Abstract
Background: Controversy continues to surround laparoscopic rectal resection for malignancy. A longer follow-up period is required to evaluate the long-term efficacy of the procedure and its impact on survival. Furthermore, no data from ongoing randomized controlled trials are yet available. The aims of this study were to compare long-term outcomes for unselected patients undergoing either laparoscopic or open rectal resection for cancer. Methods: A series of 124 unselected consecutive patients with rectal cancer, who underwent surgery by the same surgical team, have been included in this study. Patients with T1N0 tumors underwent local excision, and emergency cases were excluded from the study. Written consent was submitted by each patient, and inclusion in either group (laparoscopic or open) was left to the patient’s choice. The laparoscopic approach was chosen by 81 patients, and 43 patients chose open surgery. All the patients underwent preoperative radiotherapy (5,040 cGy), performed in selected cases with chemotherapy (for patients younger than 70 years). The following parameters were compared between the two groups: length of the surgical specimen, clearance of the margins of the specimen, number of lymph nodes identified, local recurrence rate, incidence of distant metastases, and survival probability analysis. The mean follow-up period for both groups was 43.8 months (range, l–9 years). Results: We performed 60 laparoscopic and 27 open anterior resections, as well as 21 laparoscopic and 16 open abdomino perineal resections, respectively. No mortality occurred in either group. The mean length of the resected specimens was 24.3 cm in the laparoscopic group and 23.8 cm in the open group (p = 0.47). The mean tumor-free margin was 3.0 cm in the laparoscopic group and 2.8 cm in the open group (p = 0.57), and the mean number of lymph nodes identified was 10.3 in the laparoscopic group and 9.8 in the open group (p = 0.63). Of the 124 patients, 86 (52 laparoscopic and 34 open) were included in out study. We excluded patients who underwent a palliative resection (6 laparoscopic and 6 open patients) or conversion to open surgery (n = 10) and patients who had undergone surgery in the past year (n = 16). One laparoscopic patient was lost to follow-up evaluation, whereas three laparoscopic patients and one open patient died of causes not related to cancer. No wound recurrence was observed. The local recurrence rate after laparoscopic resection was 20.8%, as compared with 16.6% after open resection (p = 0.687). Distant metastases occurred in 18.2% of the patients in the laparoscopic group, as compared with 21.2% in the open group (p = 0.528). Cumulative survival probability was 0.709 after laparoscopic resection after LR and 0.606 after open resection (p = 0.162), whereas for Dukes’ stages A, B, and C in the laparoscopic group versus the open group, it was 0.875 vs 0.889 (p = 0.392), 0.722 vs 0.584 (p = 0.199), and 0.500 vs 0.417 (p = 0.320), respectively. At this writing 20 laparoscopic patients (62.5%) and 20 open patients (60.6%) are disease free (p = 0.623). Conclusions: Oncologic surgical principles were respected. Long-term outcome after laparoscopic resection of rectal cancer was comparable with that after conventional resection. We should wait to draw conclusive scientific statements until the completion of ongoing international radomized controlled trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
During the past two decades, remarkable progress has been made in the treatment of rectal cancer. The main goal of rectal surgery for malignancy is oncologic radicality in an effort to achieve the preservation of sphincters and of sexual–urinary function. Total mesorectal excision, proposed by Heald [8] probably provides the best local control of local recurrences. Adjuvant radiochemotherapy has become an integral part of the multidisciplinary approach to rectal cancer to reduce the risk of local recurrences. Tumors have been downstaged, lymph nodes sterilized, and resectability improved, but the quality of the surgical technique still holds a prominent role [3, 10, 18].
Furthermore, several authors have recently reported their experience with laparoscopic low anterior resections and abdominal perineal resections of the rectum for cancer [1, 6, 7, 9, 11, 12, 14, 17, 19, 20, 21, 22, 24, 27, 29]. These preliminary studies have shown that the laparoscopic technique is associated with equivalency of oncologic resection and equal to better survival, as compared with the results from standard surgery. It is possible to hypothesize that a correct laparoscopic technique may allow an even more meticulous total mesorectal excision. This relevant aspect probably is related to the magnified view and to the better vision obtained by the angled optics, together with the reduced manipulation of the mesorectum during the laparoscopic pelvic dissection [16]. In addition, magnification of the image facilitates a correct nerve-sparing technique [27].
