal, and the sacral nerves and the coccygeal muscle were exposed. Obturator nerves were preserved, and the obturator artery and vein were dissected. The lateral side of the obturator space was exposed to the pelvic wall along the obturator fascia, while the inside of the pelvic wall was dissected to the lateral side of the internal iliac artery. The proximal side was dissected en block to the bifurcation of the internal and external iliac artery (Fig. 4 and video file 2).
The internal iliac lymph nodes, which were located between the internal iliac artery and the autonomic nerves (the pelvic splanchnic nerves and the pelvic plexus), were dissected (Fig. 5 and video file 3, 4).
Results
Fifty consecutive patients underwent RALLD for lower rectal cancer. Median patient age was 62 years (range 36–74 years). The demographic characteristics are presented in Table 1. The preoperative T stage was T1 in one patient, T3 in 43 patients, T4a in three patients, and T4b in three patients. For one patient with T1, LLD was performed because preoperative MRI showed lateral lymph node metastasis. There were ten patients with preoperative lateral lymph node metastasis. Neoadjuvant CRT was administered to six patients.
The operative data are presented in Table 2. Operative procedures included low anterior resections (LAR) in 27 patients, intersphincteric resections (ISR) in 16 patients, and abdominoperineal resections (APR) in seven patients. Bilateral LLD was performed in 44 patients. The median operating time was 476 min (range 320–683 min), the median console time was 309 min (range 193–550 min), and median time required for LLD was 165 min (range 85–257 min). The median blood loss was 27 mL (range 5–690 mL), and none of the patients received intraoperative blood transfusion. There were no cases of open or laparoscopic conversion.
Postoperative data are presented in Table 3. The median postoperative hospital stay was 8 days (range 6–13 days). Clavien–Dindo classification Grade II–IV complications occurred in seven patients (14.0 %). One patient had three complications. Grade III complications (ileus) occurred in only one patient (2.0 %). There were no cases of anastomotic leak. There was no perioperative mortality.
Four patients (8.0 %) had Grade II urinary retention. Three of these patients had undergone unilateral dissection of the autonomic nerves because of lateral lymph node metastasis or because of tumor invasion of pelvic plexus. These three patients were treated with intermittent self-catheterization and drugs until residual urine decreased to <50 mL. All patients recovered within 1 month after operation. At present, patients who completed follow-up of 12-months was only eight patients. One (12.5 %) of eight patients reported deterioration of sexual function.
Histopathological data are presented in Table 4. There were no positive resection margins. The median number of harvested lymph nodes was 48 (range 21–112). The median number of lateral lymph nodes was 19 (range 5–47). 10 patients (20 %) had lateral lymph node metastasis, and only four of these patients were diagnosed with lymph node metastasis preoperatively. Nine patients had lymph node metastasis in the obturator node.
Discussion
In Japan, LLD is utilized as a standard treatment for T3 or T4 lower rectal cancer, as described by the JSCCR guidelines [16]. Moriya et al. [18] previously described a case of nerve-sparing rectal resection with LLD in the 1980s. They reported that the use of nerve-sparing surgery would result in improvements in the local control rate, survival rate, rate of urinary disorders, and rate of sexual dysfunction [19]. A retrospective analysis conducted in Japan reported that lateral lymph node metastasis was present in 15.6–20.4 % of patients with lower rectal cancer and that the risk of pelvic recurrence would decrease by 50 % and the 5-year-survival rate would improve by 8 % when LLD was performed for T3–T4 lower rectal cancer [15].
The standard treatment for advanced rectal cancer in Western countries is TME and CRT. In 1982, Heald et al. [20] reported that TME reduced the local recurrence rate. Further, preoperative radiotherapy reduces the local recurrence rate and improves postoperative survival [21], but radiotherapy can be complicated by small bowel obstruction, urogenital dysfunction, and anal dysfunction [22–25]. In our study, the rate of sexual dysfunction was 12.5 %, which is better than that previously reported [25]. It is possible that pelvic nerve-sparing yields better functional outcomes. Kim et al. [14] reported that patients undergoing robotic surgery exhibited recovery of voiding and sexual function significantly faster than patients undergoing laparoscopic surgery.
