Abstract
We present an algorithm developed in MATLAB that can be applied to both normal and disordered swallowing to automatically extract a wide array of measurements from the spatiotemporal plots produced by high-resolution manometry (HRM) of the pharyngeal swallow. The algorithm was developed from data from 12 normal and 3 disordered subjects swallowing 5-ml water boluses. Automated extraction was compared to manual extraction for a subset of seven normal and the three disordered subjects to evaluate algorithm accuracy. Area and line integrals, pressure wave velocity, and pressure gradients during upper esophageal sphincter opening were also measured. Automated extraction showed strong correlations with manual extraction, producing high correlation coefficients in both normal and disordered subjects for maximum velopharyngeal pressure and maximum tongue base pressure. Timing data were also strongly correlated for all variables, including velopharyngeal pressure duration, tongue base pressure duration, and total swallow duration. Preliminary descriptive data on area and line integrals are presented. Our results indicate that the algorithm can effectively extract data automatically from HRM spatiotemporal plots. The efficiency of the algorithm makes it a valuable tool to supplement clinical and research use of HRM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Safe swallowing requires precise coordination of neuromuscular events to generate pressure gradients that propel a bolus from the mouth to the esophagus [1–3]. An inability to generate adequate pharyngeal pressure has been correlated with dysphagia. For example, delayed opening of the upper esophageal sphincter (UES) combined with elevated pharyngeal pressure can result in an outpocketing of the pharyngeal mucosa, resulting in Zenker’s diverticulum [4]. Patients with pharyngeal retention exhibit significantly lower UES resting pressure and a shorter duration of UES opening [5]. In a study of 114 patients with dysphagia, Xue et al. reported that 71% exhibited at least one manometric abnormality [6]. The predictable effects of disorders on pharyngeal pressures make manometry an attractive clinical tool for diagnosing and monitoring dysphagia.
Traditional methods of quantifying the rapidly changing pharyngeal pressure events used three to five unidirectional sensors typically positioned above, at the level of, and below the upper esophageal sphincter (UES) [7–12]. Measuring UES pressure in this way can lead to inaccurate recordings because the UES moves 2–3 cm rostrally during swallowing [13], shifting its position relative to the manometric catheter. The limited number of sensors also prevents manometric assessment along the entire length of the pharynx. While unidirectional sensors are appropriate for the symmetric esophagus, the rapid muscular contractions in the asymmetric pharynx require greater temporal and spatial resolution.
High-resolution manometry (HRM) uses 36 sensors capable of recording pressure in asymmetrical structures, offering the spatial and temporal resolution necessary to capture pharyngeal pressure events [14]. Though originally used in the esophagus, HRM has recently been applied to the pharynx as well [15–18]. Despite its exciting research potential, HRM is still in its infancy and has not been established as a standard clinical assessment. One barrier to the clinical application of HRM is its computational intensity. While HRM has the ability to provide comprehensive manometric information on the pharyngeal swallow, extracting all salient data from the spatiotemporal plot can be laborious. Having a method to automatically extract the pertinent quantitative information may help expedite patient assessment. Traditional manometry has failed to gain significant clinical acceptance in part because of the time required for data analysis. HRM uses more sensors and provides even more information, making automated analysis essential if HRM is to be applied routinely.
Employing an algorithm for automated analysis also expands our ability to extract additional parameters that cannot be extracted manually. With commercially available software (Manoview, Sierra Scientific, Oakland, CA), pressure and timing measurements are still extracted from the waveform produced by a single pressure sensor. Since structures such as the UES are mobile along the catheter during swallowing, it may be valuable to combine adjacent sensor traces to create a summary pressure trace. Previous studies, including ones performed in our laboratory [16, 19], report maximum pressures and durations, but analyzing the curve from the waveform may be a more comprehensive analysis of pressure events. Considering the area under the curve in regions of interest provides more information on total pressure generation for that area versus a single high-pressure point. Furthermore, pressure gradients can describe the pressure differentials along the pharynx that provide the force necessary for bolus propulsion. In this study we applied an algorithm, developed using MATLAB software, to the analysis of features of both normal and disordered swallowing. We hypothesized that the automatic data extraction procedure would produce data that are comparable to the manually extracted data. We also present data on measurements of line and area integral measurements and pressure gradients that elaborate on complex swallowing events.