Several other authors have reported that laparoscopic colorectal resections result in better preservation of the patient’s immune status [2, 13, 15]. On the other hand, it is suggested that laparoscopic access is related to an increased risk of port-site metastases. Despite a higher rate of port-site metastases initially reported, recent studies on port-site metastases have shown a low incidence (1%), suggesting that such seedings are related to the presence of advanced disease rather than the laparoscopic technique [26, 28].
However, laparoscopic treatment of rectal cancer still is controversial. Long-term data have been lacking, and large randomized trials must be conducted before this procedure gains larger acceptance.
The aim of this study was to compare the long-term results of the laparoscopic approach with the results of conventional open surgery in a series of 124 consecutive unselected patients with rectal cancer who underwent surgery performed by the same surgical team.
Patients and methods
From May 1992 to April 2002 all the patients with rectal cancer admitted to our institution were included in the study. Patients with tumors classified as T1N0, those who underwent local excision, and those whose operation was emergency surgery were excluded. The patients were not enrolled according to age, general condition, or oncologic stage (American Society of Anesthesiology [ASA] III patients and patients with late rectal cancer also were included in the study).
The treatment method (laparoscopic or open) was not randomized, but chosen by the patients without any pressure from the surgeon based on the stage of the disease.
All patients underwent preoperative tumor staging by barium enema, colonoscopy with macrobiopsies of the tumor (6 to 8 biopsies of normal mucosa at a distance of 1 cm from the tumor, with indian ink tattooing performed after each biopsy), endorectal ultrasound, liver ultrasound, computed tomography of the abdomen and pelvis, bone scan, and chest radiography.
According to our protocol, all the patients were treated with preoperative radiochemotherapy (chemotherapy in selected cases) according to the following schedule: Preoperative radiotherapy was performed, with an overall administration of 5,040 cGy divided over 5 weeks (28 fractions). The cycle of chemotherapy was performed only in selected patients (younger than 71 years) using 5 fluorouracil (5-FU) 500 mg/m2 per day, with 5 hours of infusion from day 1 to day 5, and from day 22 to day 26 of radiotherapy.
The operations were scheduled to be performed 50 days after completion of radiochemotherapy. The same surgical team performed all the surgical procedures following the same oncologic and clinical protocol in both the laparoscopic and open groups. For inferior rectum carcinoma located within 2 cm from the dentate line, low anterior resection or Miles abdomino perineal resection was performed. All the main oncologic concepts of traditional surgery were followed: high ligation of the inferior mesenteric vessels, extensive limphadenectomy, total mesorectal excision enbloc with the rectum, and correct clearance of the specimen’s margins. The operative specimens were removed through a 5 to 6-cm sovrapubic or subtumbilical minilaparotomy. In the case of the abdomino perineal amputation, the specimens were removed through the perineal access.
After the first 10 operations, a wound protector sleeve was used to prevent the possible implant of cancer cells. To prevent port-site recurrences, we also irrigated and vacuumed the abdominal cavity with distilled water (tumor cells’ dilution) and washed each port and the minilaparotomy with 5% povidone iodine. All insufflated gas was removed before trocar removal.
During low anterior resection, the splenic flexure was taken down routinely to achieve maximal colonic mobilization so a tension-free anastomosis could be performed. In the laparoscopic group, as well as the study reported by Franklin et al. [5], an intraoperative rectoscopy was performed in all patients who underwent a low anterior resection to identity precisely the lower line of resection. After a rectal washout with 5% povidone iodine solution, a transanal terminoterminally stapled colorectal anastomosis, according to the Knight and Griffen technique, was performed and checked by hydropneumatic testing. Both doughnuts were inspected for integrity after retrieval of the stapler. One perianastomotic extraperitoneal drain was left in place for as long as feces passed. In all patients, the anastomosis, located below the peritoneal reflection, was excluded from the abdominal cavity with an accurate laparoscopic suture of the pelvic peritoneum.
Currently, we do not routinely perform diverting ileostomy. In our series, the choice to perform a protective ileostomy was strictly an intraoperative decision in cases of concern about the anastomosis resulting from gas leakage diagnosed by hydropneumatic testing (in addition to intracorporeal stitches to reinforce the suture) when there was no guarantee of a stapled seal and a coloanal anastomosis was needed. Double stapling for anastomosis with the circular stapler was considered possible when an adequate margin could be obtained with the transection by the transverse stapler and an adequate healthy rectal stump was present. Otherwise, a hand-sewn transanal coloanal anastomosis was performed.
