Abstract
Background
This study aims to evaluate the clinical and anatomical factors, particularly pelvic dimensions that influence the difficulty of performing laparoscopic anterior resection for rectal cancer.
Methods
We studied 50 consecutive patients who underwent laparoscopic anterior resection with double-stapling technique (DST) anastomosis for rectal cancer between January 2006 and February 2010. Staging was performed by computed tomography. Five pelvic dimensions (anteroposterior and transverse diameters of pelvic inlet and outlet, and pelvic depth) were measured using three-dimensional volume-rendering images. We also examined a number of other clinical characteristics, including gender, history of laparotomy, body mass index (BMI), operator, tumor location, tumor depth, nodal involvement, and tumor diameter. Univariate and multivariate analyses were performed to determine the predictive significance of these variables on surgical difficulty based on operative time and intraoperative blood loss.
Results
Males had significantly shorter pelvic inlets and outlets and significantly greater pelvic depth than females. However, gender did not significantly affect surgical outcomes, although males did tend to experience greater blood loss. Maximum tumor diameter (p = 0.014), BMI (p = 0.001), operator (p < 0.001), and tumor location (p = 0.009) were independent predictors of operative time, which, in turn, was related to intraoperative blood loss (p < 0.001).
Conclusions
Maximum tumor diameter, BMI, operator experience, and tumor location can be used to predict the operative time required to complete laparoscopic anterior resection with DST anastomosis for rectal cancer, with no correlations between pelvic dimensions and operative time. The difficulty of the procedure was not related to patients’ pelvic dimensions, which led us to conclude that “narrow pelvis” is not a contraindication for this surgery. Based on these results, we suggest that laparoscopic anterior resection should be performed by experienced surgeons in patients with large tumors, high BMI, and/or extraperitoneal rectal cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Laparoscopic procedures for rectal cancer have been reported to be safe and effective for a number of reasons, including relatively low levels of pain and blood loss, early resumption of bowel movement, and short postoperative hospital stay [1–6]. Additionally, randomized studies have shown that laparoscopic total mesorectal excision (TME) and lymph node dissection are productive surgical techniques with survival and recurrence rates comparable to those of open procedures [4, 6–8]. However, while laparoscopic surgery is the standard treatment for colon cancer, it is not commonly performed in cases of rectal cancer because it is technically challenging and may be associated with disadvantages such as long operative time [1, 2, 4] and increased rate of positive surgical margins [9].
Rectal surgery is performed through a narrow and funneling bony inlet, which makes access and visualization difficult in the deep pelvis. Even in the relatively simpler open approach, it is difficult to maintain a clear surgical field, to recognize precise anatomy, and to accurately perform rectal mobilization and excision while preserving urogenital functions. Recent studies have suggested that the quality of open rectal surgery is influenced not only by the surgeon’s skill but also by the patient’s clinical and anatomical factors, such as gender, tumor height, and pelvic size [10–12]. Similar relationships possibly influence the outcomes when using the laparoscopic approach, but evaluation of the influence of such clinical and anatomical factors on laparoscopic rectal surgery has been limited [13, 14]. The purpose of this study is to evaluate the influence of various clinical and anatomical factors, particularly pelvic dimensions, on operative time and intraoperative blood loss, which were selected as dependent variables to represent the level of difficulty in performing laparoscopic anterior resection with double-stapling technique (DST) anastomosis for rectal cancer.
Patients and methods
Patients
We studied 50 consecutive patients who underwent laparoscopic anterior resection with DST anastomosis for rectal cancer located below the inferior edge of the S2 vertebra between January 2006 and February 2010.
The indications for laparoscopic surgery were rectal cancer without involvement of the lateral lymph nodes or invasion of the adjacent organs, as determined by computed tomography (CT) and pelvic magnetic resonance imaging (MRI) during preoperative examinations. An additional indication was evidence of metastatic disease that could not be curatively resected using open surgery.
