Abstract
As a bolus enters the pharynx during the swallow, the airway is protected by laryngeal closure, a process characterized by approximation of the vocal folds plus approximation of the arytenoid cartilages to the base of the epiglottis. The purpose of this study was to measure initiation of laryngeal closure (ILC) and laryngeal closure duration (LCD) in three groups of subjects: (1) ten stroke patients who aspirated before and during the swallow (aspirators), (2) ten stroke patients who did not aspirate (nonaspirators), and (3) ten normal control subjects. Means and standard deviations of ILC and LCD were analyzed for both 5-ml and 10-ml thin-liquid boluses using a 100-ms timer during subsequent analysis of videofluoroscopic swallowing examinations. There were significant differences between aspirators and control subjects for both ILC and LCD, and significant differences between aspirators and nonaspirators for ILC. There were no significant differences between aspirators and nonaspirators for LCD. Both delayed ILC and reduced LCD were associated with post-stroke aspiration. Delayed ILC is a significant indicator of overall risk of aspiration. Clinical implications for these findings are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
As a bolus is propelled from the oral cavity into the pharyngeal cavity, the pharyngeal stage of the swallow begins. The physiological events of the normal pharyngeal swallow are characterized by anterior-superior displacement of the hyolarynx, closure of the larynx, and downward displacement of the epiglottis with concurrent approximation of the arytenoids to the base of the epiglottis. These rapidly executed, overlapping movements contribute to laryngeal closure and protection of the airway [1]. Disturbances in the initiation and duration of these physiological events involved in laryngeal closure are likely to place individuals at risk for aspiration during swallowing.
Aspiration associated with oropharyngeal swallowing is defined as the entrance of food or liquid into the airway before, during, or after the swallow and can cause aspiration pneumonia, dehydration, malnutrition, and even death [2]. Aspiration that occurs before and during the swallow is likely to occur if the bolus passes into the pharynx before laryngeal closure or if the duration of laryngeal closure is too short. Delayed pharyngeal swallow, i.e., delayed laryngeal closure relative to bolus position in the pharynx, increases with age, i.e., in younger, healthy individuals, laryngeal closure begins when the bolus reaches the ramus of the mandible; in older, healthy individuals, laryngeal closure begins after the bolus has passed the ramus of the mandible [3–6]. However, older healthy individuals are not necessarily more likely to aspirate than younger, healthy individuals because this physiological difference in initiation of laryngeal closure is effectively compensated in older individuals. In individuals with laryngeal-pharyngeal weaknesses (e.g., stroke patients), delayed laryngeal closure before or during the swallow is associated with an increased risk for aspiration [7–9]. In addition, delayed and slowed airway closure is an indicator of risk of aspiration in older dysphagia patients [10] and in neurogenic dysphagic patients [11]. Kim and McCullough [12] reported that a delayed pharyngeal swallow in post-stroke patients in the range of 0.9-1.0 s was associated with increased aspiration before or during the swallow.
Aspiration before and during the swallow occurs because the bolus enters the laryngeal vestibule and passes below the true vocal folds before the larynx has fully closed. Aspiration during the swallow occurs because the bolus passes below the true vocal folds before laryngeal closure is complete or because laryngeal closure duration is too short. Other contributing factors include weakness in the propulsive force of the tongue on the bolus and weak contraction of the pharyngeal constrictors. The present study focused on measurements of laryngeal closure, both before and during the swallow.
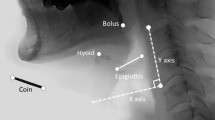
To describe aspiration before and during the swallow, this study employed a temporal measure developed by Rademaker et al. [13]. Initiation of laryngeal closure (ILC) is the time between when the bolus enters the pharynx (operationally defined as the posterior edge of the ramus of the mandible) and the first contact of the arytenoids and the epiglottis. Under normal physiological conditions, laryngeal movement starts before the bolus passes into the pharynx [5, 6]. In pathological conditions, delayed initiation of laryngeal closure may allow the bolus to enter the airway.
