Abstract
Introduction
In gastrointestinal surgery, specifically in bariatric surgery, there are many types of fixed bands used for restriction and there are a multitude reasons that might eventually be an impetus for the removal of those bands. Bands consisting of Marlex or non silastic materials can be extremely difficult to remove. Intraoperative complications removing fixed bands include the difficulty in locating the band, inability to remove all of the band, and damage to surrounding structures including gastrotomies. Removal of eroded bands endoscopically may pose less risk. Potentially, forced erosion may be an easier modality than surgery, allowing revision without having to deal with the actual band at the time of definitive revision surgery.
Methods
A retrospective case series developed from a university single institution bariatric practice setting was utilized. Endpoints for the study include success of band removal, complications, length of time the stent was present, and the type of stent.
Results
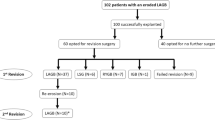
A total of 15 consecutive cases utilizing endoscopic stenting to actively induce fixed gastric band erosion for subsequent endoscopic removal were reviewed. There was an 87 % success rate in complete band removal with partial removal of the remaining bands that resolved the patient’s symptoms. A complication rate of 27 % was recorded among the 15 patients, consisting of pain and/or nausea and vomiting. The mean time period of the placement of the stent prior to removal or attempted removal was 16.3 days.
Conclusion
Endoscopic forced erosion of fixed gastric bands is feasible, safe, and may offer an advantage over laparoscopic removal. This technique is especially applicable for gastric obstruction from fixed bands, prior to large and definitive revision surgeries, or anticipated hostile anatomy that might preclude an abdominal operation altogether.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Multiple types of fixed bands, or nonadjustable gastric bands (NAGB), exist including Molina Bands, Fovey Bands, and vertical gastric bands. The NAGB was first created in 1978 by Wilkerson, but was only placed in one patient who Wilkerson felt had suboptimal results because the band was placed too low and the resulting gastric pouch was too large [1, 2]. The procedure was later adapted by several groups around the world. Dr. Molina, a surgeon out of Houston Texas, evolved the procedure and over the ensuing years, continued to modify and improve the procedure which ultimately he performed on 7,347 patients from around the world [3].
These operations had a band removal rate between 10–17 % within just a few years [4, 5]. Reasons for the removal of the various band types include esophageal dilation, band slippage, band erosion, bloating, nausea, vomiting, and failure of anticipated weight loss [6]. Upto 50 % of vertical banded gastroplasties (VBG) require need reintervention due to one or more of these problems [7]. Though Molina touts that his procedure is entirely reversible, the surgery to remove the band can be very challenging. The difficulty of the surgery is highly dependent on the materials used to construct the band. Those bands consisting of the silastic material are easier to remove technically because of the lack of incorporation into surrounding tissue or structures. On the other hand, those bands consisting of marlex or nonsilastic materials can be extremely difficult to remove because of its integration in the gastric tissue.
In addition, these surgeries were generally performed through a minilaparotomy which caused adhesions and scarring around the banded portion of the stomach. This results in a band that tends to be densely adhered to the abdominal wall and the liver. This makes locating the band along the gastric wall difficult and anxiety provoking for many surgeons. Many times, a gastrotomy is required in order to remove the band, adding further complexity and potential morbidity to the case. Efforts to reduce the complexity of this reversal were taken by Sweeny and Brunicardi who successfully avoided the dense adhesions by removing the band using a transgastric endoscopic rendezvous approach with good success [8].
We serendipitously discovered that we could actively cause an erosion when we stented across a patient with a gastric outlet obstruction that was unknowingly caused by a band. When the stent was removed, we were impressed to find a silastic band nicely adhered to the stent like a ring on a finger. With this finding, we researched everything we could to ultimately develop a reproducible and purely endoscopic solution.
Methods
A retrospective case series developed from our single University setting. Endpoints of band removal, complications, length of time the stent was present, and the type of stent were measured. All patients with known fixed bands who wanted them revised or simply removed were offered this treatment. We performed 15 consecutive cases and report the results.
The procedure is performed as follows: Once a patient is diagnosed with a fixed band that is resulting in a complication we schedule the patient for stent placement in the operating room with fluoroscopic assistance. We elect to conduct the placement under general endotracheal anesthesia because the obstructive nature of these bands can be significant and the risk of aspiration is greatly increased. After intubation, we first carefully inspect the pouch and clear it of debris.
Next, the lumen of the band is cannulated and a guide wire is placed to the pylorus. The endoscope is replaced to visualize the band stoma and a 120 mm covered, expandable stent (alveolus 22 × 120, 22 × 100 mm, Alveolus, Inc., Charlotte, NC; or Boston Scientific 125 × 23 partial, Boston Scientific Inc., Natick, Mass.) is placed over the guide wire and positioned approximately 30 mm past the band. The endoscope is retracted to allow for stent deployment and the stent is deployed under fluoroscopy. The position is verified and, when needed, adjusted by endoscopy or fluoroscopy (see Fig. 1).
In the first half of our series, a second 120 mm stent is then deployed in a similar manner to the first with a goal of achieving at least 50 mm overlap between the two stents and a 50 mm esophageal overlap with a proximal lumen that is closely approximated to the wall of the esophagus. This is done to increase circumferential pressure on the band and avoid migration of the stents (see Figs. 2, 3). The patient is then discharged home on a liquid diet.
In the second half of our series, in an effort to avoid esophageal overlap which seemed to cause pain in some patients, we used a single stent with phalanges that are placed in the same fashion as the first stent in the early part of the series.