In Japan, preoperative radiotherapy or chemotherapy is not routinely administered in the treatment of rectal cancer; it is currently being used in clinical trials or mainly in patients with locally advanced, very low tumors to increase the chance of sphincter-preserving surgery. In this study, no patients underwent preoperative radiotherapy or chemotherapy.
Data for age, gender, history of laparotomy, body mass index (BMI), tumor location, tumor size, tumor staging, operative time, amount of blood loss, conversion to open surgery, pathology, 30-day morbidity, and mortality were collected prospectively. Tumors were staged according to the sixth tumor–node–metastasis (TNM) classification of the International Union against Cancer (UICC) on the basis of the histological findings of the surgical specimens.
Surgical procedures
The surgeries were performed by an experienced expert surgeon (T.Y.) or by trainees with 3–6 years of experience, operating under the expert’s supervision.
Anterior resection is used for the treatment of early cancer located just above the dentate line and advanced cancer located >1 cm above the dentate line; these criteria enable the acquisition of adequate distal margin after rectal transection. Here, patients were placed in Lloyd–Davis position with the head and right side of the bed lowered. First, a 12-mm camera port was inserted below the umbilicus using the open method. After creation of a pneumoperitoneum, four working ports were inserted: a 5-mm port in the right and left upper abdominal quadrants each, and a 12-mm port in the right and left lower abdominal quadrants each. The mesocolon was mobilized using the mediolateral approach, and the inferior mesenteric artery was divided near its origin in order to achieve wide lymphadenectomy. This permitted TME, except in cases of intraperitoneal rectal cancer, where tumor-specific mesorectal excision (TSME) was performed instead. The rectum was transected intracorporeally using an Endo-Cutter (Ethicon Endo-Surgery, Cincinnati, OH, USA) and anastomosed with DST. No diverting stoma was created.
Pelvimetry
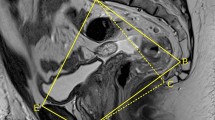
All patients underwent abdominopelvic CT (Aquilion 16; Toshiba Medical Systems Corporation, Tochigi, Japan). In most cases, the slice interval was adjusted to 5 mm. Sequences were volume-rendered using a DICOM 3D viewer, INTAGE Realia (KGT Inc., Tokyo, Japan). Volume-rendering (VR) images were obtained from the extracted volume data using INTAGE Volume Player (KGT Inc., Tokyo, Japan). A single observer (S.O.) blinded to all clinical information regarding the patients made all measurements in the VR images. Five pelvic dimensions were measured: anteroposterior and transverse diameters in the pelvic inlet (the axis from the superior aspect of the pubis symphysis to the sacral promontory and the longest lateral axis in the iliopectineal line), anteroposterior and transverse diameters in the pelvic outlet (the axis from the inferior aspect of the pubis symphysis to the tip of the coccyx and the distance between the tips of the ischial spines, i.e., interspinous distance), and the pelvic depth (the distance between the sacral promontory to the tip of the coccyx) (Fig. 1).
Statistical analysis
The sample size was calculated to detect moderate correlation (correlation coefficient: |r| = 0.4) with α of 0.05 (two-sided) and β of 0.2 (power of 80%), suggesting a total study population of 47 patients. All statistical analyses were performed using SPSS version 15.0 (Statistical Package for Social SciencesTM; SPSS, Inc., Chicago, IL, USA). Statistical significance was defined as p < 0.05. Where appropriate, we used Fisher’s exact test, chi-square test, Student’s t test, Welch’s test, or Pearson’s product-moment correlation coefficient to investigate relationships between patients’ clinical and anatomical characteristics and surgical difficulties. Multivariate analysis was performed using a multiple linear regression model with a stepwise method (significance level to enter = 0.05; significance level to stay = 0.1).
