Abstract
Background
Recent advances in endoscopic surgery have allowed laparoscopic harvesting of the omental flap with minimal deformity of the donor site. This study aimed to assess the safety and long-term complication rate for laparoscopic harvest of the omental flap (LHOF).
Methods
From April 2002 to December 2008, 96 patients underwent LHOF for immediate reconstruction after breast cancer surgery. All the patients were evaluated for operating time, length of hospital stay, and the short- and long-term complications associated with LHOF.
Results
The omental flap was harvested successfully in 95 of 96 cases, and the total success rate for harvesting of the omental flaps was 99% without conversion to open surgery. The median operative time for harvesting of the omental flap was approximately 1 h. Five cases of partial graft necrosis (5.2%) and two cases of vascular injury (2.1%) to the gastroepiploic artery and vein occurred, and the graft survival rate was 96.8% (93 of 95 cases). Laparoscopy-associated complications occurred in eight cases (8.3%), including one incisional hernia.
Conclusions
As a safe and minimally invasive procedure, LHOF has a low incidence of short- and long-term complications. This technique can expand the indications and usefulness of the omental flap.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recently, the omentum has attracted much attention for its immunologic and angiogenic features. In 1929, Knazozovicky first used the omentum from the abdominal cavity to perform arthroplasty [1]. Since then, many applications for the omentum have been reported because it protects against infection and ischemia. Kiricuta [2, 3] was the first to use pedicled omental flaps for a range of indications. Surprisingly, various new indications (e.g., chronic spinal cord injury and Alzheimer’s disease) also have emerged [4]. Nevertheless, the omental flap has not obtained widespread popularity because it requires a laparotomy.
Recent advances in endoscopic surgery have allowed laparoscopic harvesting of the omental flap with less donor-site deformity and morbidity [5], making the omental flap more attractive. Many reports have described laparoscopic harvesting of the omental flap, but most are case reports or small series [6–8]. The true complication rates and long-term results for this minimally invasive procedure are not clear.
Since April 2002, we have managed 96 cases of laparoscopically harvested omental flaps (LHOFs) for reconstruction after breast cancer surgery [9]. We report our laparoscopic techniques for harvesting the omental flap as well as the short- and long-term complications.
Materials and methods
The study was approved by the hospital ethics committee, and all the patients provided written informed consent. From April 2002 to December 2008, 96 patients with stage 0, 1, or 2 breast cancer underwent breast cancer surgery using LHOF. The LHOF was applied as a volume replacement tissue after breast-conserving surgery or nipple-sparing mastectomy. For breast-conserving surgery, LHOF was used when a 30% or wider region of breast tissue was resected or the cosmetic result was poor due to the location of the tumor in the lower inner quadrant.
Patients with a history of either intraabdominal malignancy or upper abdominal laparotomy were excluded from the study. However, a history of laparoscopic surgery (i.e., laparoscopic cholecystectomy) and a history of lower abdominal surgery (i.e., cesarean section) were not criteria for exclusion. Patients with a body mass index (BMI) of 35 kg/m2 or more also were excluded.
Surgery was performed with the patient in the supine position under general anesthesia. A camera port (5 or 10 mm, 30º) was inserted under the umbilicus, with the surgeon positioned on the right side of the patient. Pneumoperitoneum was maintained at 10 mmHg. Two 5-mm ports for the surgical instruments were inserted from the right abdominal wall: the one through the right upper quadrant at the level of a wedge of the rectus muscle and the other through the right lateral lower quadrant. One 5-mm port for the assistant was inserted through the left lateral lower quadrant, and an additional 5-mm port was added through the left upper quadrant when needed.
First, the omentum was evaluated for size and adhesion, then moved cephalad for dissection from the transverse colon. Usually, a site slightly left of the transverse colon’s center was the most suitable place for starting dissection because it provided easier access to the lesser sac.
After entrance to the lesser sac, the dissection was advanced leftward while appropriate tension was maintained between the omentum and the transverse colon. Care was taken not to injure the transverse colon, especially near the splenic flexure. When the splenic flexure was reached, the omentum was transected toward the lower pole of the spleen. Because the right rather than the left gastroepiploic artery (GEA) predominates, we always selected the right gastroepiploic artery and vein (GEAV) as a pedicle, dividing the left GEAV near the spleen with the Harmonic Scalpel (Ethicon Endo-surgery Inc., Cincinnati, OH, USA).