The patient is then brought 3 weeks later for the second stage of the procedure. This stage is done in the operating room with the patient intubated for airway protection. The stents are removed using a pair of rat toothed graspers and the stomach is inspected endoscopically to locate the eroded band (see Fig. 4). The band is cut using endoscopic sheers or similar cutting devices (see Fig. 5). Once cut, the band is pulled through by grabbing one end with the graspers and applying gentle force until the band releases and is removed through the esophagus (see Fig. 6). The stomach is inspected and a chest X-ray is conducted in recovery to ensure the absence of free air. They are discharged home from recovery and maintained on proton pump inhibitors.
Results
A total of 15 consecutive patients during the year of 2010–2011 had an endoscopic stent placed in anticipation of forced gastric band erosion and removal. This was performed by three different surgeons utilizing the same technique. 6 (40 %) of these were silastic tubing placed at the time of a vertical banded gastroplasty, 3 (20 %) were Gortex Molina bands, 5 (33 %) were Dacron Molina bands, and 2(14 %) were Marlex Molina bands. The demographics and results are listed in Tables 1, 2.
There was an 87 % success rate in complete endoscopic band removal. The remaining two bands were partially removed and the patients both experienced a resolution of their gastric outlet obstruction. The first incomplete removal was a Dacron Molina band that partially eroded, but could not be completely freed. The band was cut and the exposed portion of the band was removed. The second incomplete band removal was for a vertical banded gastroplasty which the patient ultimately elected to convert to a bypass.
There were complications in 5 (33 %) of the patients. These included 2 (20 %) complaints of substernal chest pain which required early removal, 1 (7 %) migration of the stent resulting in nausea and pain that required removal, 1 (7 %) had severe nausea and vomiting, and 1 (7 %) developed a stricture two weeks after the procedure that required intervention. The mean time period of the presence of the stent prior to removal or attempted removal was 16 ± 9 days.
Discussion
These are the first cases where intentional erosion was created with a goal of future endoscopic retrieval of fixed gastric bands. The logical complications of perforation or stenosis do not appear to be a significant issue. The same scar tissue that encompasses the bands and makes the laparoscopic retrieval difficult may very well be protecting the patient after the gastric wall erosion. Furthermore, the stomach appears to heal quickly from the procedure with evidence from our own endoscopic follow up.
The complications from our series are minor and improved when changing our practice to bridging the stenosis itself without overlapping the esophagus. All of the substernal chest pain and nausea complications were in patients with stents that overlapped the esophagus. Once we were no longer placing the proximal portion of the stent in the esophagus, the patients generally tolerated the stents well and reported a resolution of their obstructive symptoms.
It would seem that the remaining bed from which the band is removed would be ideal for scar tissue formation and stenosis. In this series, stenosis only occurred in one patient just two weeks after removal of a silastic band in a patient suffering severe gastric obstruction 15 years after a vertical banded gastroplasty. Major complications were not seen and may be related to the small sample size in our series. The results are promising and certainly indicate that further efforts to study this technique are needed.
With hundreds of thousands of NAGB in place today and their continued use, compounded by their high reintervention rate, most bariatric surgeons are going to be challenged with the removal of these bands. The more modalities we have to treat NAGB patients, the more individualized the treatment and the better the outcomes will be. While forced erosion may not be ideal for all patients who desire removal of their fixed band, it may present itself as great option for many. This is true whether the patient only wants the band removed or if they desire a revision to another operation.
Conclusion
These are the first cases where intentional erosion was created with a goal of future endoscopic retrieval of fixed gastric bands. The endoscopic forced erosion of fixed gastric bands is feasible, appears safe, and may offer an advantage over laparoscopic removal and the technique should be considered when removal of a fixed band is anticipated.
References
Linner JH (1984) Gastric operations: specific techniques. In: Linner JH (ed) Surgery for morbid obesity. Springer, New York, pp 65–91
Wilkinson LH (1980) Reduction of gastric reservoir capacity. Am J Clin Nutr 33:151–157
Oria HE (2009) Gastric segmentation: nonadjustable banding by minilaparotomy: historical review. Surg Obes Relat Dis 5:365–370
Wolf AM, Kortner B, Kuhlmann HW (2001) Results of bariatric surgery. Int J Obes Relat Metab Disord 25(Suppl 1):S113–S114
Vassallo C, Andreoli M, La Manna A, Turpini C (2001) 60 reoperations on 890 patients after gastric restrictive surgery. Obes Surg 11(6):752–756
Steffen R (2008) The history and role of gastric banding. Surg Obes Relat Dis 4:S7–S13
Miller K, Pump A, Emanuel H (2007) Vertical banded gastroplasty versus adjustable gastric banding: prospective long-term follow up study. Surg Obes Relat Dis 3(1):84–90
Karmali S, Sweeney JF, Yee K, Brunicardi C, Sherman V (2008) Transgastric endoscopic rendezvous technique for removal of eroded Molina gastric band. Obes Surg 4(4):559–562
Disclosures
Drs. Todd D. Wilson, Nathan Miller, Nicholas Brown, Brad E. Snyder, and Erik B. Wilson, have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wilson, T.D., Miller, N., Brown, N. et al. Stent induced gastric wall erosion and endoscopic retrieval of nonadjustable gastric band: a new technique. Surg Endosc 27, 1617–1621 (2013). https://doi.org/10.1007/s00464-012-2638-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-012-2638-0