To assess intraobserver variation, measurements of the pelvic dimensions of 10 patients were repeated after an interval of 4 weeks, with the observer blinded to the initial results [10, 14]. According to the Pearson’s product-moment correlation coefficient, the intraobserver variation was 0.946. The two sets of measurements were highly correlated (p < 0.001), indicating that they accurately described pelvic anatomy.
Results
Patient and tumor characteristics are summarized in Table 1. Anastomosis height was significantly greater in males than in females (p = 0.014). All five pelvic dimensions differed significantly between male and female patients. Females had significantly longer measurements for the pelvic inlet and outlet (all p < 0.003), while males had significantly greater pelvic depth (p < 0.001). Overall, this indicated that male pelvises were significantly narrower and deeper than female pelvises.
Although males tended to experience more blood loss during surgery (p = 0.11), there were no significant differences between the genders in surgical outcomes (Table 2). In no case was there conversion to open surgery, death or positive circumferential resection margins (CRM). Complications were identified in two male patients: one wound infection and one anastomotic leakage. The overall morbidity rate was 4%, and the anastomotic leakage rate was 2%.
Univariate analysis showed that age (p = 0.012), BMI (p = 0.009), operator (p = 0.006), and maximum tumor diameter (p = 0.003) were significantly associated with operative time (Table 3). Although operative time tended to increase as anteroposterior pelvic inlet diameter decreased (p = 0.151) and pelvic depth increased (p = 0.103), these relationships were not significant.
Stepwise linear regression analysis showed that the optimal model for predicting operative time included maximum tumor diameter, BMI, operator, and tumor location (p < 0.001, Table 4). Operative time increased as maximum tumor diameter and BMI increased, but decreased with an expert performing the operation and for intraperitoneal tumor location. Operative time, in turn, was the only factor significantly associated with blood loss (p < 0.001); no other variables had any relationship with blood loss.
Discussion
In this study, multivariate analysis showed that larger maximum tumor diameter, higher BMI, trainee performing the operation, and extraperitoneal tumor location were significantly associated with longer operative time in laparoscopic anterior resection with DST anastomosis for rectal cancer, while pelvic dimensions had no correlations with operative time. Furthermore, operative time was the only factor significantly associated with intraoperative blood loss. The present findings are valuable in suggesting that pelvic dimensions were not definitive factors as compared with maximum tumor diameter, BMI, operator experience, and tumor location in predicting the difficulty of performing this procedure.
Interest in pelvimetry began with attempts to predict cephalopelvic disproportion in pregnant women prior to labor. Pelvimetry has been utilized for patients with rectal cancer, using MRI [10–12] and CT [13, 14] images; in these cases, measurements were made on two-dimensional reconstructed (axial and sagittal) images. However, these cross-sectional images only permit measurement of distances between points that exist in the same orthogonal coordinate axis. We preferred the use of three-dimensional VR images, because they allow precise measurements along any axis and can be especially beneficial in cases with anatomically strained pelvis or mismatched alignments between patients and imaging devices. The precision and sensitivity of this technique were demonstrated by its ability to correctly indicate that male pelvises are narrower and deeper than female pelvises, as well as by the strong correlation between the two sets of measurements in the intraobserver variation test (r = 0.946, p < 0.001).
In this study, we chose to evaluate cases of rectal cancer that underwent laparoscopic anterior resection with DST anastomosis, because intracorporeal rectal transection and anastomosis is one of the most difficult procedures in laparoscopic rectal surgery and therefore should be considered separately from cases that undergo abdominoperineal resection or intersphincteric resection with transanal hand-sewn anastomosis. We selected operative time and intraoperative blood loss as dependent variables representing technical difficulties during this procedure. Other variables, including complications, anastomotic leakage, conversion, mortality, and positive CRM, occurred at such low rates that they could not be analyzed. This indicates that the procedure can be performed safely and without morbidity or conferring any oncologic disadvantage.
