Abstract
Background
We have evaluated the safety and feasibility of combining median-to-lateral and anterior-to-median (MLAM) approaches to perform laparoscopic complete mesocolic excision (CME) with radical lymph node dissection along the gastrocolic trunk of Henle (GTH) for right hemicolon cancer.
Patients and methods
We retrospectively analyzed data obtained from a prospectively maintained database on 31 consecutive patients who had undergone laparoscopic CME with radical lymph node dissection for right hemicolon cancer between January 2010 and December 2013. We used video recordings of the procedure to assess the quality of the surgery and completeness of CME. We also assessed operative data, pathological findings, length of large bowel resected, complications, BMI, operative time by experience of surgeon, and length of hospital stay.
Results
All patients had undergone en bloc resection of the enveloped parietal planes and radical lymph node dissection along the surgical trunk without any serious intraoperative complications. Twenty six and five patients graded mesocolic and intra-mesocolic plane, respectively. Five, three, eleven, and thirteen patients had T1, T2, T3, and T4 tumors, respectively. The median number of lymph nodes retrieved was 25, lymph node metastasis being identified in 11 patients. The mean length of large bowel resected was 21.8 cm. The mean operative time and intraoperative blood loss were 269 min and 39 mL, respectively. No intraoperative complications occurred in any patient. Three patients had postoperative complications. The mean BMI was 22.6 kg/m2. The mean operative time for patients stratified by BMI of <22 or ≥22 was 225 and 297 min, respectively. There were no correlations with operative time by experience of surgeon. The median postoperative hospital stay was 13 days.
Conclusions
Laparoscopic CME conducted by fusion fascia exposure with radical lymph node dissection along the GTH via a combination of MLAM approaches is a safe and feasible procedure for right hemicolon cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Laparoscopic colectomy has become a standard surgical treatment for colon cancer, and many large randomized trials have shown short-term benefits with more rapid postoperative recovery and improved quality of life compared with open colectomy [1–3]. The procedure is oncologically safe for managing colon cancer [4–6]. Complete mesocolic excision (CME) with central vascular ligation (CVL) is now a standard form of colon cancer surgery: it has a reduced local recurrence rate and improved long-term survival compared with previously performed procedures [7]. The feasibility and safety of CME in both open and laparoscopic surgery have been demonstrated [8–18].
When performing laparoscopic surgery, it is very important to understand the embryologic basis of adult anatomy. Until 12 weeks, the primary midgut loop rotates 270° counterclockwise around the primary superior mesenteric artery, and until 20 weeks, the greater omentum and transverse mesocolon overlay the frontal surface of the mesoduodenum [19–21]. The peritoneal membrane at the attachment site fuses and degenerates to form membranous connective tissue called the fusion fascia [21]. Here, we describe laparoscopic CME based on embryologic considerations and involving detachment of the fusion fascia with radical lymph node dissection for right hemicolon cancer.
Patients and methods
Patients analyzed
In this retrospective study, data on 31 consecutive patients (14 men, 17 women) of mean age 71.9 years (range: 52–92 years) obtained from a prospectively maintained database at Kagoshima University hospital in Japan were analyzed. All patients had undergone laparoscopic CME with CVL and radical lymph node dissection for right hemicolon cancer between January 2010 and December 2013. Patients were excluded if they had T4b cancer or bulky tumors, small bowel obstruction or perforation due to the primary tumor, dense adhesions, or American Society of Anesthesiologists scores of 4 or 5. Video recordings of the procedures were used to assess the quality of the surgery and completeness of CME with CVL, the level of which was graded as mesocolic, intra-mesocolic, or intra-muscularis propria plane according to standard definitions [8]. Pathological findings, operative data, complications, BMI, operative time by experience of surgeon, and length of hospital stay were also assessed. The patients were stratified by BMI (< 22 or ≥22) and differences in operative time and results of histopathologic evaluation assessed. The surgeons were also stratified into three categories: highly experienced laparoscopic colorectal surgeons, experienced laparoscopic surgeons, and laparoscopic surgeons with little experience, after which the mean operative times according to category of surgeon were calculated. All mesenteric lymph nodes were retrieved and fixed in formalin, after which each specimen was assessed by two pathologists.
