Abstract
Objective
To explore the feasibilities between operational approaches for laparoscopic complete mesocolic excision (CME) to right hemicolon cancer.
Methods
This prospective randomized controlled trial included patients admitted to a Shanghai minimally invasive surgical center to receive laparoscopic CME from September 2011 to January 2013 randomized into two groups: hybrid medial approach (HMA) and completely medial approach (CMA). The feasibilities and strategies of the two techniques were studied and compared. Furthermore, the operation time and vessel-related complications were designed to be the primary end points, and other operational findings, including the classification of the surgical plane and postoperative recovery, were designed to be the secondary end points for this study.
Results
After screening, 50 cases were allocated to the HMA group and 49 to the CMA group. Within the HMA group, there were 48 cases graded with mesocolic plane and 2 with intramesocolic plane. For the CMA group, there were 42 cases graded with mesocolic plane and seven with intramesocolic plane. The differences between the two were insignificant, as were the number of lymph nodes retrieved. The mean±standard deviation total operation time for the CMA group was 128.3 ± 36.4 min, which was significantly shorter than that for the HMA group, 142.6 ± 34.8 min. For the CMA group, the time involved in central vessel ligations and laparoscopic procedures was 58.5 %, 14.1 and 81.2 ± 23.5 min, respectively, which were shorter than the HMA group. The vessel-related complication rate was significantly higher in the HMA group.
Conclusions
Laparoscopic CME via the total medial approach is technically feasible after the precise identification of the surgical planes and spaces for the right hemicolon. The procedure has a shorter operation time and fewer vessel-related complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Research has demonstrated that complete mesocolic excision (CME) has the potential to become the standard procedure for colon cancer surgery as a result of its effectiveness in reducing local recurrence rate and improving prognosis [1, 2]. Two modes of access characterize this procedure: lateral and medial [3–5]. Most traditional laparoscopic procedures use lateral access [1]. Previous research has demonstrated that technically, laparoscopic CME via medial access is comparable to a laparoscopic procedure [6, 7]. Accordingly, intermesenteric space (IMS) is located posterior to the greater omentum and superior to the transverse colon mesentery. It communicates with the transverse retrocolic space (TRCS) by passing behind the root of the transverse colon mesentery [8]. Therefore, dissection of the transverse colon mesentery requires the entrance of the IMS.
Our medical center proposed two approaches for medial access: a hybrid medial approach (HMA) and a completely medial approach (CMA). HMA involves the entrance to the IMS by an incision of the gastrocolonic ligament followed by the dissection of the middle colic vessels and the Henle trunk in a top-to-bottom fashion. The approach is capped by the dissection of the inferior edge of the pancreas, requiring the blending of both top-to-bottom and bottom-to-top approaches. CMA, on the other hand, involves a bottom-to-top approach in every step, including the entrance of IMS through TRCS; dissection of the middle colic vessels and the Henle trunk; and dissection of the inferior edge of the pancreas.
The main goal of this study was to investigate the technical feasibility and strategies of the two approaches.
Methods
Study design
This prospective randomized controlled trial included 102 of 113 consecutive patients admitted to a Shanghai minimally invasive surgical center, Ruijin Hospital affiliated to Shanghai Jiaotong University School of Medicine, to receive laparoscopic CME for right hemicolon cancer from September 2011 to January 2013 who had signed informed consent to participate in the study, which was approved by the ethics committee of Ruijin Hospital. Patients were randomized into two groups, HMA and CMA. Eleven of 113 patients were excluded from the study because they did not agree to the terms of the consent form.
The inclusion criteria were as follows: patients (1) who had carcinoma of cecum, ascending colon, or hepatic flexure identified by preoperative histopathological findings; (2) whose preoperative tumor staging was I, II, or III according to the 6th edition of UICC tumor classification I; (3) whose tumor diameter was less than 7 cm; and (4) who received elective surgery. The exclusion criteria were as follows: patients (1) who had malignant lymphoma and benign tumor for right hemicolon; (2) whose preoperative tumor staging was stage IV; (3) whose tumor was exceedingly large (7 cm or larger), infiltrated tissues in vicinity, and/or invaded important vessels; and (4) who had emergent presentation. After the selected patients provided informed consent, each drew 1 of 2 opaque envelops to be assigned to a random grouping. Patients who drew an envelope containing the letter H were assigned to the HMA group; those who drew the letter C were assigned to the CMA group. Fifty-two and 50 patients were assigned to the HMA and CMA groups, respectively.