To describe aspiration during the swallow, this study measured laryngeal closure duration (LCD), described by Perlman et al. [14]. LCD refers to the duration of approximation of the arytenoid cartilages to the base of the epiglottis. Kendall et al. [15] reported that older normal individuals did not differ in laryngeal LCD when compared to younger individuals. Bisch et al. [16] reported that LCD was shorter in neurogenic dysphagic patients than in age-matched control subjects. However, Power et al. [17] found no differences in LCD among post-stroke patients and normal controls did not identify the incidence of aspiration. Since aspiration occurs when the bolus enters the laryngeal vestibule and true vocal folds, it is necessary to have more clinical data to determine whether reduced LCD is a sole indicator associated with aspiration during the swallow.
The purpose of this investigation is to evaluate whether delayed initiation or reduced duration of laryngeal closure is different among stroke patients who aspirate and those who do not aspirate compared to neurologically normal participants using refined radiographic analyses.
Method
Subjects
Thirty subjects’ high-quality video clips from videofluoroscopic swallowing examinations (VFSEs) were exported for analysis from a prior investigation [18]. Individuals patients who experienced cerebrovascular events consistent with stroke who aspirated (n = 10 aspirators) and who did not aspirate (n = 10 nonaspirators) were examined and compared to 10 neurologically normal volunteers who did not aspirate (n = 10 normal controls). All aspirators were observed during videofluoroscopic examination to aspirate at least one thin liquid either before or during the swallow. Among the stroke patients, seven had a left hemisphere stroke, six had a right hemispheric stroke, and seven had bilateral lesions.
The records of the ten age-matched normal control subjects from a prior investigation [19] were analyzed for comparison to aspirators and nonaspirators. Control and stroke subjects were age-matched because the previous temporal studies showed that older subjects had delayed initiation and reduced duration of laryngeal closure compared to younger subjects. The mean age was 69 years for aspirators, 65 for nonaspirators, and 70 for aspirations. The control group was screened for neurological or structural abnormalities which would interfere with swallowing using a comprehensive questionnaire and administering an oral motor examination by a speech-language pathologist. The Ohio University Institutional Review Board (IRB) approved this study of temporal measurements, conducted using videofluoroscopic films.
Videofluoroscopic Swallowing Examination (VFSE)
Videofluoroscopic swallowing examination (VFSE) data were collected on stroke patients [18] and the control group [19] using the same methodology. The fluoroscopic tube was focused in the lateral plane on the oral cavity (the lips anteriorly to the pharyngeal wall posteriorly) and the nasopharynx (superiorly) to just below the UES area (inferiorly). Each subject swallowed an array of consistencies including bolus sizes from 5 ml to 3 ounces and consistencies from thin liquid to solid. For this investigation, however, data were analyzed for only two 5-ml boluses and two 10-ml boluses of thin liquid. This was done because thin liquids create the primary risk for aspiration. The boluses were a mixture of water and barium sulfate powder (50/50 water and E-Z M barium sulfate powder for suspension) [15, 16]. VFSE began with two swallows of each of the 5-ml and 10-ml thin liquid. Each subject was instructed by the clinician to swallow after putting the liquid in his/her mouth by pill cup. VFSEs for stroke patients were adjusted due to “bailout criteria” if aspiration appeared to place them at risk. All other aspects of VFSEs were identical for control subjects and stroke patients. A total of 120 swallows (30 subjects × 4 thin-liquid swallows) were submitted for this investigation.
Procedures for Temporal Measurement
To accurately analyze ILC and LCD, the VFSE video files were digitized on an external hard drive with an attached 100-ms digital video timer. Adobe Premier Pro 1.5 (San Jose, CA), a video-editing program, was used for digitization with a Sony DVMC-DA1 Media Converter (Tokyo, Japan).
Data regarding the occurrence of aspiration by subjects in this investigation were already recorded and analyzed in order to place participants into one of the three groups. The verification of aspiration agreed with the previous study’s classification by McCullough et al. [18]. After VFSE, the investigator recorded the presence or absence of aspiration in order to put the subjects into one of three groups. In addition, for aspirators, timing of aspiration (before, during, or after the swallow) was also verified. All aspiration in this investigation occurred either before or during the swallow. Aspiration was defined as the entry of the liquid below the true vocal folds. Laryngeal closure in this investigation referred to laryngeal vestibule closure rather than true vocal folds closure. However, laryngeal vestibule closure has been and continues to be the accepted and encouraged methodology for analyzing temporal measure of laryngeal closure [5, 6, 15].