Surgical procedure
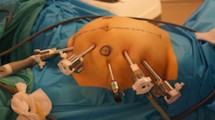
All patients were administered general anesthesia and placed in the lithotomy position. The operator and camera operator stood on the left and the assistant on the right side. First, a trocar for a 10-mm flexible laparoscope was inserted through the umbilicus using an open technique (point a in Fig. 1). A pneumoperitoneum was maintained at 10 mm Hg using CO2. Under laparoscopic guidance, 12- and 5-mm trocars for the operator were placed in the left lower abdomen and two 5-mm trocars for the assistant in the right lower and left upper abdomen. The operator used the left middle trocar (point c in Fig. 1) with the right hand and the left lower trocar (point d in Fig. 1) with the left hand during the median-to-lateral approach, and the left upper trocar (point b in Fig. 1) with the right hand and left middle trocar (point c in Fig. 1) with the left hand during the anterior-to-median approach. The abdominal cavity was initially explored, laparoscopic instruments were inserted, and a median-to-lateral approach was achieved using an ultrasonically activated device. Dissection was then started behind the pedicle of the ileocolic vessels (Fig. 2A), and proceeded along the superior mesenteric vein (SMV) using a short pitch technique. The ileocolic vessels were then severed at their roots (Fig. 2B). After the embryological tissue planes comprising Told’s and prerenal fascia had been exposed, a wide separation between the pancreatic head and transverse colon was achieved (Fig. 2C). Dissection then proceeded along the SMV, exposing the gastrocolic trunk of Henle (GTH) (Fig. 2D). Next, the middle colic artery was identified arising from the superior mesenteric artery (SMA) and severed at the root of its right branch. This was accompanied by lymph node dissection, taking care to preserve the left branch of the middle colic artery. Simultaneously, the middle colic vein was identified and severed at the root of its right branch (Fig. 2D). Next, an anterior-to-median approach was performed by dissecting the right side of the greater omentum (Fig. 3A). The fusion fascia was detached between the omentum and transverse mesocolon and the hepatic flexure mobilized (Fig. 3B). The accessory middle colic veins were carefully dissected accompanied by lymph node dissection (Fig. 3C), and the transverse mesocolon dissected below the lower edge of the pancreas, uncovering the SMV (Fig. 3D). Next, a minilaparotomy was performed via the umbilicus, the incision being approximately 4 cm in diameter. The excised specimen was extracted through this incision with wound protection, after which extracorporeal functional end-to-end anastomosis was performed.
Skin incision of trocar placement diagrammatic representation of skin incisions for placement of a 12-mm trocar in the umbilicus for a 10-mm flexible laparoscope (A), a 5-mm trocar in the left upper quadrant for an assistant or operator (B), a 12-mm trocar in the left mid abdomen for an operator (C), a 5-mm trocar in the left lower quadrant for an operator or assistant (D), and a 5-mm trocar in the right upper quadrant for an assistant (E)
Median-to-lateral approach Intraoperative photographs of the median-to-lateral approach showing: A Dissection starting dorsal to the ileocolic vessels. B Lymph node dissection along the SMV with CVL of the ileocolic artery (ICA) and vein (ICV). C Detaching the fusion fascia between the mesocolon and prerenal fascia. D Ligation at the roots of the right branches of the middle colic artery (MCA) and vein
Anterior-to-median approach Intraoperative photographs of the anterior-to-median approach showing: A Dissection of the right side of the greater omentum. B Detaching fusion fascia between the omentum and transverse mesocolon. C Dissection of the accessory middle colic veins (AMCV). D Final view after radical lymph node dissection along the GTH and SMV. RGEV, right gastroepiploic vein; ASPDV, anterior superior pancreaticoduodenal vein
Statistical analyses
The results were analyzed using SPSS for windows, version 15.0 (SPSS, Chicago, IL, USA). Differences between means were analyzed using Student’s t test. A P value of less than 0.05 was considered statistically significant.