The following parameters were assessed in our protocol: length of surgical specimen, clearance of the margins of the specimen, number of lymph nodes identified, local recurrence rate, incidence of distant metastases, and survival probability analysis. Dukes D patients were included in the study for completeness, but were not included in the survival probability analysis.
Detailed pathologic examination of the resected specimens, performed by three pathologists according to standardized techniques, focused on tumor grading of the preradiotherapy biopsies and on staging of the surgical specimens according to Dukes classification. The pathologists were not informed about the surgical technique used (laparoscopic or open).
The t-test was used to compare the lengths of the surgical specimens, the clearances of the specimen margins, and the number of lymph nodes, while chi-square test was used for local recurrence rates and the incidence of distant metastases.
This study was designed to analyze a minimum follow-up period of 1 year. All the patients were followed up prospectively by clinical examination, oncologic markers, colonoscopy, and liver ultrasonography (every 6 months for the first 3 years, then every year), as well as chest radiography, abdomino pelvic computed tomography (CT) scan, and bone scan every year to evaluate local or systemic recurrence of the neoplastic disease.
The survival probability analysis was performed in both groups by the Kaplan-Meier method. Significant differences in survival probability between strata were assessed by the log-rank test. A level of 5% was used as the criterion for statistical significance. For statistical analysis, SAS software was used.
Results
Of the 124 elective consecutive patients in the current study, 81 chose the laparoscopic approach and 43 chose open surgery. The laparoscopic group consisted of 45 men and 36 women, whose mean age was 64.3 years (range, 32–86). In this group, 30 of the patients (37%) were older than 70 years, and 5 (6.1%) were older than 80 years. Anagraphic data and the patients’ distribition among ASA classes of risk were similar in the two groups (Table 1).
In the laparoscopic group, 45 patients underwent neoadjuvant radiochemotherapy, and 30 patients underwent preoperative radiotherapy alone. In the open group, radiochemotherapy was performed in 17 patients and radiotherapy alone in 21 patients. Patients with synchronous metastases did not receive neoadjuvant treatment.
We performed 60 laparoscopic and 27 open anterior resections as well as 21 laparoscopic and 16 open abdominoperineal resections.
In the laparoscopic group, there were 10 conversions to open surgery (12.3%; 8 anterior resections and 2 abdominoperineal resections), because of difficulties rectum isolation (n = 7), anastomotic defects (n = 2), and hemorrhage (n = 1). These patients were excluded from evaluation of the two groups and underwent a separate follow-up evaluation as a “converted open group.”
In the laparoscopic group, 65 patients were treated with a curative intent, whereas 6 patients (8.4%) with synchronous liver metastases underwent a palliative resection. Open palliative resection was performed in six cases (14%). We did not observe any case of postoperative mortality in either group. Of 52 patients treated with laparoscopic low anterior resection, 7 underwent protective ileostomy. In three of these patients, there was no guarantee of stapled seal, and in two patients, gas leakage was diagnosed by hydropneumatic test. In the remaining two patients, the ileostomy was performed as protection during coloanal anastomosis. Among these seven patients, two experienced anastomotic leakage, which resolved with conservative treatment.
Among the 45 patients who underwent laparoscopic low anterior resection without protective ileostomy, five anastomotic leakages required reoperation with protective ileostomy (3 of the 5 reoperations were performed laparoscopically) and four resolved with conservative treatment.
The 40 patients (77%) discharged without protective ileostomy had an uneventful postoperative course.
In 27 open low anterior resections, protective ileostomy was performed for 5 patients. Among the remaining 22 patients in this group, we observed 5 anastomotic leakages. Two required reoperation with protective ileostomy, and three resolved with conservative treatment.
The mean length of the resected specimens was 24.3 cm in the laparoscopic group and 23.8 cm in the open group (p = 0.47). The mean tumor-free margin was in the laparoscopic group and 3.0 cm and 2.8 cm in the open group (p = 0.57). The mean number of lymph nodes identified was 10.3 in the laparoscopic group and 9.8 in the open group (p = 0.63).