Materials and Methods
Data Collection
Equipment
A solid-state high-resolution manometer was used for all data collection (ManoScan360 High Resolution Manometry System, Sierra Scientific Instruments, Los Angeles, CA). The manometric catheter has an outer diameter of 4 mm and 36 circumferential pressure sensors spaced 1 cm apart. Each sensor spans 2.5 mm and receives input from 12 circumferential sectors. These inputs are averaged and a mean pressure is recorded as the pressure detected by that individual sensor. The system is calibrated to record pressures between –20 and 600 mmHg, with fidelity of 2 mmHg. Data were collected at a sampling rate of 50 Hz (ManoScan Data Acquisition, Sierra Scientific Instruments). Prior to calibration, the catheter was covered with a protective sheath to preserve sterility without the need to sterilize the catheter between uses (ManoShield, Sierra Scientific Instruments). The catheter was calibrated before each participant according to the manufacturer’s specifications.
Participants
Fifteen subjects participated in this study with the approval of the Institutional Review Board of the University of Wisconsin-Madison. Twelve subjects (age = 20.9 ± 1.8 years; range = 19–25) were without swallowing, neurological, or gastrointestinal disorders, while three had a swallowing disorder (age = 68.7 ± 20.2 years; range = 47–87). Clinical descriptions of the disordered subjects are presented in Table 1. Participants were instructed not to eat for 4 h and not to drink liquids for 2 h prior to testing to avoid any potential confounding effect of satiety.
Procedure
Topical 2% viscous lidocaine was applied to the nasal passages with a cotton swab and participants gargled a solution of 4% lidocaine (1–2 cc) for several seconds. The manometric catheter was lubricated with 2% viscous lidocaine to ease passage of the catheter through the pharynx. Once the catheter was positioned within the pharynx, participants rested for 5–10 min to adjust to the catheter before performing the experimental swallows.
For the nondisordered subjects, a 5-ml water bolus was swallowed five times with the head in the neutral position. Each water bolus was delivered to the oral cavity via syringe. Disordered participants swallowed 5-ml boluses between two and four times. Sixty swallows were analyzed for normal subjects and nine swallows were analyzed for disordered subjects.
Data Analysis
Variables of Interest
Maximum pressure, pressure rise rate, and duration of pressure above baseline were calculated for the velopharynx and tongue base. Maximum preopening and postclosure pressure, minimum pressure, and opening duration were calculated for the UES. Additional measured variables such as area and line integrals, pressure gradients, and pressure wave velocity are described further below.
Automated Data Extraction
Pressure and timing data were extracted using a customized MATLAB program (The MathWorks, Inc., Natick, MA), which locates peak pressures in areas of interest (velopharynx, tongue base, and UES) and then calculates relevant parameters based on those points. The basic workflow is automated, with the program suggesting the locations of the significant points. However, in cases where the automated analysis fails to accurately identify regions of interest, the user may override program suggestions and manually select the correct manometric sensors and temporal location of the areas of interest.
Regions of interest were defined manometrically, as in McCulloch et al. [16]. The velopharynx is the region of swallow-related pressure change just proximal to the area of continuous nasal cavity quiescence and extending 2 cm distally. The tongue base is the area of swallow-related pressure change, with a high-pressure zone approximately midway between the nasopharynx and UES, and with its epicenter at the high-pressure point and extending 2 cm proximal and distal to that point. The UES is the midpoint of stable high pressure just proximal (rostral) to the baseline low esophageal pressure zone, extending to a point of low esophageal pressure distally and low baseline pharyngeal pressure proximally. During swallowing, the UES is mobile along the catheter, moving rostrally as much as 4 cm.