It is not immediately clear why anastomosis height was significantly greater in males than in females. Unlike previous authors [13, 15], we did not find that operative outcome differed significantly between the two genders. These results may partly be explained by the fact that pelvic procedures can be completed more easily in wider and shallower female pelvises, but may also be disrupted by the presence of the uterus.
Patients in this study had BMI ranging from 12.0 to 27.8 kg/m2; these values are lower than those in Western populations. Nevertheless, our results agreed with previous reports that found a positive association between operative time and BMI [14]. This is likely associated with greater mesorectal volume, which restricts the pelvic working space for the procedures. Therefore, visceral fat may be an even better predictor of surgical difficulty than BMI [16, 17]. Further, larger maximum tumor diameter reflects larger tumor volume, which again restricts the pelvic working space. Space can also be restricted by the location of tumors; i.e., when tumors are positioned extraperitoneally, surgeons have a narrower space in which to perform rectal dissection, transection, and anastomosis, since the pelvic width becomes narrower as one approaches deeper into the pelvis. Thus, cumulatively, higher BMI, larger maximum tumor diameter, and extraperitoneal tumor location impact operative time by limiting pelvic free space for the procedures and reducing visibility, maximum retraction, and access to the depths of the pelvis via the pelvic inlet.
In keeping with our previous finding, operative time was longer when procedures were performed by trainees [18]. Pelvic space cannot be expanded by pneumoperitoneum, as can be done in the upper abdomen, and limited working space directly affects the difficulty of safe and quick access, required to optimize visibility and retraction. We presume that these issues do not present as great a problem to expert surgeons because they have more experience in creating an appropriate surgical field and obtaining a good view for identifying and dissecting anatomical structures even in a limited pelvic working space.
Although pelvic depth tended to correlate with operative time, we did not find any significant patterns linking pelvic dimensions with operative outcomes. These results are contrary to those previously reported elsewhere [13, 14]. We hypothesize that this is because BMI, maximum tumor diameter, and tumor location have greater effect on pelvic working space than do pelvic dimensions. Additionally, this study included cases of both intraperitoneal and extraperitoneal rectal cancer, while a similar previous study focused only on extraperitoneal rectal cancer [14]. Furthermore, the procedures in our study were performed by both experts and trainees, rather than experts only [14]. Thus, these differences in inclusion criteria may explain why we did not detect any significant correlations between pelvic dimensions and operative time.
Our study has certain limitations. The sample size of this study was small, although the study was not statistically underpowered. However, future examinations with larger sample size would elucidate our results further. Additionally, other variables, including complications, anastomotic leakage, conversion, mortality, and positive CRM, should be examined to generalize the present findings.
In summary, our results indicate that “narrow pelvis” is not a contraindication for laparoscopic resection of rectal cancer. We also recommend that this procedure be performed by experienced surgeons in patients with large tumors, high BMI, and/or extraperitoneal rectal cancer.