The distribution of patients according to Dukes stage after laparoscopic and open resection was similar, as shown in Table 2.
We observed a significant number of cases in which the tumor was downstaged as a result of preoperative radiochemotherapy, and the rate of downstaging was similar in the two arms of the study. In the laparoscopic group, three patients classified as T2N0 turned out to be pT0N0. Four Dukes B and six Dukes C patients were downstaged to Dukes A, whereas two Dukes C patients were downstaged to Dukes B. In the open group seven Dukes C patients were downstaged: to Dukes B in five cases and to Dukes A in two cases. The percentage of downstaging therefore was 23% in the laparoscopic group and 24.3% in the open group.
Of the 124 consecutive patients, excluding those receiving a palliative resection (n = 12), those who underwent conversion to open surgery (n = 10), and those who underwent surgery in the preceding year (n = 16), 86 patients (52 in the laparoscopic group and 34 in the open group) were left for evaluation and served as the object of this study. The mean follow-up period in the laparoscopic and open groups was 43.8 months (range, 1–9 years) (Table 3). One patient in the laparoscopic group (2%) was lost to follow-up. evaluation. Three patients in the laparoscopic group (5.7%) and one patient in the open group (2.9%) died of unrelated causes. No trocar-site or wound-site recurrence was observed.
In the laparoscopic group, 10 patients (20.8%) experienced a local recurrence and 8 patients (16.6%) experienced metachronous metastases (4 liver, 2 lung, and 2 bone metastases). In the open group, six patients (18.2%) experienced local recurrences, and seven patients (21.2%) experienced hepatic (n = 5) and lung (n = 2) metastases. No statistically significant difference was observed between the two groups in the local recurrence rate (p = 0.687) and in the occurrence of metachronous metastases (p = 0.528).
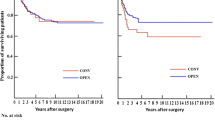
The cumulative survival probability at 60 months was 0.709 in the laparoscopically resected cases and 0.606 in the open cases (Fig. 1), and the disease free survival rate was 62.5% and 60.6%, respectively (Table 3).
The cumulative survival probability curves according to Dukes stages A, B, and C for laparoscopic as compared with open surgery are reported in Figs. 2, 3 and 4.
The follow-up evaluation of the 10 patients who underwent conversion to open surgery demonstrated the following results: three Dukes A patients were alive, respectively, at 27, 49, and 85 months; of two Dukes B patients, one was alive at 21 months and one 92-year-old patient died of cardiac failure at 61 months; of five Dukes C patients, three were alive, respectively, at 24, 93, and 99 months, one died of peritoneal carcinomatosis at 14 months, and one died of hepatic metastases at 26 months. The two cancer-related deaths observed at follow-up assessment (40% of the Dukes C patients) in this group of converted cases project a mortality rate comparable with that observed in the open group and with the mortality rate reported in the literature after rectal surgery for this advanced stage. Because of the limited number of subjects, we do not report the Kaplan-Meier curves for these converted cases.
Discussion
The aim of current study was to compare the results of laparoscopic and open resections for rectal cancer in an unselected consecutive series of patients. Our study was not a randomized investigation. Rather, the choice of procedure (laparoscopic or open) was based on the patients’ decision. However, the resulting distribution of patients in the two groups was similar in terms of gender, age, and clinical conditions (Table 1). The two groups also were similar in terms of stage distribution, as shown in Table 2.
An unexpected finding for us was that the conversions in this study were not related to tumor stage, but mainly resulted from difficulty identifying the anatomic landmarks because of obesity and inflammatory adhesions. The use of preoperative radiotherapy on the pelvis did not cause any specific difficulty in open or laparoscopic surgical dissection.
Wide mobilization of the splenic flexure was key for adequate resection with tension-free anastomosis. Often perianastomotic drainage and extraperitonealization of the anastomosis allow conservative treatment of a possible leakage. In our series of 45 consecutive low anterior resections without a protective stoma, we observed five clinical anastomotic dehiscences and reported the possibility of treating three of these patients with leaks using minimally invasive relaparoscopic peritoneal lavage and ileostomy.