After division of the left GEAV, its branches were divided at a site as close to the stomach wall as possible, little by little, until the pyloric ring was passed (Fig. 1). Attention was paid to the main branches of the GEAV because they sometimes ran very close to the stomach wall and could be difficult to identify when abundant fat deposit of the omentum was evident. The dissection from the right side of the transverse colon then was advanced. Fusion between the posterior leaf of the gastrocolic ligament and the anterior leaf of the transverse mesocolon was carefully divided toward the anterior capsule of the pancreas head. With upward traction of the omentum maintained, careful sharp and blunt dissection between the gastrocolic ligament and the transverse mesocolon was advanced until the roots of the GEAV were confirmed. To avoid the subsequent complication of ventral hernia, we found it better to resect as much fat tissue around the root of the GEAV as possible for the thin pedicle of the flap.
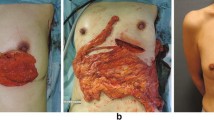
All the dissection and resection was performed using the Harmonic Scalpel without ligation or clipping. However, when a free flap was planned, the root of the GEAV was clipped and divided. With the use of a pedicled flap, a subcutaneous tunnel approximately two fingers wide was prepared from an incision along the medial half of the inframammary fold toward the xyphoid process. When it reached the white line, a longitudinal incision two or two and one-half fingers wide was made to communicate with the abdominal cavity. The forceps were inserted into the abdominal cavity, and the pedicled omental flap was carefully withdrawn, avoiding twisting (Fig. 2). When a wider abdominal incision was needed for a large omental flap, reclosure of the abdominal incision was necessary after the flap was pulled out to avoid incisional hernia. Otherwise a free flap generally was the better choice. With the use of a free flap, a transverse incision approximately 4 cm was made in the right or left lower abdominal wall similar to that for appendectomy, and the flap was removed.
The procedure for breast surgery (breast-conserving surgery or nipple-sparing mastectomy) and reconstruction with the omental flap has been described previously [9]. All patients were evaluated for outcome of the operation, length of hospital stay, and the early and late postoperative complications.
Results
All the patients underwent LHOF as an immediate volume replacement procedure after breast cancer surgery. The mean BMI was 22, and 28 of the patients (29.2%) had undergone prior abdominal surgery (Table 1).
All the omental flaps could be harvested without conversion to open surgery. Adhesions between the omentum and the peritoneum of the lower abdomen or around the liver bed due to prior surgeries were identified in several cases, but adhesiolysis performed laparoscopically was possible without any difficulties. The median operative time for harvesting the omental flap was approximately 1 h. Blood loss attributable to laparoscopic procedures was not great enough to count in most cases (Table 2).
In only one case, the flap lost its arterial blood flow after being withdrawn from the abdominal cavity, likely due to injury of the GEA not noticed during the laparoscopic procedure, and LHOF was abandoned. One additional injury of the GEA occurred. However, it was minor and could be treated with hemostasis. Ultimately, the omental flaps were successfully harvested in 95 of 96 cases, and the total success rate for harvesting omental flaps was 99% (Tables 2, 3).
In the early postoperative period, five cases of partial graft necrosis occurred and could be treated conservatively. However, two of these necroses lost most of their initial volume after repeated debridement for associated graft infections (Table 3). The total graft survival rate was 96.8% (93 of 95 cases). There was no incidence of late graft necrosis.
The sole late laparoscopy-associated complication (intraabdominal complication) was a case of epigastric hernia, in which the volume of the omental flap was extremely large, and a wider laparotomy incision was needed to withdraw the flap from the abdominal cavity. To date, no other late laparoscopy-associated complications (e.g., small bowel obstruction) have occurred (Table 3).
Two intraabdominal diseases were experienced during the follow-up period. The one was gastric lymphoma with a lymph node metastasis, diagnosed by laparoscopic wedge resection of the stomach wall and intraabdominal lymph node biopsy. The other disease was sigmoid colon cancer, which was resected using open sigmoidectomy. Both of these procedures could be performed easily because there was no adhesion in the abdominal cavities.
