Abstract
The aim of this study was to evaluate leakage of liquids, i.e., water and saliva, past low-pressure cuffs of tracheostomy tubes. Three different types of tracheostomy tubes, TRACOE® vario (TRACOE Medical GmbH, Germany), Rüsch Ultra-Tracheoflex® (Rüsch GmbH, Germany), and Portex Blue Line Ultra™ (Smiths Medical, UK) were tested in isolated pig tracheas. Sixty samples (10 tubes each of 7- and 8-mm inner diameter of each type) were used. Four different experiments were devised: type 1 (water and artificial ventilation), type 2 (water and no artificial ventilation), type 3 (saliva and artificial ventilation), and type 4 (saliva and no artificial ventilation). Six milliliters of water or artificial saliva were infused over the cuff and the volume of fluid that leaked past the cuff was measured after 5, 10, and 15 min. Intracuff pressure was also measured three times. The saliva experiments resulted in less leakage than the water experiments. Leakage after treatment with water or artificial saliva is higher without artificial ventilation than with ventilation. The amount of leakage among the tubes with respect to manufacturer showed statistically significant results. However, there were no differences among tracheostomy tubes with respect to internal diameter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients with a tracheostomy tube may experience swallowing disorders. There are physiologic factors that may contribute to the development of dysphagia in tracheostomized patients, including reduced laryngeal elevation, reduced pharyngeal sensation, reduced cough response, and atrophy of the laryngeal musculature [1–4]. Cameron et al. [5] found that aspiration was detected in 69% of the patients who had had a tracheostomy. A cuffed tracheostomy tube is placed to prevent aspiration of secretions, aspiration of liquids and food, and aspiration of gastric contents [1,6,7].
Because of longitudinal channels caused by folds in the cuff wall material [8,9], however, the cuff does not effectively prevent leakage of fluid into the lower airway. These folds always occur in high–volume, low-pressure (HVLP) cuffs upon inflation because the diameter of the cuff has to be greater than that of the trachea for intracuff pressure to be equal to tracheal wall pressure.
Nevertheless, differences exist between different types of tracheostomy tubes [9–12]. Young et al. [9] assessed a range of HVLP cuffed tubes in a benchtop experiment and found that all the cuffs did not protect the lower airway from contamination. Asai and Shingu [10] and Young and Blunt [12] tested the leakage of fluid around tracheal tubes and ascertained different amounts of leakage. While Young and Blunt [12] found that the Portex Soft Seal tracheal tube prevented leakage effectively, Asai and Shingu [10] found that the Portex Soft Seal and the Mallinckrodt Hi-Lo tracheal tubes prevented leakage of fluid to a similar degree at the minimum effective intracuff pressure. The laboratory findings of Oikkonen and Aromaa [11] suggested that most of the commonly used low-pressure tracheal tubes will not guard against aspiration of fluid.
The above-mentioned examinations [9–12] were in-depth studies on the leakage of fluid around cuffed tracheal tubes but none tested the amount of leakage of fluids of different viscosities; presumably the level of viscidity will affect the rate of leakage. We hypothesized that viscosity would influence the results; thus, leakage of artificial saliva around the cuff should be relatively low compared with leakage of water. Furthermore, we hypothesized that the leakage varies with different factors such as “artificial ventilation (yes/no),” “thickness of the tracheal tube (7mm/8mm),” and “manufacturer (Tracoe/Portex/Rüsch).” Therefore, we studied the efficacy of tracheostomy tubes from three different manufacturers using four different experiments: type 1 (water, artificial ventilation), type 2 (water, no artificial ventilation), type 3 (saliva, artificial ventilation), and type 4 (saliva, no artificial ventilation).