To accurately analyze the temporal sequence of events, slow motion, frame-by-frame analyses was performed using a 100-ms video timer. First, each liquid swallow was analyzed for the following points of incidence: (1) bolus passing the ramus of mandible, (2) first contact of arytenoids and epiglottis, and (3) final contact of arytenoids and epiglottis. Second, the times for each of the above-mentioned markers were recorded and used to calculate the two measures of laryngeal closure.
Statistical Analysis
Statistical comparisons were be made by two-way analysis of variance (ANOVA), with independent variables being the three groups and two volumes of bolus. Significance level was set at p < 0.05. A post-hoc test (Tukey) was performed to test significant main group differences (p < 0.05) in each temporal measure. All of the swallows for each subject on each bolus volume were analyzed separately.
Results
Reliability
For interjudge reliability, a second independent judge analyzed the designated swallows of six randomly selected subjects (24 swallows, 20%). The measurements of the principal investigator and second judge were compared using Pearson’s correlation coefficient. A significant correlation was observed (r = 0.90, p < 0.01). For intrajudge reliability, the principal investigator reanalyzed the same six subjects a second time. Intrajudge reliability was also significant (r = 0.93, p < 0.01).
Initiation of Laryngeal Closure (ILC)
Table 1 gives the mean duration and the standard deviation of ILC for the three subject groups. The mean ILC for each group was progressively shorter from aspirators to nonaspirators to control group (Fig. 1). The stroke patients who aspirated clearly showed longer delays in ILC than the nonaspirating stroke patients and the control group. On two-way ANOVA, aspirators were significantly different from both the nonaspirators and the control group [F(2, 114) = 0.93, p < 0.01]. The post-hoc test revealed that aspirators were significantly different from nonaspirators and control group on ILC. Nonaspirating stroke patients and the normal control subjects were also significantly different from each other. There was no bolus volume effect or bolus volume × group interaction on ILC.
Laryngeal Closure Duration (LCD)
Table 2 gives the mean duration and the standard deviation of LCD for the three subject groups. The mean LCD for each group was progressively longer from aspirators to nonaspirators to control group (Fig. 2). The stroke patients who aspirated showed reduced laryngeal closure compared with the other groups. On two-way ANOVA, both stroke patient groups were significantly different from the control group [F(2, 114) = 17.6, p < 0.01]. However, there was no significant difference between the stroke patients groups. There was no bolus volume effect or bolus volume × group interaction on LCD.
Discussion
Timely and appropriate laryngeal closure is important for protecting the airway during the swallow [1]. This investigation revealed that stroke patients who aspirated had delayed laryngeal closure and reduced duration of laryngeal closure compared to control subjects. Delayed and reduced duration of laryngeal closure allows the bolus to enter the laryngeal vestibule and places the stroke patient at increased risk for aspiration [20].
Coupled with hyolaryngeal excursion, arytenoids elevate and contact the descending epiglottis. This biomechanical movement provides effective airway protection as the bolus safely passes through the pharynx [1]. Extrinsic and intrinsic laryngeal muscles may be affected after a stroke and affect mobility of the epiglottis and arytenoid cartilages during the swallow [21]. In post-stroke patients, aspirators had longer delays to initiate laryngeal closure during the pharyngeal swallow. For those aspirators, as the bolus entered the pharynx, the epiglottis and arytenoid cartilages did not begin to close the laryngeal vestibule to protect the airway in a timely manner, compared with controls. Based on the ILC data of this study, we can estimate that an ILC of approximately 1.5 s may indicate a considerable risk of aspiration. However, with such a small sample, these results can be used only to alert clinicians to the potential risk of aspiration in the natural circumstances.
The finding of ILC is consistent with previous studies of pharyngeal delay of post-stroke patients [8, 11, 22]. The difference between this study and those previous studies is that the latter focused on hyoid excursion rather than epiglottic-arytenoid contact. Temporal measurements using laryngeal closure may be a useful indicator for discriminating stroke patients who aspirate and those who do not.