Discussion
The omentum is a useful reconstructive tissue because of its rich vascularity, angiogenic capacity, and significant antimicrobial properties [4, 10, 11]. Based on these properties, the omental flap is indicated for the following:
-
1.
Prevention and treatment of postoperative septic complications caused by dead spaces with and without radionecrosis [12]; pneumonectomy and empyema caused by tuberculosis, tumor, or aspergillosis [1]; and cardiac and lung surgery reoperation, especially deep sternomediastinisis [13]
-
2.
Prevention and treatment of anastomotic leaks and fistula formation [12] after intestinal or esophagointestinal anastomoses or perforations [1] and threatening bronchial leaks after pneumonectomy [1]
-
3.
Plastic repair on the surface of the body [12] (e.g., for extensive face and head injuries [14] or breast reconstruction [6, 9])
-
4.
Supply for vascular deficiencies [12] (e.g., for Buerger’s [4] or for Alzheimer’s disease [4]).
Nevertheless, use of the omental flap is limited because of its serious disadvantages. The omental flap’s greatest disadvantage is laparotomy-associated morbidity [15], which can be greatly decreased by improvements in laparoscopic surgery [5]. All the procedures for harvesting a pedicled flap can be conducted using only a Harmonic Scalpel without ligation or clipping.
The operating time for harvesting the omental flap was comparatively short (~1 h), and the success rate was almost 99% in the current study. However, because obese patients (BMI > 35) were basically excluded from this study, further examination is needed to determine whether the omental flap also can be safely harvested laparoscopically in patients with morbid obesity.
Cases with a history of lower laparotomy or laparoscopic surgery, even in the upper abdomen, were not excluded from the current study. As a result, the conversion rate to an open laparotomy was 0%, although adhesiolysis was required in several cases. Hereafter, cases with such histories may be actively included in the indications for LHOF.
The early postoperative period had five cases (5.2%) involving partial necrosis of the flap. The reported incidence of partial necrosis of open omental flaps varies from 2% to 16% [15], and our finding was within this range. This may have been due to partial necrosis of damaged or resected epiploic vessels, which branch from the GEAV and descend in the omentum because of anatomic misidentification during flap harvesting or trauma when the flap is drawn out of the abdominal cavity. Damage to the epiploic vessels may form a partial induration of the flap even if it does not cause necrosis.
However, little possibility exists for the flap to become necrotic during the late postoperative period. Although irradiation was applied to the breasts reconstructed with the omental flap in 31 cases (32.3%), few changes in the shape or hardness of the irradiated breasts occurred, which suggests that the omental flap was resistant to the irradiation.
Laparoscopic harvesting is associated with less postoperative pain and a considerably shorter time until resumption of bowel function. It allows food intake and ambulation the day after the operation [6]. In the current study, the mean hospital stay was 8 days, which was influenced mainly by the duration of axillary drainage after lymph node dissection. The LHOF procedure itself would require only 2 or 3 days in the hospital.
A fairly high incidence of incisional hernia after open omental flap harvesting has been reported [15–17]. Van Gardenren et al. [15] reported that 7 of 35 (20%) extraabdominal pedicled omentoplasties resulted in incisional hernias. Contant et al. [17] reported 9 incisional hernias experienced by 34 patients (26%) for whom the pedicled omental flap was used to reconstruct defects in the chest wall. In our laparoscopic harvesting cases, only one patient (1%) experienced an incisional hernia, which usually is rare with laparoscopic surgery. However, it is a problem when the omental flap is used as an extraabdominal pedicled flap.
All our patients underwent surgery for breast cancer. In such cases, cosmetic issues are very important, and it is better to make as few incisions on the abdominal wall as possible. Most of the omental flaps were pulled out through the subcutaneous tunnel into the dead space of the breast. The laparotomy incisions on the white line were a maximum of two and one-half fingers wide to avoid incisional hernia. However, when the volume of the omentum is great, it is difficult to withdraw the flap through the small laparotomy incision, and the possibility of vascular injury to the flap increases. In such cases, it would be better to make an appropriate longitudinal incision on the abdominal wall. The pedicle of the flap must be made thinner by defatting after it is withdrawn from the abdominal cavity, and the laparotomy incision should be closed to less than two fingers wide. It also is important to detach the omentum from the stomach past the pyloric ring and to confirm the root of the GEAV to make a long and slender pedicle. A free flap is another choice for a large omentum or for obese patients.