Results
All patients underwent en bloc resection of the enveloped parietal planes with radical lymph node dissection along the surgical trunk. Twenty six and five patients graded mesocolic and intra-mesocolic plane, respectively. Five, three, eleven, and thirteen patients had histological T1, T2, T3, and T4 tumors, respectively. The median number of lymph nodes retrieved was 25, lymph node metastasis being identified in 11 patients. According to the Union for International Cancer Control staging criteria, 5, 12, 10, and 4 patients had stage I, II, III, and IV tumors, respectively. Twenty-two, four, two, two, and one patient had well-differentiated tubular, moderately differentiated tubular, poorly differentiated, mucinous, and papillary adenocarcinoma, respectively (Table 1). The mean length of large bowel resected was 21.8 cm. The mean operative time and intraoperative blood loss were 269 min (range: 165–420 min) and 39 mL (range: 0–270 mL), respectively. Mean time to commencing postoperative oral intake was 4 days (range: 3–5 days). No patients had any serious intraoperative complications. Three patients had postoperative complications. One patient, aged 67, developed anastomotic bleeding that was successfully managed by achieving hemostasis via colonoscopy and was discharged 23 days after surgery. Another patient, aged 78, developed ileus, which resolved when oral intake was stopped for a few days; this patient was discharged 8 days after surgery. The third patient, aged 52, developed cholangitis, recovered with conservative treatment, and was discharged 18 days after surgery. No other postoperative complication, such as anastomotic leakage, intra-abdominal abscess, wound infection, or pulmonary infection, occurred in any patient. There was no intraoperative or postoperative mortality. The mean BMI was 22.6 kg/m2: 12, 10, and 9 patients had BMI of <22, 22–24, or >24, respectively. The mean operative time was significantly shorter in patients with BMI < 22 than in those with BMI ≥ 22 (mean 225 vs. 297 min; P < 0.002). The mean blood loss was significantly less in patients with BMI < 22 than in those with BMI ≥ 22 (mean 15.8 vs. 54.1 min; P < 0.033) (Table 2). There were no significant differences in operative times between highly experienced and less experienced surgeons (mean 250 vs. 282 min; P = 0.492). The median postoperative hospital stay was 13 days.
Discussion
Cancer surgery based on embryologic considerations plays a central role in the meaningful management of colorectal surgery. Patients with T3–T4 right colon cancer are suitable candidates for lymph node dissection with high ligation [22]. There is global consensus that total mesorectal excision is the standard for rectal cancer surgery: this procedure focuses on achieving intact block resection of the mesorectum, tumor, and lymphatic drainage [23–25]. CME with CVL, in which the embryologic tissue planes are resected along the entire enveloped mesocolon, has been found to reduce local recurrence and improve long-term survival compared with previously performed procedures [7–9]. A retrospective analysis of surgical quality and its correlation with prognosis in 399 patients with colon cancer found that improving the plane of dissection may improve survival, especially in patients with stage III disease [8]. Moreover, laparoscopic CME with CVL for colon surgery has been shown to be technically feasible and effective, surgical results being optimized through a combination of surgical proficiency, institutional caseload, and expertise [12–18]. However, a safe strategy for vascular ligation around the GTH and middle colic vessels with radical lymph node dissection has not yet been described. In this study, we describe laparoscopic CME involving detachment of the fusion fascia accompanied by radical lymph node dissection via a combination of median-to-lateral and anterior-to-median (MLAM) approaches for right hemicolon cancer.