Fujita et al. [17] described the short-term results of bilateral LLD in patients undergoing the open surgery in Japan and reported that the median operative time was 360 min, the median column of blood loss was 576 mL, and the complication (grade 3–4) rate was 22 %. Surgical time was longer with robotic surgery (476 min) in our study than with open surgery (360 min) in previous study [17], but the volume of blood loss and the complication rate were lower with robotic surgery than with open surgery. The longer operative time for robotic surgery in this study likely resulted from the initial learning curve. When considering cases performed by a single surgeon, the mean operative time decreased from 638 to 366 min and the mean time required from for bilateral LLD decreased from 219 to 116 min when comparing the first three cases to the last three cases, which suggests that a learning curve phenomenon had occurred.
According to a report by Sugihara et al. [15], the median number of dissected lymph nodes in patients undergoing laparotomy and bilateral LLD was 17 (range 0–66). By contrast, the median number of dissected lymph nodes in the present study was 19 (range 5–47), which is comparable to the median number of dissected lymph nodes in the context of open surgery. This seems to suggest the oncological safety of RALLD.
According to reports of laparoscopic LLD by Park et al. [26] and by Liang et al. [27], the average blood loss was 188 mL, the number of dissected lymph nodes in the lateral lymph node was 6–9.1, and the complication rate was 20.6–25.0 %. By contrast, the use of RALLD in the present study was associated with less blood loss, a greater number of dissected lymph nodes, and a lower complication rate. Liang et al. [27] suggested that laparoscopic lateral pelvic lymph node dissection is a very complex procedure, and Park et al. [28] reported the short-term outcomes of RALLD and laparoscopic LLD [26], in which RALLD was associated with a shorter operative time and less blood loss when compared with laparoscopic LLD. Robotic surgery enables accurate surgery, even in the narrow pelvic cavity, and is therefore useful for LLD.
In conclusion, RALLD is a safe and feasible method, but further studies are needed to evaluate long-term oncological outcomes associated with this strategy.
References
Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ (2010) Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg 97:1638–1645
Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Pahlman L, Cuesta MA, Msika S, Morino M, Lacy A, Bonjer HJ (2009) Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 10:44–52
Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W, Nelson H (2007) Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST study group trial. Ann Surg 246:655–662
Lacy AM, Delgado S, Castells A, Prins HA, Arroyo V, Ibarzabal A, Pique JM (2008) The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg 248:1–7
Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Pahlman L, Cuesta MA, Msika S, Morino M, Lacy AM (2005) Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 6:477–484
Lacy AM, Garcia-Valdecasas JC, Delgado S, Castells A, Taura P, Pique JM, Visa J (2002) Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet 359:2224–2229
Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365:1718–1726
Pigazzi A, Ellenhorn JD, Ballantyne GH, Paz IB (2006) Robotic-assisted laparoscopic low anterior resection with total mesorectal excision for rectal cancer. Surg Endosc 20:1521–1525
Corcione F, Esposito C, Cuccurullo D, Settembre A, Miranda N, Amato F, Pirozzi F, Caiazzo P (2005) Advantages and limits of robot-assisted laparoscopic surgery: preliminary experience. Surg Endosc 19:117–119
Delaney CP, Lynch AC, Senagore AJ, Fazio VW (2003) Comparison of robotically performed and traditional laparoscopic colorectal surgery. Dis Colon Rectum 46:1633–1639
Baik SH, Kwon HY, Kim JS, Hur H, Sohn SK, Cho CH, Kim H (2009) Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol 16:1480–1487
Patriti A, Ceccarelli G, Bartoli A, Spaziani A, Biancafarina A, Casciola L (2009) Short- and medium-term outcome of robot-assisted and traditional laparoscopic rectal resection. JSLS 13:176–183