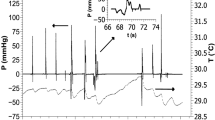
An example of the automated analysis algorithm screen is shown in Fig. 1a, b. To locate the regions of interest, the program first locates the peak pressure values on each sensor channel. These pressure maximums form a gradient that typically extends from the velopharynx to the UES, although peaks outside these bounds are also sometimes detected. The program attempts to eliminate these extraneous pressure peaks by selecting the largest continuous range of sensors containing a peak as the relevant range. After the initial suggestion, the user is then given the option of correcting the range to eliminate falsely identified peaks or of using the algorithmically defined range. Once the range is determined, the program identifies which peaks best represent the velopharynx and tongue base. This determination is made on the basis of the profile of the peaks present within the range of interest. The velopharynx is detected by comparing the most proximal (rostral) peaks of the range, as the peaks increase continually until maximal velopharyngeal pressure is reached. After the sensor containing the maximal velopharyngeal pressure is identified, the peaks of the sensors immediately caudal to the maximum continually decrease to a local minimum. The tongue base is then detected by comparing the sensors immediately below this local minimum, which increase until another local maximum is reached, the maximum tongue base pressure. The location of the UES is determined not by the calculated pressure profile but rather by computing the average resting pressure of each sensor and selecting the sensor with the highest value. Additional pressure maximums before the opening and after the closing of the UES are also of interest. To locate these maximums, allowing for the inherent movement of the UES during swallowing, the program considers up to three sensors immediately rostral to the detected UES sensor. For these sensors there are two peaks corresponding to the pre- and postswallow UES pressure maximums on that channel, and the highest among the candidate peaks are chosen as the true pre- and postswallow UES pressure maximums. Minimum UES pressure is also calculated at this time by finding the point of minimum pressure between the detected pre- and postswallow UES pressure maximums.
High-resolution manometry spatiotemporal plot of one normal swallow (a) and corresponding automated extraction of salient parameters (b). A maximum velopharyngeal pressure, B velopharyngeal pressure integral, C maximum tongue base pressure, D tongue base pressure integral, E maximum preswallow upper esophageal sphincter (UES) pressure, F minimum UES pressure, G UES pressure integral, H maximum postswallow UES pressure, I pressure gradient through maximum preswallow UES pressure J pressure gradient through descending UES pressure K pressure gradient through minimum UES pressure
Once the velopharynx and tongue base pressure maximums are identified, timing information is calculated by marking the onset and offset of elevated pressure on the relevant sensor channel. Relevant parameters, including durations and the rate of pressure increase, are determined based on these onset and offset points. UES activity time is calculated similarly, by calculating the difference between the postswallow UES pressure peak and the point at which the UES pressure begins to fall. Total swallow duration is defined as the time lapse between onset of velopharyngeal pressure and the postswallow UES pressure peak.
Novel Parameters
Integrals
While maximum pressure can provide valuable information on swallowing physiology that can easily be compared to previous manometric investigations, it does not provide a complete picture of pharyngeal pressure events. Measuring the total pressure created in a specific region offers more information and, when combined with durative data, reveals more about the shape of the pressure curve. Measuring these integrals also utilizes the multisensor array capabilities of HRM. Integrals are calculated of the area beneath the velopharynx and tongue base pressure curves as well as above the UES minimum, with the UES resting pressure as an upper limit. Temporal bounds in all cases are the onset and offset of pressure elevation or depression determined previously. Partial integrals with an upper limit of a certain percentage of the maximum in a region of interest are also considered in the velopharynx and tongue base, with the goal of both characterizing the shape of the pressure curve and perhaps identifying spikes in pressure that are affecting the maximum pressure reading for that region while not contributing significantly to overall pressure generation. We consider ten such partial integrals for the two regions of interest, with upper limits corresponding to 10, 20%, etc., of the maximum pressure in that region.
To provide additional information on pressure pattern, we also measured line integrals of the velopharynx, tongue base, and UES. Line integrals for the velopharynx and tongue base were calculated as the distance along the pressure curve from pressure onset to offset. The UES line integral was calculated as the distance along the pressure curve bounding the UES area integral (from resting UES pressure to minimum UES pressure and back to resting pressure).
Pressure Gradients
In comparison to measuring single points of pressure, the force utilized for bolus propulsion can be better described by a series of pressure gradients that more accurately characterize the pressure differential along the entire length of the pharynx. Measuring these gradients is a task well suited to the multisensor capabilities of HRM. We measure three pressure gradients, one at the time of UES minimum pressure, another at the onset of UES pressure descent, and one at a time midway between those two points. Gradients were calculated by subtracting UES pressure from maximum pressure along the catheter at the three times of interest.