Video and photographs of the operation and the resected samples were assessed by three independent professional observers to evaluate the quality of the approaches. Two patients in the HMA group and one patient in the CMA group were excluded as a result of the lack of clear video or photographs sufficient to evaluate the quality of the surgery. Operation time and vessel-related complications were designed to be the primary end points; other operational findings, including the classification of the surgical plane and postoperative recoveries, were designed to be secondary end points.
Operational approaches
Both HMA and CMA require mesocolon excision and central vessel ligation [1, 2].
HMA strategies
The IMS is entered via an incision of the gastrocolonic ligament, followed by the dissection of the middle colic vessels and the Henle trunk in a top-to-bottom fashion. The approach is capped by dissection at the inferior edge of the pancreas, requiring the blending of both top-to-bottom and bottom-to-top approaches.
CMA strategies
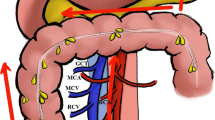
The dissection starts at the ileocolic vessel and proceeds along the superior mesenteric vein (SMV) to enter the TRCS in bottom-to-top fashion. The dissection of the TRCS is extended laterally to the right retrocolic space (RRCS) and superiorly enter the IMS, followed by the dissection of middle colon vessels, the Henle trunk, and pancreatic lower edge in a bottom-to-top fashion (Fig. 1).
Complete mesocolon excision for CMA. A Initiation. B Dissection of surgical trunk. C TRCS exploration. D RRCS exploration. E Interspace between TRCS and IMS via transverse mesocolon. F extraperitoneal space (EPS) and prerenal fascia. G Middle colic vessels and Henle trunk in bottom-to-top fashion. H Middle colic vessels and Henle trunk in top-to-bottom fashion
Comparisons
Operational assessments
We referred to the evaluation system of West et al. [9] to assess the quality of the operation, as follows: (1) muscularis propria plane (defined as a poor plane of surgery—little mesocolon excised with the incision extending down onto the muscularis propria); (2) intramesocolic plane (moderate plane of surgery—partial mesocolon excised with irregular shape but incisions do not reach down to the muscularis propria); and (3) mesocolon plane (good plane of surgery—intact mesocolon excised without defects and high ligation of the supply vessels) (Fig. 2).
Central vessel ligation time
The central vessel ligation time was part of the whole operation time, which represented the duration required for procedures starting from the dissection of the pedicle to ileocolic vessel via the ligations of ileocolic vessels, right colon vessels, Henle trunk, and middle colon vessels to the establishment of the connections between the IMS and the TRCS.
Laparoscopic procedure time
The laparoscopic procedure time was part of the total operation time, from insertion of the trocars to complete the vessel ligations and mobilization of the colon.
Statistical analysis
All calculations and analyses were performed by SPSS software, version 15.0 (SPSS, Chicago, IL). Quantitative data were expressed as mean ± standard deviation. Student’s t test was used to compare the differences between the two groups; P < 0.05 was considered statistically significant.
Results
General information
Forty-nine and 50 patients were assigned to the CMA and HMA groups, respectively (Table 1). The sex, tumor locations, tumor classifications, and body mass index of both groups were not significantly different.
Operational assessment
Forty-eight and 2 cases were evaluated for the mesocolic and intramesocolic planes, respectively, in the CMA group, whereas 42 and 7 cases were assessed for the mesocolic and intramesocolic planes, respectively, in the HMA group. The differences between the two groups were insignificant.
Evaluation in lymph node resections
The mean resection sample length in the CMA group was 22.3 ± 6.3 cm, which was not different from the HMA group, 23.1 ± 6.1 cm. The number of lymph nodes retrieved in the CMA group was 20.3 ± 5.8. In the stage III group, there were seven cases (26.9 %) of lymph nodes located at the root of the vessels. Among 23 patients with tumor located at the hepatic flexure who underwent subpyloric lymph nodes resection, lymph node metastasis was found in five (21.7 %), with three cases (6.1 %) of positive lymph nodes observed in the greater omentum along the greater curvature. In the HMA group, the mean number of lymph nodes collected was 19.2 ± 6.7. In the stage III group, there were eight cases (26.9 %) of lymph nodes located at the root of the vessels. Among 18 patients with tumor located at the hepatic flexure who underwent subpyloric lymph node resection, lymph node metastasis was found in four cases (22.2 %), with four cases (8.0 %) of positive lymph nodes observed in greater omentum along the greater curvature. The above data from both groups were statistically the same (Table 2).