There were vascular anomalies noted around the GTH and middle colic vessels in any of the patients. The superior right colic vein exits and drains from the right flexure to the GHT in 89 % of cases [26]. Several cadaver studies have clarified the three-dimensional venous anatomy of the GTH and reported that the GTH is a constant and conspicuous anatomic entity [27–29]. Preoperative 3D-CT is informative and helpful for surgeons performing laparoscopic CME for right colon cancer as the venous tributaries of the right colon are variable [30]. However, because of its tight relationship to the right colon arteries and their variations, it is not easily accessible [26–29]. Although in both total mesorectal excision and CME detachment of the fusion fascia is performed by techniques derived from embryologic considerations, in adults, the fusion planes around the SMV and SMA are complicated because of secondary modification and simplification during vascular development [21]. An understanding of the embryologic basis for anatomy is very important for laparoscopic surgeons. Until 20 weeks, the mesocolon is attached to the renal fascia covering the right kidney and the greater omentum and transverse mesocolon overlie the frontal surface of the mesoduodenum: these fuse together and degenerate to form the membranous connective tissue called the fusion fascia [19–21]. We here propose a novel form of laparoscopic surgery for right hemicolon cancer based on embryologic considerations and consisting of CME with CVL via a combination of MLAM approaches. We use a median-to-lateral approach to uncover the SMV, ileocolic vessels, duodenum, pancreas head, GTH, and middle colic vessels and detach the fusion fascia. In addition, we dissect the ileocolic vessels and right branch of the middle colic vessels together with regional lymph nodes. Following this, we use an anterior-to-median approach to dissect the greater omentum and detach the fusion fascia of the greater omentum and transverse mesocolon, after which we carefully dissect the accessory middle colic veins together with lymph node dissection. When the carcinomas were located in the caecum and proximal ascending colon, we did not dissect the accessory middle colic veins. Because the GTH can have variations involving the accessory middle colic veins, anterior superior pancreaticoduodenal vein, right gastroepiploic vein, and sometimes the middle colic vein, it is necessary to have a three-dimensional grasp of the surgical anatomy. Because the combination of MLAM approaches allows us to recognize the GTH from median and anterior, our procedure ensures three-dimensional visualization of the anatomy around the GTH. Thus, we perform CME with radical lymph node dissection along the SMV and en bloc resection of the mesocolon. This formalized technique yields good short-term outcomes, including minimal blood loss, retrieval of sufficient numbers of lymph nodes, and few perioperative complications. Our procedure provides patients safety and oncological clearance.
One limitation of this study is that it included only 31 patients; however, none had any intraoperative complications, and only three had postoperative complications. A second limitation is that during our procedure the operators must change position when they move from the median-to-lateral approach to the anterior-to-median approach: this is not complex. Third, the mean duration of surgery was 269 min. We attribute this relatively long duration to the number of different surgeons who performed it: one highly experienced laparoscopic colorectal surgeon, three experienced laparoscopic surgeons, and two laparoscopic surgeons with little experience. There were no significant differences in duration of surgery between the highly experienced and less experienced surgeons. We also assessed the effect of BMI. Although longer operative times were significant correlated with high BMI, there were no intraoperative complications in this series. We attribute our lack of such complications to the fact that we performed CME by detaching the fusion fascia along the embryologic tissue plane, dissected carefully using a short pitch technique, had a good grasp of the 3-dimensional anatomy of the GTH and used the combined MLAM approach. We assessed the level of CME with CVL using the video recording, and graded as mesocolic plane, intra-mesocolic plane or intra-muscularis propria plane according to standard definitions [8]. We verified that 26 and 5 patients graded mesocolic and intra-mesocolic plane, respectively. In these 5 patients graded intra-mesocolic plane, who had Stage I of 2 and Stage IV of 3, all patients underwent high ligation of its root of vessels, but mesocolic resection was not sufficient. In contrast, we are unable to provide data on the length of vascular pedicle, the length of small bowel, and the area of mesentery, because we were unable to assess these parameters from the available video recordings of the procedures. Indeed, in all we have had only 3 years of experience with this procedure, suggesting that our team has not yet reached a plateau in our learning curve. Another limitation of our study is that it was a retrospective analysis of data on 31 consecutive patients with right hemicolon cancer obtained from a prospectively maintained database. Short- and long-term randomized trials are required to assess the effectiveness of this procedure.