Pressure Wave Velocity
The pharyngeal swallow can be thought of as a traveling pressure wave, with peak pressure traveling caudally and ending at the UES (Fig. 1b). Automated analysis can be used to calculate the velocity of this pressure wave, defined here as the distance from the velopharyngeal pressure peak to the maximum postswallow UES pressure peak divided by the time lapse between these two points.
Sensor Combination
We present two possible methods of creating a summary pressure trace: averaging three consecutive sensor traces into one trace or combining discrete sections of traces from three consecutive sensors into one trace in a piecewise manner.
Validation
Comparison to Manual Extraction
Though automated analysis significantly reduces the time required for analysis, it must also perform as accurately as manual analysis to be clinically valuable. To determine the accuracy of this automated analysis, seven normal subjects and three disordered subjects were analyzed using both manual and automated extraction. All manual analyses were done by one author (MRH). The manual analysis method used in this study was developed by three authors (TMM, MRH, MRC) and implemented previously. All automated analyses were done by one author (JDM). Prior to developing the algorithm and applying it to the data, he was trained on manual analysis to learn typical shapes of pressure curves in areas of interest. Five swallows of a 5-ml bolus for each normal subject and all 5-ml swallows available for each disordered subject were analyzed. Means were calculated per subject for each parameter and were compared to the values determined using the automated analysis. Pearson product-moment tests and linear regression analyses were performed. A significance level of α = 0.05 was used for all tests.
Results
Summary data from normal and disordered subjects are presented in Table 2.
Comparison of Automated and Manual Extraction—Normal Subjects
Summary data and statistics are presented in Table 3. Close correlations were observed for all parameters evaluated. Pressure events were more closely linked than timing events, as demonstrated by maximum velopharyngeal pressure (r = 0.968) and tongue base pressure (r = 0.998). Velopharyngeal pressure duration (r = 0.938) and tongue base pressure duration (r = 0.942) were still correlated, though to a lesser degree than maximum pressure values. Total swallow duration was less correlated than duration of any single area of interest, but still significantly linked (r = 0.88). UES pressures showed strong correlations, including maximum preswallow UES pressure (r = 0.971), maximum postswallow UES pressure (r = 0.927), and minimum UES pressure (r = 0.993).
Comparison of Automated and Manual Extraction—Disordered Subjects
Summary data and statistics are presented in Table 4. The close correlations observed for normal subjects were preserved for disordered subjects. Pressures such as maximum velopharyngeal (r = 0.982), maximum tongue base (r = 0.999), maximum preswallow UES (r = 0.994), maximum postswallow UES (r = 1.000), and minimum UES pressure (r = 1.000) showed high correlations. Timing events such as velopharyngeal pressure duration (r = 1.000), tongue base pressure duration (r = 0.999), UES opening duration (r = 0.967), and total swallow duration (r = 0.975) also showed good agreement.
Integrals
Figure 2 shows the percentage of total pressure generated at each of the ten partial integrals, or percentages of maximum pressure, at the tongue base. At 70% of maximum pressure for both the velopharynx and tongue base, percentage of total pressure generated approaches 90%, meaning a pressure spike may be affecting maximum pressure measurement while not contributing significantly to the total pressure generated in the region. At 60% of maximum pressure, percentage of total pressure generated drops significantly.
Line integrals displayed variable differences across normal and disordered subjects, with differences most evident for the velopharynx and tongue base pressure line integrals. A discernible difference was not present for the UES.
Pressure Gradients
Figure 3 depicts pressure gradients at three key times during UES opening. A large negative gradient is turned into a large positive gradient during UES opening. This change becomes irregular during disordered swallowing. Deviation from normal in the disordered subjects was most evident at maximum preopening UES pressure and during opening. The pressure gradient at the time of minimum UES pressure did not differ between normal and disordered subjects.
Pressure Wave Velocity
Mean velocity was 9.99 ± 1.85 cm/s for normal subjects and 10.12 ± 1.25 cm/s for disordered subjects. Intrasubject consistency was higher for normal subjects.