Comparing the data for laparoscopic and open surgery, no statistically significant differences were found in the length of the surgical specimen, the clearance of the specimen margins and the number of lymph nodes identified. However, despite the standard techniques used for both laparoscopic and open rectal resections and the pathologic examination of the specimens, the mean number of lymph nodes harvested was 10.3 for the laparoscopic group and 9.8 for the open group, but varied, respectively, from 0 to 32 and from 0 to 28. Scott and Grace [25] recommended that at least 13 lymph nodes be histologically examined for reliable assessment of the nodal stage in colorectal cancer [25]. In the current series of patients with rectal cancer, neoadjuvant therapy caused approximately a 23% to 24% downstaging rate, and probably impaired the number of lymph nodes found on resected specimens in both the laparoscopic and open groups. Simply counting lymph nodes in the specimen after neoadjuvant therapy does not prove that an inadequate oncologic rectal resection has been performed. The afore-mentioned consideration should be taken into account.
In our experience, rectoscopic determination of the lower margin of resection in the laparoscopic group did not decrease the anastomotic recurrence rate, in contrast to that reported by Franklin [5]. Apparently the local recurrence rate reported in the current study is higher than the data reported in the recent literature. However, it must be remembered that according to the protocol, all the patients with T1 tumors were excluded from the laparoscopic and open groups because they underwent local excision, and that the distribution of patients according to the different Dukes stages, as reported in Table 2, is based on the pathologist’s report, including the patients’ downstaging related to the use of neoadjuvant therapy.
Port-site recurrences have become a major cause of concern for surgeons performing laparoscopic surgery for malignancy. Several reports have described tumor seeding at the abdominal wall, which did not occur in our series.
In the current series of patients, no statistically significant differences were observed between the laparoscopic and open groups in the incidences of local recurrences (20.8% vs 18.2%) and distant metastases (16.6% vs 21.2%) after surgery.
Regarding survival probability, this report clearly shows that the long-term outcome after the laparoscopic technique is comparable with the outcome after conventional rectal resection, and that these outcomes occur not only in patients with an early stage of rectal cancer (Dukes A or B), but also in Dukes C stage (Figs. 1,2,3,4), as reported by other authors [4].
On the basis of these results and considering that a similar disease-free survival rate was observed in both groups (62.5% in the laparoscopic group and 60.6% in the open group), we suggest that the laparoscopic approach to rectal cancer may in fact be a therapeutic improvement.
Initially, we observed a difference between the laparoscopic and open cumulative survival curves, probably because of minor immunosuppression after laparoscopic procedures, whereas, at a 3-year follow-up assessment, the curves instead tend to get close, possibly as an expression of disease progression. We believe that the well-known less aggressive immunosuppression after laparoscopic colonic resections, as compared with that after the open approach, could improve the oncologic results for laparoscopically treated patients. In fact, several reports investigating various components of the immune system indicate that laparoscopic colorectal resection is less immunosuppressive than the open approach [2, 13, 15]. Ordermann et al. [20] reported that these findings may have important implications, especially for patients with colorectal cancer who undergo laparoscopic resection. As a result of minor postoperative immunosuppression, lower incidence rates are reported for postoperative intraabdominal abscesses, urinary tract infections, pneumonia, and perinal wound sepsis after laparoscopic colonic resection than after open surgery [23]. However, the exact role of this mechanism in cost response to tumors is yet unclear. This immunologic effect should have an impact on the midterm oncologic results for laparoscopically treated patients. To draw conclusive scientific statements, we should wait until the completion of the ongoing international randomized controlled trials.
The results of the current study add to a growing body of literature supporting the laparoscopic procedure for the treatment of rectal cancer. It is beyond doubt that skilled surgeons can laparoscopically perform exactly the same steps used for open oncologic rectal resections.
The main limitation, of our study was the small number of patients enrolled. Furthermore, the absence of a strict randomization between the laparoscopic and open groups does not allow us to draw a definitive conclusion.