In conclusion, we propose that laparoscopic CME with detachment of fusion fascia and radical lymph node dissection along the GTH via a combination of MLAM approaches is a safe and feasible surgical procedure for patients with right hemicolon cancer. This procedure also offers an improved oncological strategy for cancer surgery. Although our results suggest that this strategy is acceptable for right hemicolon cancer, the effectiveness and feasibility of this procedure should be assessed in randomized trials.
References
Clinical Outcomes of Surgical Therapy Study Group (2004) A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 350:2050–2059
Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM, MRC CLASICC trial group (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365:1718–1726
Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy AM, Colon cancer Laparoscopic or Open Resection Study Group (COLOR) (2005) Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 6:477–484
Lacy AM, Delgado S, Castells A, Prins HA, Arroyo V, Ibarzabal A, Pique JM (2008) The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg 248:1–7
Laurent C, Leblanc F, Wütrich P, Scheffler M, Rullier E (2009) Laparoscopic versus open surgery for rectal cancer: long-term oncologic results. Ann Surg 250:54–61
Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy A, Bonjer HJ, Colon Cancer Laparoscopic or Open Resection Study Group (2009) Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 10:44–52
Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S (2009) Standardized surgery for colonic cancer: complete mesocolic excision and central ligation: technical notes and outcome. Colorectal Dis 11:354–364
West NP, Morris EJ, Rotimi O, Cairns A, Finan PJ, Quirke P (2008) Pathology grading of colon cancer surgical resection and its association with survival: a retrospective observational study. Lancet Oncol 9:857–865
West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P (2010) Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol 28:272–278
Bertelsen CA, Bols B, Ingeholm P, Jansen JE, Neuenschwander AU, Vilandt J (2011) Can the quality of colonic surgery be improved by standardization of surgical technique with complete mesocolic excision? Colorectal Dis 13:1123–1129
West NP, Kobayashi H, Takahashi K, Perrakis A, Weber K, Hohenberger W, Sugihara K, Quirke P (2012) Understanding optimal colonic cancer surgery: comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J Clin Oncol 30:1763–1769
Gouvas N, Pechlivanides G, Zervakis N, Kafousi M, Xynos E (2012) Complete mesocolic excision in colon cancer surgery: a comparison between open and laparoscopic approach. Colorectal Dis 14:1357–1364
Feng B, Sun J, Ling TL, Lu AG, Wang ML, Chen XY, Ma JJ, Li JW, Zang L, Han DP, Zheng MH (2012) Laparoscopic complete mesocolic excision (CME) with medial access for right-hemi colon cancer: feasibility and technical strategies. Surg Endosc 26:3669–3675
Adamina M, Manwaring ML, Park KJ, Delaney CP (2012) Laparoscopic complete mesocolic excision for right colon cancer. Surg Endosc 26:2976–2980
Takemasa I, Uemura M, Nishimura J, Mizushima T, Yamamoto H, Ikeda M, Sekimoto M, Doki Y, Mori M (2013) Feasibility of single-site laparoscopic colectomy with complete mesocolic excision for colon cancer: a prospective case-control comparison. Surg Endosc 28(4):1110–1118.