Sensor Combination
Figure 4a shows pressure traces from multiple sensors that are used to create composite curves made of averaged pressures (Fig. 4b) and of discrete portions of multiple curves (Fig. 4c). While the former may be more beneficial in displaying the summary manometric activity in an area of interest, the latter is more valuable when extracting local maximum and minimum pressure values.
Discussion
An algorithm for automated extraction of pressure and timing data from HRM spatiotemporal plots is presented. Strong correlations were observed between data extracted using automated and manual methods, demonstrating the accuracy of the algorithm. Generally, pressure values were computed more consistently than timing values. Extracting timing measurements is more subjective than pressure measurements, as pressure rise onset and pressure fall offset can be slightly ambiguous. The lowest correlation coefficients recorded were for tongue base pressure rise rate in normal subjects and velopharynx pressure rise rate in disordered subjects. However, results from the two methods were still highly linked, with correlation coefficients of 0.869 and 0.825, respectively. Because an automated algorithm eliminates the subjectivity inherent in manual extraction, implementation could lead to more consistent measurements. Optional user intervention, while needed rarely in this study, ensures that the correct sensors are analyzed. Intervention occurrence is dependent on factors such as the nature of particular swallowing disorders and also erroneous sensor readings. For example, the data trace of one subject was marked by several periods of a false high-pressure band which caused errors in the detection of the velopharyngeal region. Overall, the rate of user intervention in this study was approximately 10%. Both automated and manual analyses are subject to biases and limitations. Automated analysis is limited by our descriptions of how complex physiological phenomena may appear and by a potential inability to operate predictably when faced with an extremely disordered swallow. Manual analysis is limited by the subjectivity and potential interrater reliability issues that plague all methods of manual data interpretation and extraction. Such biases cannot be controlled and are not consistent across raters; however, automated analysis uses the same process every time and thus may be more reliable.
Automated analysis also provides the opportunity to evaluate parameters that cannot be measured manually, such as the area and line integrals evaluated in this study. Area integrals were rather stable within subjects compared to the intrasubject variability of maximum pressure measurements. Total pressure generated is likely more physiologically meaningful than maximum pressure, reflective of the overall bolus propulsion force. Measurement of pressure maximums is subject to including pressure spikes of short duration that may not significantly contribute to bolus transit. Integrals provide a more comprehensive manometric picture that is immune to such phenomena. Poor clinical decisions potentially could be made if based solely on local maximum pressure data. For example, a tongue base pressure curve of a patient with muscle weakness may mask the deficit present and make the patient appear normal. Conversely, a pressure spike in a healthy patient with otherwise normal pressure generation may cause a clinician to diagnose the patient with muscle hypercontractility.
Line integrals may be valuable in evaluating patients who can generate but not sustain pressures at either the velopharynx or the tongue base. This would produce a large value for the line integral but small value for the area integral (Fig. 5a, b). Partial area integrals are also useful in characterizing the shape of a pressure curve. If near total pressure generated is reached at approximately 75% of maximum pressure (Fig. 5c), a pressure spike may be skewing maximum pressure measurement while not generating meaningful physiological pressure for swallowing. Though not observed in this study, one could expect differences in pressure wave velocity between normal subjects and patients with disorders affecting pressure propagation. Modifying and combining the novel parameters measured in this study into potentially clinically useful parameters and ratios will be the subject of future investigations.
Pressure gradients represent the force underlying bolus propulsion. We observed that a large negative gradient immediately preceding UES opening became a large positive gradient at the time of maximal UES opening, indicated by minimum UES pressure. The three disordered subjects in this study displayed different irregularities in this pattern. For example, subject 1 had a positive gradient at maximum UES preswallow pressure, possibly indicative of high velopharyngeal activity trying to compensate for UES dysfunction. Data from subjects 2 and 3 were closer to those of normal subjects; however, subject 2 may have cricopharyngeal hyperactivity due to the large negative gradient and smaller positive gradients. Subject 3 may have slight UES hypofunction due to the reversal of the trend seen in subject 2, relative to normal subjects.