References
Leung KL, Kwok SP, Lam SC, Lee JF, Yiu RY, Ng SS, Lai PB, Lau WY (2004) Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet 363:1187–1192
Law WL, Lee YM, Choi HK, Seto CL, Ho JW (2006) Laparoscopic and open anterior resection for upper and mid rectal cancer: an evaluation of outcomes. Dis Colon Rectum 49:1108–1115
Morino M, Allaix ME, Giraudo G, Corno F, Garrone C (2005) Laparoscopic versus open surgery for extraperitoneal rectal cancer: a prospective comparative study. Surg Endosc 19:1460–1467
Braga M, Frasson M, Vignali A, Zuliani W, Civelli V, Di Carlo V (2005) Laparoscopic vs. open colectomy in cancer patients: long-term complications, quality of life, and survival. Dis Colon Rectum 48:2217–2223
Zhou ZG, Hu M, Li Y, Lei WZ, Yu YY, Cheng Z, Li L, Shu Y, Wang TC (2004) Laparoscopic versus open total mesorectal excision with anal sphincter preservation for low rectal cancer. Surg Endosc 18:1211–1215
Ströhlein MA, Grützner KU, Jauch KW, Heiss MM (2008) Comparison of laparoscopic vs. open access surgery in patients with rectal cancer: a prospective analysis. Dis Colon Rectum 51:385–391
Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, Heath RM, Brown JM, UK MRC CLASICC Trial Group (2007) Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol 25:3061–3068
Kuhry E, Schwenk W, Gaupset R, Romild U, Bonjer J (2008) Long-term outcome of laparoscopic surgery for colorectal cancer: a Cochrane systematic review of randomised controlled trials. Cancer Treat Rev 34:498–504
Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM, MRC CLASICC Trial Group (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365:1718–1726
Salerno G, Daniels IR, Brown G, Norman AR, Moran BJ, Heald RJ (2007) Variations in pelvic dimensions do not predict the risk of circumferential resection margin (CRM) involvement in rectal cancer. World J Surg 31:1313–1320
Baik SH, Kim NK, Lee KY, Sohn SK, Cho CH, Kim MJ, Kim H, Shinn RK (2008) Factors influencing pathologic results after total mesorectal excision for rectal cancer: analysis of consecutive 100 cases. Ann Surg Oncol 15:721–728
Boyle KM, Petty D, Chalmers AG, Quirke P, Cairns A, Finan PJ, Sagar PM, Burke D (2005) MRI assessment of the bony pelvis may help predict resectability of rectal cancer. Colorectal Dis 7:232–240
Targarona EM, Balague C, Pernas JC, Martinez C, Berindoague R, Gich I, Trias M (2008) Can we predict immediate outcome after laparoscopic rectal surgery? Multivariate analysis of clinical, anatomic, and pathologic features after 3-dimensional reconstruction of the pelvic anatomy. Ann Surg 247:642–649
Akiyoshi T, Kuroyanagi H, Oya M, Konishi T, Fukuda M, Fujimoto Y, Ueno M, Miyata S, Yamaguchi T (2009) Factors affecting the difficulty of laparoscopic total mesorectal excision with double stapling technique anastomosis for low rectal cancer. Surgery 146:483–489
Hidalgo JM, Targarona EM, Martinez C, Hernandez P, Balague C, Trias M (2010) Laparoscopic rectal surgery: does immediate outcome differ in respect to sex? Dis Colon Rectum 53:438–444
Seki Y, Ohue M, Sekimoto M, Takiguchi S, Takemasa I, Ikeda M, Yamamoto H, Monden M (2007) Evaluation of the technical difficulty performing laparoscopic resection of a rectosigmoid carcinoma: visceral fat reflects technical difficulty more accurately than body mass index. Surg Endosc 21:929–934
Tsujinaka S, Konishi F, Kawamura YJ, Saito M, Tajima N, Tanaka O, Lefor AT (2008) Visceral obesity predicts surgical outcomes after laparoscopic colectomy for sigmoid colon cancer. Dis Colon Rectum 51:1757–1765
Ogiso S, Yamaguchi T, Hata H, Kuroyanagi H, Sakai Y (2010) Introduction of laparoscopic low anterior resection for rectal cancer early during residency: a single institutional study on short-term outcomes. Surg Endosc 24:2822–2829
Acknowledgements
We thank Naoki Sakane, MD, PhD, for his help in statistical analyses.
Disclosures
Authors Satoshi Ogiso, Takashi Yamaguchi, Hiroaki Hata, Meiki Fukuda, Iwao Ikai, Toshio Yamato, and Yoshiharu Sakai have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogiso, S., Yamaguchi, T., Hata, H. et al. Evaluation of factors affecting the difficulty of laparoscopic anterior resection for rectal cancer: “narrow pelvis” is not a contraindication. Surg Endosc 25, 1907–1912 (2011). https://doi.org/10.1007/s00464-010-1485-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-010-1485-0