Feng B, Ling TL, Lu AG, Wang ML, Ma JJ, Li JW, Zang L, Sun J, Zheng MH (2013) Completely medial versus hybrid medial approach for laparoscopic complete mesocolic excision in right hemicolon cancer. Surg Endosc 28(2):477–483
Storli KE, Søndenaa K, Furnes B, Eide GE (2013) Outcome after introduction of complete mesocolic excision for colon cancer is similar for open and laparoscopic surgical treatments. Dig Surg 30:317–327
Galizia G, Lieto E, De Vita F, Ferraraccio F, Zamboli A, Mabilia A, Auricchio A, Castellano P, Napolitano V, Orditura M (2014) Is complete mesocolic excision with central vascular ligation safe and effective in the surgical treatment of right-sided colon cancers? A prospective study. Int J Colorectal Dis J 29:89–97
Shinohara H, Kurahashi Y, Kanaya S, Haruta S, Ueno M, Udagawa H, Sakai Y (2013) Topographic anatomy and laparoscopic technique for dissection of no. 6 infrapyloric lymph nodes in gastric cancer surgery. Gastric Cancer 16:615–620
Sadler TW (2009) Langman’s medical embryology, 11th edn. Lippincott, Baltimore
Jeong YJ, Cho BH, Kinugasa Y, Song CH, Hirai I, Kimura W, Fujimiya M, Murakami G (2009) Fetal topohistology of the mesocolon transversum with special reference to fusion with other mesenteries and fasciae. Clin Anat 22:716–729
Kobayashi H, Enomoto M, Higuchi T, Uetake H, Iida S, Ishikawa T, Ishiguro M, Kato S, Sugihara K (2011) Clinical significance of lymph node ratio and location of nodal involvement in patients with right colon cancer. Dig Surg 28:190–197
Heald RJ (1988) The ‘Holy Plane’ of rectal surgery. J R Soc Med 81:503–508
Morino M, Parini U, Giraudo G, Salval M, Brachet Contul R, Garrone C (2003) Laparoscopic total mesorectal excision: a consecutive series of 100 patients. Ann Surg 237:335–342
Law WL, Chu KW (2004) Anterior resection for rectal cancer with mesorectal excision: a prospective evaluation of 622 patients. Ann Surg 240:260–268
Gundara JS, Gill AJ, Hugh TJ, Samra JS (2013) Redefining the apical lymph node at right hemicolectomy. Eur J Surg Oncol 39:662–665
Ignjatovic D, Spasojevic M, Stimec B (2010) Can the gastrocolic trunk of Henle serve as an anatomical landmark in laparoscopic right colectomy? A postmortem anatomical study. Am J Surg 199:249–254
Jin G, Tuo H, Sugiyama M, Oki A, Abe N, Mori T, Masaki T, Atomi Y (2006) Anatomic study of the superior right colic vein: its relevance to pancreatic and colonic surgery. Am J Surg 191:100–103
Ignjatovic D, Stimec B, Finjord T, Bergamaschi R (2004) Venous anatomy of the right colon: three-dimensional topographic mapping of the gastrocolic trunk of Henle. Tech Coloproctol 8:19–21
Ogino T, Takemasa I, Horitsugi G, Furuyashiki M, Ohta K, Uemura M, Nishimura J, Hata T, Mizushima T, Yamamoto H, Doki Y, Mori M (2014) Preoperative evaluation of venous anatomy in laparoscopic complete mesocolic excision for right colon cancer. Ann Surg Oncol 21:429–435
Acknowledgments
The authors deeply appreciate the contributions of all the surgeons, coworkers, and friends who participated in this study and thank the editors and reviewers for their help with this manuscript.
Disclosures
Authors Shinichiro Mori, Kenji Baba, Masayuki Yanagi, Yoshiaki Kita, Shigehiro Yanagita, Yasuto Uchikado, Takaaki Arigami, Yoshikazu Uenosono, Hiroshi Okumura, Akihiro Nakajo, Kosei Maemura, Sumiya Ishigami and Shoji Natsugoe have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mori, S., Baba, K., Yanagi, M. et al. Laparoscopic complete mesocolic excision with radical lymph node dissection along the surgical trunk for right colon cancer. Surg Endosc 29, 34–40 (2015). https://doi.org/10.1007/s00464-014-3650-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-014-3650-3