Operational findings
Table 3 shows the operational findings between the CMA and HMA groups. The total operation time for the CMA group was 128.3 ± 36.4 min, which was significantly shorter than the HMA group, 142.6 ± 34.8 min. Also, the time required for the central vessel ligation and the laparoscopic procedure was 58.5 ± 14.1 and 81.2 ± 23.5 min, respectively, for CMA; these times were significantly shorter than HMA, 68.3 ± 15.2 and 78.2 ± 28.7 min, respectively. Blood loss, postoperative flatus recovery time, postoperative liquid intake time, and hospitalization were not significantly different between the two groups.
Operational complications
Postoperative that complications occurred in CMA group included 1 case of pneumonia, 1 case of hemorrhage, and 1 case of chylous leakage, all of which were effectively treated by relevant conservative treatments. The number of postoperative complications was statistically equal between the two groups. CMA had relatively fewer vessel-related complications, which implicated the superior mesenteric, ileocolic, right colic, middle colic, right gastroepiploic, and pancreaticoduodenal vessels and the Henle trunk; however, this did not reach statistical significance. HMA had relatively more hemorrhages caused by injuries of the pancreaticoduodenal vessels (Table 4).
Discussion
Anatomic strategies for CMA to CME
Traditional laparoscopic CME utilizes lateral access, which starts by mobilizing the right hemicolon in a lateral-to-medial fashion [1, 2]. Sharp dissection between the visceral fascia covering the pancreas plus mesentery and the parietal fascia covering the retroperitoneal tissues was applied until the vessels that supplied the colon near the superior mesenteric artery were revealed. On the other hand, laparoscopic CME facilitates medial access, which completes dissection of the surgical trunk and central vessel ligation followed by mobilization of the colon in an inferior medial-to-superior lateral fashion [6]. The mobilization of the right hemicolon is based on three potential avascular surgical spaces bound by one surgical plane, the guts, and a nearby structure, the prerenal fascia, which provides a smooth surgical plane for the mobilization of the right hemicolon [8]. Furthermore, the RRCS, TRCS, and IMS, located between the transverse mesocolons, produce a surgical space, IMS, for the mobilization of right hemicolon. The IMS is situated posterior to greater omentum and superior to transverse mesocolon. It commutes with the TRCS posteriorly to the root of the transverse mesocolon. Therefore, the mobilization of transverse mesocolon requires the entrance of the IMS (Fig. 3).
According to the anatomical theories behind CMA, complete transverse mesocolon excision is achieved by the direct cranial extension of the TRCS to enter the IMS. With such a strategy, the mesocolon is dissected in a top-to-bottom fashion at once. In theory, this fashion complies better to the principles of CME. In the meantime, it can avoid repetitive flipping of the colon and the mesocolon, which lead to confusion in recognizing anatomical structures, which further causes failure in complying with the requirements of CME. In addition, this strategy can dissect the inferior margin of the pancreas with associated branched vessels under direct vision, which results in less blood loss. The present study demonstrated that CME requires less time for both central vessel ligation and laparoscopic procedures. Meanwhile, it reduces vessel-related complications, especially the pancreaticoduodenal vessels. Overall, CMA is a preferred choice for laparoscopic CME.
Strategic techniques and difficulties in the CMA
The recognition and extension of the TRCS
The TRCS is located between the transverse mesocolon and the inferior edge of the pancreas. By the inferior margin of the duodenal third portion, the caudal portion of the TRCS extends into the RRCS. By the root of the transverse mesocolon, the cranial portion of the TRCS extends into the IMS. The recognition and extension of the TRCS is one of the most important steps in CMA.
We suggest two possible ways to recognize the TRCS. First, not only is the SMV the boundary between the ascending mesocolon and enteric mesentery, but it is also the middle boundary of and the entrance to the TRCS. Therefore, successful entrance to the TRCS can be achieved after sharp lateral dissection of the ascending mesocolon along the surface of the SMV sheath. Second, the recognition of the ileocolic vessel leads to a successful entrance to the inferior part of the RRCS. The following superior extension enters the TRCS via the dissection of the duodenal third portion and ventral part of the pancreas. Either way can direct into the IMS and the RRCS with cranial and right extensions, respectively, to complete the mobilization of the mesocolon.