No other late complications (e.g., small bowel obstruction) occurred in the current study. The findings in two cases of reoperation for other abdominal disease suggest that the LHOF procedures would cause fewer incidences of small bowel obstruction than open omental flap procedures.
The abdominal scars from LHOF were almost the same as those from laparoscopic cholecystectomy. Although LHOF has another disadvantage (i.e., preoperative estimation of the omental volume is not possible), it is more attractive because it results in minimal deformities of the donor site when it is applied for plastic reconstruction [6, 9].
The LHOF procedure is a safe and minimally invasive approach that has a low incidence of short- and long-term complications. This technique can expand the indications and usefulness of the omental flap.
References
Lieberman-Meffert D, White H (1983) The greater omentum: anatomy, physiology, pathology, surgery, with a historical survey. Springer, New York
Kiricuta I (1963) L’emploi dugrand epiploon dans la chirurgie du sein cancereux. Press Med 71:15–17
Kiricuta I, Goldstein AM (1972) The repair of extensive vesicovaginal fistulas with pedicled omentum: a review of 27 cases. J Urol 108:724–727
Goldsmith HS (1990) The omentum: research and clinical applications. Springer, New York
Salz R, Stowers R, Smith M, Gadacz TR (1993) Laparoscopically harvested omental free flap to cover a large soft tissue defect. Ann Surg 217:542–546
Cothier-Savey I, Tamtawi B, Dohnt F, Raulo Y, Baruch J (2001) Immediate breast reconstruction using laparoscopically harvested omental flap. Plast Reconstr Surg 107:1156–1163
Nishimura T, Kanehira E, Tsukatani T, Furukawa M (2002) Laparoscopically harvested omental flap for head and neck reconstruction. Laryngoscope 112:930–932
Ferron G, Garrido I, Martel P, Gesson-Paute A, Classe JM, Letourneur B, Querleu D (2007) Combined laparoscopically harvested omental flap with meshed skin grafts and vacuum-assisted closure for reconstruction of complex chest wall defects. Ann Plast Surg 58:150–155
Zaha H, Inamine S, Naito T, Nomura H (2006) Laparoscopically harvested omental flap for immediate breast reconstruction. Am J Surg 192:556–558
Cartier R, Brunette I, Hoshimoto K, Bourne WM, Schaff HV (1990) Angiogenic factor: a possible mechanism for neovascularization produced by omental pedicles. J Thorac Cardiovasc Surg 99:264–268
Beelen RHJ (1991) The greater omentum: physiology and immunological concepts. Neth J Surg 43:145–151
Liebermann-Meffert D (2000) The greater omentum: anatomy, embryology, and surgical applications. Surg Clin North Am 8:275–293
Yasuura K, Okamoto H, Morita S, Ogawa Y, Sawazaki K, Seki A, Matsumoto H, Matsuura A, Maseki T, Torii S (1998) Results of omental flap transposition for deep sternal wound infection after cardiovascular surgery. Ann Surg 41:455–459
Arnold PG, Irons GB (1981) One-stage reconstruction of massive craniofacial defect with gastroomental flap. Ann Plast Surg 6:26–33
Garderen Van, Wiggers TH, Van Geel AN (1991) Complications of the pedicled omentoplasty. Neth J Surg 43:1171–1174
Obaid SI, Morris DJ (2001) Laser Doppler perfusion imager use in incisional hernia repair following omental breast reconstruction. Plast Reconstr Surg 109:2006–2008
Contant CME, Van Geel AN, Van der Holt B, Wiggers T (1996) The pedicled omentoplasty and split skin graft (POSSG) for reconstruction of large chest wall defect: a validity study of 34 patients. Eur J Surg Oncol 22:532–537
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zaha, H., Inamine, S. Laparoscopically harvested omental flap: results for 96 patients. Surg Endosc 24, 103–107 (2010). https://doi.org/10.1007/s00464-009-0533-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-009-0533-0