Three disordered subjects were analyzed in this study. No modifications were made to the algorithm to accommodate analysis of these subjects. The few problems that occurred were problems occasionally experienced when analyzing normal subjects as well. Pre- and postswallow UES pressure maximums were lower than the average of the normal subjects but were typically within a range seen in normal subjects with lower pressures. In some situations, the lower pressures required user intervention to correct the suggestions of the program. Maximum tongue base pressure remains the most difficult to locate, for both normal and disordered subjects. This may be a consequence of the simplicity of our detection algorithm; further refinements will focus on improving performance while not sacrificing ease of use or processing speed. No formal statistical analyses were performed to determine the presence of differences between normal and disordered swallowing since we tested a limited number of disordered subjects and such a comparison is beyond the scope of this study. Several observable differences for parameters such as velopharyngeal pressure rise rate, velopharyngeal pressure integral, maximum UES preswallow pressure, minimum UES pressure, and total swallow duration will be the subject of future investigations.
One interesting feature found in the spatiotemporal plots of normal subjects which appears absent in those from the disordered subjects is a linear pattern connecting the pressure peaks within the swallow. While pressure peaks created by the velopharynx, tongue base, and UES align linearly in normal swallows, this linearity is lost in disordered swallows (Fig. 6). This may be caused by cricopharyngeal dysfunction. Hyperfunction could result in a common cavity event, with uniform pressure from the velopharynx to tongue base. Prolonged UES opening could also disturb the linear pattern. While we tested a variety of types of dysphagia to evaluate the robustness of our automated analysis algorithm, systematic comparisons between normal and disordered subjects may benefit from standardizing the type of dysphagia present.
Spatiotemporal plots from three disordered subjects. Subject 1 (left) displayed high velopharyngeal pressure (A), a short duration of tongue base pressure and small tongue base pressure integral (B), low preswallow upper esophageal sphincter (UES) pressure (C), and high postswallow UES pressure (D). Subject 2 (middle) displayed high velopharyngeal pressure (E), a large tongue base pressure line integral (F), and low preswallow UES pressure. Subject 3 (right) displayed low velopharyngeal pressure (H), low preswallow UES pressure (I), and high minimum UES pressure (J)
To extend automated analysis to patients with more severe dysphagia and more atypical pressure patterns, our algorithm may need to be altered. The algorithm currently locates the UES first. Patients with cricopharyngeal hypofunction may have low resting UES pressures or display different UES activity during swallowing. As UES pre- and postswallow pressure maximums are used to calculate timing events, both timing and pressure parameters may be affected. Also, although our algorithm is still effective at relatively low pressure values, patients with severely reduced tongue base retraction may be unable to generate a great enough pressure differential to be recognized. Solving this problem could be done either by modifying the current algorithm or by creating a set of disorder-specific algorithms that could be applied when appropriate. At this time, applying the algorithm and relying on user intervention to correct program suggestions when necessary, particularly in the tongue base region, could be employed. While the automated algorithm shows promise, this is a preliminary study. The ability to analyze data obtained from a wide variety of clinical patients must be demonstrated before the algorithm can be applied clinically.
The large amount of data generated by HRM is well-suited for automated analysis. While one may be able to look at a swallow and perceptually determine that it is abnormal, determining which treatment is appropriate demands knowing how that swallow is abnormal. The potential number of variables that can be analyzed is infinite and automated analysis is needed if data extraction is to remain feasible. As more complex disorders are evaluated, the absence of certain key events (e.g., tongue base pressure peak) may become as significant as their presence in normal subjects. Furthermore, additional parameters such as gradients, integrals, and pressure wave velocity can be calculated with automated analysis and may provide a more robust picture of overall swallowing patterns. Though not specifically measured, the time required for the automated analysis was much shorter than for a manual extraction, even without the additional parameters. While manually extracting all data from a single swallow takes approximately 3 min, it can be done in a few seconds using our automated algorithm. In the case of user intervention to correct sensor identification, only an additional 15–20 s is required, though this time is dependent on the experience level of the user. Automation also allows for more precise pressure measurements because the possibility of users selecting an incorrect maximum is removed. The efficiency and accuracy of our algorithm make it a potentially valuable tool if HRM is to be applied routinely to patient assessment.