“Climbing” the inferior edge of the pancreas
CMA requires the bottom-to-top extension of the TRCS that enters the IMS via the root of the transverse mesocolon. Potential complications of the CMA include mistakenly entering the posterior part of the pancreas and hemorrhage caused by the injuries to the pancreas. Therefore, recognizing the inferior edge of the pancreas and “climbing” are key steps. We believe that the emergence of the Henle trunk after the dissection of the SMV suggests that the extension is close to the inferior margin of the pancreas. In the meantime, the extension should convert to a superior direction for the climbing. Also, it is easier to enter the IMS after dissection toward the left of the right gastroepiploic vein, followed by the emergence of the vein.
According to National Comprehensive Cancer Network guidelines and Japanese general rules for clinical and pathological studies on cancer of the colon, rectum, and anus [10], stage I tumor of right hemicolon does not require resection at root of the vessels. In the medical center, preoperative tumor classifications were performed mainly by computed tomography and colonoscopy. The tumor of each patient was thought to be stage II or higher before the operation. Therefore, laparoscopic CME was provided to these patients, even though a few patients in both groups had stage I tumor as confirmed by postoperative pathological examinations (Table 1).
The success of CME to right hemicolon cancer surgery is based on a thorough understanding of embryology and surgical oncology [11]. CMA is technically feasible for laparoscopic CME. It can only take place after properly recognizing the surgical planes and spaces of the right hemicolon under laparoscopic vision. In addition, CMA for laparoscopic CME for the right hemicolon can further reduce operation time and vessel-related complications. This method should be widely encouraged and disseminated.
References
Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S (2009) Standardized surgery for colonic cancer: complete mesocolic excision and central ligation—technical notes and outcome. Colorectal Dis 11:354–364
West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P (2010) Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol 28:272–278
Eiholm S, Ovesen H (2010) Total mesocolic excision versus traditional resection in right-sided colon cancer—method and increased lymph node harvest. Dan Med Bull 57:A4224
Pramateftakis MG (2010) Optimizing colonic cancer surgery: high ligation and complete mesocolic excision during right hemicolectomy. Tech Coloproctol 14(Suppl 1):S49–S51
Zheng MH, Feng B, Lu AG, Li JW, Wang ML, Mao ZH, Hu YY, Dong F, Hu WG, Li DH, Zang L, Peng YF, Yu BM (2005) Laparoscopic versus open right hemicolectomy with curative intent for colon carcinoma. World J Gastroenterol 11:323–326
Feng B, Sun J, Ling TL, Lu AG, Wang ML, Chen XY, Ma JJ, Li JW, Zang L, Han DP, Zheng MH (2012) Laparoscopic complete mesocolic excision (CME) with medial access for right-hemi colon cancer: feasibility and technical strategies. Surg Endosc 26:3669–3675
Adamina M, Manwaring ML, Park KJ, Delaney CP (2012) Laparoscopic complete mesocolic excision for right colon cancer. Surg Endosc 26:2976–2980
Zhang C, Ding ZH, Yu HT, Yu J, Wang YN, Hu YF, Li GX (2011) Retrocolic spaces: anatomy of the surgical planes in laparoscopic right hemicolectomy for cancer. Am Surg 77:1546–1552
West NP, Morris EJ, Rotimi O, Cairns A, Finan PJ, Quirke P (2008) Pathology grading of colon cancer surgical resection and its association with survival: a retrospective observational study. Lancet Oncol 9:857–865
Japanese Society for Cancer of the Colon and Rectum (2009) Japanese classification of colorectal carcinoma, 2nd edn. Kanehara, Tokyo
West NP, Kobayashi H, Takahashi K, Perrakis A, Weber K, Hohenberger W, Sugihara K, Quirke P (2012) Understanding optimal colonic cancer surgery: comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J Clin Oncol 30:1763–1769
Acknowledgments
We gratefully acknowledge the National High Technology Research and Development Program of China (863 Program), the Science and Technology Commission of Shanghai Municipality, and the Shenkang Center of Hospital Development for financial support (2012AA021103, 1141195070, 11411950701, SHDC12010116). In addition, we appreciate the contributions of their coworkers and friends to this study, as well as the editors and reviewers for their input.
Disclosures
Bo Feng, Tian-Long Ling, Ai-Guo Lu, Ming-Liang Wang, Jun–Jun Ma, Jian-Wen Li, Lu Zang, Jing Sun, and Min-Hua Zheng have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Feng, B., Ling, TL., Lu, AG. et al. Completely medial versus hybrid medial approach for laparoscopic complete mesocolic excision in right hemicolon cancer. Surg Endosc 28, 477–483 (2014). https://doi.org/10.1007/s00464-013-3225-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-013-3225-8