References
E Barlehner T Decker S Anders B Heukrodt (2001) ArticleTitleLaparoscopic surgery of rectal carcinoma: radical oncology and late results. Zentralbl Chir 126 302–306 Occurrence Handle10.1055/s-2001-14745 Occurrence Handle11370393
M Bressler RL Whelan A Holversan MR Treat R Nwygrod (1994) ArticleTitleIs immune function better preserved after laparoscopic vs open colon resections? Surg Endosc 8 881–883
HJ Chang JJ Jian SH Cheng MC Liu SY Leu FM Wang SY Tsai MH Tsao HH Lin AT Huang JL Sung (1998) ArticleTitlePreoperative concurrent chemotherapy and radiotherapy in rectal cancer patients. J Formos Med Assoc 97 32–37 Occurrence Handle1:STN:280:DyaK1c7kt1Slsw%3D%3D Occurrence Handle9481062
ME Franklin GB Kazantsev D Abrego-Medina A Diaz J Balli JL Glass (2000) ArticleTitleLaparoscopic surgery for stage III colon cancer. Surg Endosc 14 612–616 Occurrence Handle10.1007/s004640000169 Occurrence Handle1:STN:280:DC%2BD3M7gvFWmsw%3D%3D Occurrence Handle10948295
ME Franklin D Rosenthal D Abrego-Medina JP Dorman JL Glass R Norem A Diaz (1996) ArticleTitleProspective comparison of open vs laparoscopic colon surgery for carcinoma: five years results. Dis Colon Rectum 10 S35–S46
JE Hartley BJ Mehigan AW Mac Donald AW Lee JR Monson (2000) ArticleTitlePatterns of recurrence and survival after laparoscopic and conventional resections for colorectal carcinoma. Ann Surg 2 181–186 Occurrence Handle10.1097/00000658-200008000-00005
JE Hartley BJ Mehigan AE Qureshi GS Duthie AW Lee JR Monson (2001) ArticleTitleTotal mesorectal excision: assessment of the laparoscopic approach. Dis Colon Rectum 44 315–321 Occurrence Handle1:STN:280:DC%2BD3M7pvFyktw%3D%3D Occurrence Handle11289275
RJ Heald (1995) ArticleTitleTotal mesorectal excision is optimal surgery for rectal cancer: a Scandinavian consensus. Br J Surg 82 1297–1299 Occurrence Handle1:STN:280:BymD1MjotVw%3D Occurrence Handle7489148
T Ichihara Y Nagahata H Nomura S Fukumoto T Urakawa N Aoyama Y Kuroda (2000) ArticleTitleLaparoscopic lower anterior resection is equivalent to laparotomy for lower rectal cancer at the distal line of resection. Am J Surg 179 97–98 Occurrence Handle10.1016/S0002-9610(00)00273-7 Occurrence Handle1:STN:280:DC%2BD3cvhs1CktA%3D%3D Occurrence Handle10773141
E Kapiteijn CA Marijnen ID Nagtegaal H Putter WH Steup T Wiggers HJ Rutten L Pahlman B Glimelius JH Van Krieken JW Leer CJ Van De Velde (2001) ArticleTitlePreoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 30 638–646 Occurrence Handle10.1056/NEJMoa010580
TM Khalili PR Fleshner JR Hiatt TP Sokol M Manookian G Tsushima EH Phillips (1998) ArticleTitleColorectal cancer: comparison of laparoscopic with open approach. Dis Colon Rectum 41 832–838 Occurrence Handle1:STN:280:DyaK1czktFGjtQ%3D%3D Occurrence Handle9678367
F Kockerling (1998) ArticleTitleProspective multicenter study of the quality of oncologic resections in patients undergoing laparoscopic colorectal surgery for cancer. Col Rect 8 963–970
C Kuntz F Wunsch J Windeler F Glaser C Herfarth (1998) ArticleTitleProspective randomized study of stress and immune response after laparoscopy vs conventional colonic resections. Surg Endosc 7 963–967 Occurrence Handle10.1007/s004649900757
D Lanvin A Elhage B Henry E Leblanc D Querleu A Delobelle-Deraide (1997) ArticleTitleAccuracy and safety of laparoscopic lymphadenectomy: an experimental prospective randomised study. Gynecol Oncol 1 83–87 Occurrence Handle10.1006/gyno.1997.4823
KL Leung Pb Lai RL Ho WC Meng RY Yiu JF Lee WY Lau (2000) ArticleTitleSystemic cytokine response after laparoscopic-assisted resection of rectosigmoidocarcinoma: a prospective randomised trial. Ann Surg 231 506–511 Occurrence Handle10.1097/00000658-200004000-00008 Occurrence Handle1:STN:280:DC%2BD3c3htlaitg%3D%3D Occurrence Handle10749610