Popescu I, Vasilescu C, Tomulescu V, Vasile S, Sgarbura O (2010) The minimally invasive approach, laparoscopic and robotic, in rectal resection for cancer. A single center experience. Acta Chir Iugosl 57:29–35
Kim JY, Kim NK, Lee KY, Hur H, Min BS, Kim JH (2012) A comparative study of voiding and sexual function after total mesorectal excision with autonomic nerve preservation for rectal cancer: laparoscopic versus robotic surgery. Ann Surg Oncol 19:93–2485
Sugihara K, Kobayashi H, Kato T, Mori T, Mochizuki H, Kameoka S, Shirouzu K, Muto T (2006) Indication and benefit of pelvic sidewall dissection for rectal cancer. Dis Colon Rectum 49:1663–1672
Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, Ishiguro M, Kanemitsu Y, Kokudo N, Muro K, Ochiai A, Oguchi M, Ohkura Y, Saito Y, Sakai Y, Ueno H, Yoshino T, Fujimori T, Koinuma N, Morita T, Nishimura G, Sakata Y, Takahashi K, Takiuchi H, Tsuruta O, Yamaguchi T, Yoshida M, Yamaguchi N, Kotake K, Sugihara K (2012) Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol 17:1–29
Fujita S, Akasu T, Mizusawa J, Saito N, Kinugasa Y, Kanemitsu Y, Ohue M, Fujii S, Shiozawa M, Yamaguchi T, Moriya Y (2012) Postoperative morbidity and mortality after mesorectal excision with and without lateral lymph node dissection for clinical stage II or stage III lower rectal cancer (JCOG0212): results from a multicentre, randomised controlled, non-inferiority trial. Lancet Oncol 13:616–621
Moriya Y, Hojo K, Sawada T, Koyama Y (1989) Significance of lateral node dissection for advanced rectal carcinoma at or below the peritoneal reflection. Dis Colon Rectum 32:307–315
Moriya Y, Sugihara K, Akasu T, Fujita S (1995) Nerve-sparing surgery with lateral node dissection for advanced lower rectal cancer. Eur J Cancer 31A:1229–1232
Heald RJ, Husband EM, Ryall RD (1982) The mesorectum in rectal cancer surgery-the clue to pelvic recurrence? Br J Surg 69:613–616
Trial Swedish Rectal Cancer (1997) Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish rectal cancer trial. N Engl J Med 336:980–987
Guckenberger M, Flentje M (2006) Late small bowel toxicity after adjuvant treatment for rectal cancer. Int J Colorectal Dis 21:209–220
Peeters KC, van de Velde CJ, Leer JW, Martijn H, Junggeburt JM, Kranenbarg EK, Steup WH, Wiggers T, Rutten HJ, Marijnen CA (2005) Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients: a Dutch colorectal cancer group study. J Clin Oncol 23:6199–6206
Bruheim K, Guren MG, Skovlund E, Hjermstad MJ, Dahl O, Frykholm G, Carlsen E, Tveit KM (2010) Late side effects and quality of life after radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 76:1005–1011
Lange MM, Marijnen CA, Maas CP, Putter H, Rutten HJ, Stiggelbout AM, Meershoek-Klein Kranenbarg E, van de Velde CJ (2009) Risk factors for sexual dysfunction after rectal cancer treatment. Eur J Cancer 45:1578–1588
Park JS, Choi GS, Lim KH, Jang YS, Kim HJ, Park SY, Jun SH (2011) Laparoscopic extended lateral pelvic node dissection following total mesorectal excision for advanced rectal cancer: initial clinical experience. Surg Endosc 25:3322–3329
Liang JT (2011) Technical feasibility of laparoscopic lateral pelvic lymph node dissection for patients with low rectal cancer after concurrent chemoradiation therapy. Ann Surg Oncol 18:153–159
Park JA, Choi GS, Park JS, Park SY (2012) Initial clinical experience with robotic lateral pelvic lymph node dissection for advanced rectal cancer. J Korean Soc Coloproctol 28:265–270
Disclosures
Hiroyasu Kagawa, Yusuke Kinugasa, Akio Shiomi, Tomohiro Yamaguchi, Syunsuke Tsukamoto, Hiroyuki Tomioka, Yushi Yamakawa, and Sumito Sato have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (WMV 39297 kb)
Supplementary material 2 (WMV 57665 kb)
Supplementary material 3 (WMV 34292 kb)
Supplementary material 4 (WMV 58463 kb)
Rights and permissions
About this article
Cite this article
Kagawa, H., Kinugasa, Y., Shiomi, A. et al. Robotic-assisted lateral lymph node dissection for lower rectal cancer: short-term outcomes in 50 consecutive patients. Surg Endosc 29, 995–1000 (2015). https://doi.org/10.1007/s00464-014-3760-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-014-3760-y