References
Kim SM, McCulloch TM, Rim K. Pharyngeal pressure analysis by the finite element method during liquid bolus swallow. Ann Otol Rhinol Laryngol. 2000;109:585–9.
McConnel FM. Analysis of pressure generation and bolus transit during pharyngeal swallowing. Laryngoscope. 1988;98:71–8.
Cook IJ. Normal and disordered swallowing: new insights. Baillieres Clin Gastroenterol. 1991;5:245–67.
McConnel FM, Hood D, Jackson K, O’Connor A. Analysis of intrabolus forces in patients with Zenker’s diverticulum. Laryngoscope. 1994;104:571–81.
Olsson R, Kjellin O, Ekberg O. Videomanometric aspects of pharyngeal constrictor activity. Dysphagia. 1996;11:83–6.
Xue S, Katz PO, Castell JA, et al. Upper esophageal sphincter and pharyngeal manometry: which patients? Gastroenterology. 2000;118:A410 (Abstract).
Logemann JA, Kahrilas PJ, Kobara M, Vakil NB. The benefit of head rotation on pharyngoesophageal dysphagia. Arch Phys Med Rehabil. 1989;70:767–71.
Lazarus C, Logemann JA, Song CW, Rademaker AW, Kahrilas PJ. Effects of voluntary maneuvers on tongue base function for swallowing. Folia Phoniatr Logop. 2002;54:171–6.
Boden K, Hallgren A, Witt Hedstrom H. Effects of three different swallow maneuvers analyzed by videomanometry. Acta Radiol. 2006;47:628–33.
Hind JA, Nicosia MA, Roecker EB, Carnes ML, Robbins J. Comparison of effortful and noneffortful swallows in healthy middle-aged and older adults. Arch Phys Med Rehabil. 2001;82:1661–5.
Bulow M, Olsson R, Ekkberg O. Supraglottic swallow, effortful swallow, and chin tuck did not alter hypopharyngeal intrabolus pressure in patients with pharyngeal dysfunction. Dysphagia. 2002;17:197–201.
Bulow M, Olsson R, Ekberg O. Videomanometric analysis of supraglottic swallow, effortful swallow, and chin tuck in healthy volunteers. Dysphagia. 1999;14:67–72.
Isberg A, Nilsson ME, Schiratzki H. The upper esophageal sphincter during normal deglutition. A simultaneous cineradiographic and manometric investigation. Acta Radiol Diagn (Stockh). 1985;26:563–8.
Fox MR, Bredenoord AJ. Oesophageal high-resolution manometry: moving from research into clinical practice. Gut. 2008;57:405–23.
Takasaki K, Umeki H, Enatsu K, Tanaka F, Sakihama N, Kumagami H, Takahashi H. Investigation of pharyngeal swallowing function using high-resolution manometry. Laryngoscope. 2008;118:1729–32.
McCulloch T, Hoffman MR, Ciucci MR. High resolution manometry of pharyngeal swallow pressure events associated with head turn and chin tuck. Ann Otol Rhinol Laryngol. 2010;119(6):369–76.
Umeki H, Takasaki K, Enatsu K, Tanaka F, Kumagami H, Takahashi H. Effects of a tongue-holding maneuver during swallowing evaluated by high-resolution manometry. Otolaryngol Head Neck Surg. 2009;141:119–22.
Takasaki K, Umeki H, Kumagami H, Takahashi H. Influence of head rotation on upper esophageal sphincter pressure evaluated by high-resolution manometry system. Otolaryngol Head Neck Surg. 2010;142:214–7.
Hoffman MR, Ciucci MR, Mielens JD, Jiang JJ, McCulloch TM. Pharyngeal swallow adaptations to bolus volume measured with high resolution manometry. Laryngoscope. 2010;120(12):2367–73.
Acknowledgments
This study was supported by a grant from the Department of Surgery, University of Wisconsin School of Medicine and Public Health. The authors thank Dr. Glen Leverson for his assistance with the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mielens, J.D., Hoffman, M.R., Ciucci, M.R. et al. Automated Analysis of Pharyngeal Pressure Data Obtained with High-Resolution Manometry. Dysphagia 26, 3–12 (2011). https://doi.org/10.1007/s00455-010-9320-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-010-9320-2