E Lezoche F Feliciotti AM Paganini M Guerrieri R Campagnacci A De Sanctis (2000) ArticleTitleLaparoscopic colonic resections versus open surgery: a prospectivenonrandomized study on 310 unselected cases. Hepatogastroenterology 47 697–708
SA Lord SW Larach A Ferrara PR Williamson CP Lago MW Lube (1996) ArticleTitleLaparoscopic resection for colorectal carcinoma: a three-years experience. Dis Colon Rectum 39 148–154 Occurrence Handle1:STN:280:BymB38znt1I%3D Occurrence Handle8620780
D Medich J McGinty D Parda S Karlovits C David P Caushaj B Lembersky (2001) ArticleTitlePreoperative chemoradiotherapy and radical surgery for locally advanced distal rectal adenocarcinoma: pathologic findings and clinical implications. Dis Colon Rectum 44 1123–1128 Occurrence Handle1:STN:280:DC%2BD3MvptFOjug%3D%3D Occurrence Handle11535851
JW Milsom B Bohm KA Hammerhofer V Fazio E Steiger P Elson (1998) ArticleTitleA prospective, randomized trial comparing laparoscopic versus conventional techniques in colorectal surgery: a preliminary report. J Am Coll Surg 187 46–57 Occurrence Handle1:STN:280:DyaK1czitFClsw%3D%3D Occurrence Handle9660024
J Ordemann CA Jacobi W Schwenk R Stosslein JM Muller (2001) ArticleTitleCellular and humoral inflammatory response after laparoscopic and conventional colorectal resections. Surg Endosc 15 600–608
AJ Pikarsky R Rosenthal EG Weiss SD Wexner (2002) ArticleTitleLaparoscopic total mesorectal excision. Surg Endosc 16 558–562 Occurrence Handle10.1007/s00464-001-8250-3 Occurrence Handle1:STN:280:DC%2BD383jsVeksA%3D%3D Occurrence Handle11972187
EC Puolin J Mamazza CM Schlachta R Gregoire N Roy (1999) ArticleTitleLaparoscopic resection does not adversely affect early survival curves in patients undergoing surgery for colorectal adenocarcinoma. Ann Surg 4 487–492 Occurrence Handle10.1097/00000658-199904000-00006
EC Puolin CM Schlachta PA Seshadri MO Cadeddu R Gregoire J Mamazza (2001) ArticleTitleSeptic complications of elective laparoscopic colorectal resection. Surg Endosc 15 203–208 Occurrence Handle11285969
H Scheidbach C Schneider J Konradt E Barlehner L Kohler Ch Wittekind F Kockerling (2002) ArticleTitleLaparoscopic abdominoperineal resection and anterior resection with curative intent for carcinoma of the rectum. Surg Endosc 16 7–13
KW Scott RH Grace (1989) ArticleTitleDetection of lymph node metastases on colorectal carcinoma before and after fat clearance. Br J Surg 76 1165–1167 Occurrence Handle1:STN:280:By%2BD1cbpsFc%3D Occurrence Handle2688803
H Tomita PW Marcello JW Milsom (1999) ArticleTitleLaparoscopic surgery of the colon and rectum. World Surg 4 397–405
MR Weiser JW Milsom (2000) ArticleTitleLaparoscopic total mesorectal excision with autonomic nerve preservation. Semin Surg Oncol 19 396–403 Occurrence Handle10.1002/ssu.10 Occurrence Handle1:STN:280:DC%2BD3MzitlGjsw%3D%3D Occurrence Handle11241922
SD Wexner (2000) Trocar-site recurrences: myth or real concern? Postgraduate course: laparoscopy in the management of malignancy. SAGES 2000, Atlanta Georgia, USA Occurrence Handle1:STN:280:DC%2BD3M%2Fkt1Ghtw%3D%3D Occurrence Handle11089603
SD Wexner JF Latulippe (1998) ArticleTitleLaparoscopic colorectal surgery and cancer. Dig Surg 15 117–123 Occurrence Handle10.1159/000018604 Occurrence Handle1:STN:280:DyaK1M%2Fmt1Wisg%3D%3D Occurrence Handle9845573
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Feliciotti, F., Guerrieri, M., Paganini, A. et al. Long-term results of laparoscopic versus open resections for rectal cancer for 124 unselected patients . Surg Endosc 17, 1530–1535 (2003). https://doi.org/10.1007/s00464-002-8874-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-002-8874-y