Power et al. [16] reported that there was no significant difference in LCD between aspirating stroke patients and nonaspirating stroke patients and control groups. However, our study found that both aspirating and nonaspirating stroke patients showed significantly shorter laryngeal closure durations than those of the control group. The difference may be a result of the timing of aspiration. Power et al. [16] did not indicate the timing of aspiration in stroke patients. However, our study included stroke patients who aspirated before or during the swallow. Reduced LCD is considered an important indicator of risk of aspiration during the swallow. It is critical to report the timing of aspiration to present the temporal indicators of aspiration.
This study highlights interesting relationships between ILC and LCD in stroke patients. In general, aspirating stroke patients have longer delays than nonaspirating stroke patients. However, a nonaspirating stroke patient, No. 18, had delays of over 2 s in ILC and did not aspirate. We suspect that this may be related to a longer LCD. Even though this patient was at risk for aspiration due to the longer delay and poor initial laryngeal protection, the longer LCD may have compensated for the delay and prevented him from aspirating. On the other hand, aspirating stroke patient No. 17 exhibited a shorter delay than other aspirating stroke patients. His aspiration occurred during the swallow rather than before the swallow. Perhaps a shorter ILC is able to compensate for aspiration when LCD is inadequate. LCD may not be a sole indicator to predict risk of aspiration, but if it is considered in conjunction with ILC and pharyngeal delay, it may provide us with another valuable tool in the armamentarium of clinical indicators.
Improving early initiation and proper duration of laryngeal closure may be a viable objective for rehabilitation exercises. Since hyolaryngeal excursion influences laryngeal closure, the effortful swallow and lingual exercises may be useful for these patients [23]. In addition, compensatory strategies such as chin tuck can enhance airway protection by facilitating laryngeal closure. Supraglottic swallows have been reported to reduce aspiration in head and neck cancer patients [24]. However, this strategy should be used cautiously with post-stroke patients and those with coronary artery disease. More research regarding the effectiveness of both rehabilitation and compensatory strategies is needed.
This study has several limitations such as a small number of subjects and VFSE data derived from a previous investigation. Future study should focus on finding the temporally specific indicators of aspiration. These measures should help clinical decision-making in the diagnosis and treatment of dysphagia and may help identify and prevent aspiration for post-stroke patients.
Conclusions
This study demonstrates that temporal measurements of laryngeal closure can be valid tools to help understand the physiological damage in swallowing for post-stroke patients. Initiation of laryngeal closure (ILC) offers important information for predicting risk of aspiration and for developing treatment strategies for helping patients, clinicians, and caregivers enhance swallowing rehabilitation. It is necessary that a more comprehensive investigation of temporal indicators associated with aspiration be performed in order to determine the degree of risk of aspiration. In addition, dysphagia rehabilitation exercises and compensatory strategies should focus on the specific physiological impairments of the patients with delayed and reduced laryngeal closure.
References
Logemann JA, Kahrilas PJ, Cheng J, Pauloski BR, Gibbons PJ, Rademaker AW, et al. Closure mechanisms of laryngeal vestibule during swallowing. Am J Physiol Gastrointest Liver Physiol. 1992;262(2 Pt 1):G338–44.
Gordon C, Hewer RL, Wade DT. Dysphagia in acute stroke. Br Med J (Clin Res Ed). 1987;295(6595):411–4.
Tracy JF, Logemann JA, Kahrilas PJ, Jacob P, Kobara M, Krugler C. Preliminary observations on the effects of age on oropharyngeal deglutition. Dysphagia. 1969;4:90–4. doi:10.1007/BF02407151.
Robbins JA, Hamilton JW, Lof GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992;103:823–9.
Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. J Speech Lang Hear Res. 2000;43(5):1264–74.
Logemann JA, Pauloski BR, Rademaker AW, Kahrilas PJ. Oropharyngeal swallow in younger and older women: videofluoroscopic analysis. J Speech Lang Hear Res. 2002;45(3):434–45. doi:10.1044/1092-4388(2002/034).
Mann G, Hankey GJ, Cameron D. Swallowing function after stroke: prognosis and prognostic factors at 6 months. Stroke. 1999;10:744–8.
Robbins J, Levine RL. Swallowing after unilateral stroke of the cerebral cortex: preliminary experience. Dysphagia. 1988;3:11–7. doi:10.1007/BF02406275.
Robbins J, Levine RL, Maser A, Rosenbek JC, Kempster GB. Swallowing after unilateral stroke of the cerebral cortex. Arch Phys Med Rehabil. 1993;74:1295–300. doi:10.1016/0003-9993(93)90082-L.
Kendall KA, Leonard RJ. Bolus transit and airway protection coordination in older dysphagic patients. Laryngoscope. 2001;111:2017–21. doi:10.1097/00005537-200111000-00028.
Kahrilas PJ, Lin S, Rademaker AW, Logemann JA. Impaired deglutitive airway protection: a videofluoroscopic analysis of severity and mechanism. Gastroenterology. 1997;113(5):1457–64. doi:10.1053/gast.1997.v113.pm9352847.
Kim Y, McCullough GH, Asp CW. Stage transition duration in patients post-stroke. Dysphagia. 2007;22(4):299–305. doi:10.1007/s00455-007-9085-4.
Rademaker AW, Pauloski BR, Logemann JA, Shanahan TK. Oropharyngeal swallow efficiency as a representative measure of swallowing function. J Speech Hear Res. 1994;37:314–25.
Perlman AL, Booth BM, Grayhack JP. Videofluoroscopic predictors of aspiration in patients with oropharyngeal dysphagia. Dysphagia. 1994;9:90–5. doi:10.1007/BF00714593.
Kendall KA, Leonard RJ, McKenzie S. Airway protection: evaluation with videofluoroscopy. Dysphagia. 2004;19:65–70.
Power ML, Hamdy S, Singh S, Tyrrell PJ, Turnbull I, Thompson DG. Deglutitive laryngeal closure in stroke patients. J Neurosurg Psychiatry. 2007;78:141–6. doi:10.1136/jnnp.2006.101857.
Bisch EM, Logemann JA, Rademaker AW, Kahrilas PJ, Lazarus CL. Pharyngeal effects of bolus volume, viscosity, and temperature in patients with dysphagia resulting from neurologic impairment and in normal subjects. J Hear Speech Lang Sci. 1994;37(5):1041–59.
McCullough GH, Wertz RT, Rosenbek JC. Sensitivity and specificity of clinical/bedside examination signs for detecting aspiration in adults subsequent to stroke. J Commun Disord. 2001;34:55–72. doi:10.1016/S0021-9924(00)00041-1.
McCullough GH, Wertz RT, Rosenbek JC. Age, gender, size, consistency effects on swallowing function in adults between 21 and 99 years of age. Albuquerque, NM: the 10th Annual Dysphagia Research Society; 2001.
Sellars C, Campbell AM, Stott DJ, Stewart M, Wilson JA. Swallowing abnormalities after acute stroke: a case control study. Dysphagia. 1999;14(4):212–8. doi:10.1007/PL00009608.
Curtis DJ, Sepulveda GU. Epiglottic motion: video recording of muscular dysfunction. Radiology. 1983;148:473–7.
Daniels SK, Schroeder MF, McClain M, Corey DM, Rosenbek JC, Foundas AL. Dysphagia in stroke: development of a standard method to examine swallowing recovery. J Rehabil Res Dev. 2006;43(3):347–56. doi:10.1682/JRRD.2005.01.0024.
Robbins J, Kays SA, Gangnon RE, Hind JA, Hewitt AL, Gentry LR, et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88(2):150–8. doi:10.1016/j.apmr.2006.11.002.
Logemann JA, Pauloski BR, Rademaker AW, Colangelo L. Super-supraglottic swallow in irradiated head and neck cancer patients. Head Neck. 1997;19(6):535–40. doi:10.1002/(SICI)1097-0347(199709)19:6<535::AID-HED11>3.0.CO;2-4.
Acknowledgment
This work was performed at the School of Hearing, Speech and Language Sciences, Ohio University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, T., Kim, Y., Ko, DH. et al. Initiation and Duration of Laryngeal Closure During the Pharyngeal Swallow in Post-Stroke Patients. Dysphagia 25, 177–182 (2010). https://doi.org/10.1007/s00455-009-9237-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-009-9237-9