Material and Methods
We tested tracheostomy tubes of three manufacturers in two different sizes [internal diameter (i.d.) = 7 mm and 8 mm] for a total of 60 tubes. The tubes used were TRACOE® vario (TRACOE Medical GmbH, Germany), Rüsch Ultra-Tracheoflex® (Rüsch/Teleflex Medical, Germany), and Portex Blue Line Ultra™ (Smiths Medical, UK). TRACOE vario tubes are made of transparent synthetic material, are spiral reinforced and therefore kink resistant, and have a low-pressure polyvinylchloride (PVC) cuff at the patient end. Cuff membrane thickness is 50–80 μm for both 7- and 8-mm-i.d. tubes. The Rüsch Ultra-Tracheoflex tubes consist of a highly flexible armored tracheostomy cannula made of RÜSCHELIT® (PVC synthetic material) with a thin-walled (50 μm for both 7- and 8-mm i.d.), cylindrical, low-pressure widescope PVC cuff. The Portex Blue Line Ultra tracheostomy tubes are manufactured from clear biocompatible PVC. The material is heat-sensitive and has sufficient initial rigidity for insertion. It is equipped with a profile soft-seal PVC cuff with a thickness of 30–50 μm (for both 7- and 8-mm i.d.) at the patient end. The Rüsch Ultra-Tracheoflex and the TRACOE vario tubes are highly pliable which makes them comfortable for mobile patients, but experience is necessary for insertion because initial stiffness is not guaranteed, especially with the Ultra-Tracheoflex, which is more pliable than the TRACOE vario tubes.
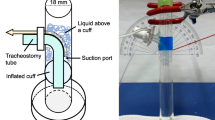
We decided to use organic material for the model trachea, as Young and Blunt [12] did. The pig tracheas had an average coronal diameter of 22 mm and an average sagittal diameter of 23 mm. The range of normal coronal and sagittal diameters is 25–27 mm in men and 21–23 mm in women [13]. The pig tracheas we used are comparable with the dimensions of female human tracheas. Tracheas with anatomic variations were excluded. To lower costs, we used each trachea for two experiments. No wear-out was visible after the first experiment. After slaughtering and dissection, the tracheas were frozen in citric acid until needed. Every trachea was completely defrosted in the same manner. The trachea was fixed at a 45° angle to the horizontal. Similar studies did not position the tracheas uniformly. Young and Blunt [12] selected a 60° angle to the horizontal, Oikkonen and Aromaa [11] decided to put the trachea at a 45° angle to the horizontal, and Asai and Shingu [10] set the trachea at a 90° angle to the horizontal. We chose a 45° angle to the horizontal, like Oikkonen and Aromaa [11], because we felt that tracheostomized patients are often immobile and bedridden and lie in bed at about a 45° angle to the horizontal, not upright or at 60°.
Intracuff pressure was manually set at 25 cmH2O with a cuff inflator (Rüsch GmbH, Germany), and was measured after 5, 10, and 15 min. Oikkonen and Aromaa [11] and Asai and Shingu [10] measured the volume of water running beyond the cuff at 5 min. Young and Blunt [12] decided that if no dye had leaked after 15 min then no leak was recorded. They did not give exact milliliter results, just leak or no leak.
The breathing system was attached to the tracheostomy tube. The model lung was ventilated using a ventilator (transPAC 2d, SIMS pneuPAC Ltd., Smithfield House, Luton, Bedfordshire) with a tidal volume of 700 ml and a respiratory rate of 12 breaths/min. Compliance was adjusted to 20 cmH2O. This would be a reasonable pressure for simulating ventilation in many intensive care patients without acute lung injury. We did not test higher peak pressures that might be more appropriate for patients with decreased lung compliance. Young and Blunt [12] and Asai and Shingu [10] used a compliance of 20 cmH2O. We tested leakage around the cuff while the model was artificially ventilated as well as without ventilation since many tracheostomized patients are not ventilated.
Six milliliters of 1% methylene blue solution were infused within 30 s through a catheter over the cuff. We used two different consistencies of test material: water and artificial saliva. After 5, 10, and 15 min, the amount of fluid that leaked past the cuff was measured. As with trachea positioning, there was no particular amount of liquid used in other studies. Young and Blunt [12] used 3.5 ml of blue dyed water, Asai and Shingu [10] used 10 ml of methylene blue solution, and Oikkonen and Aromaa [11] used 20 ml of water. We infused 6 ml of liquid (water or saliva) above the cuff. Injecting more than 6 ml proved impossible. When we injected more, the liquid would leak out of the stoma and the outside part of the trachea because the space between cuff and stoma was limited. Compared with the 7-mm-i.d. TRACOE vario and the 7-mm-i.d. Portex Blue Line Ultra, the 7-mm-i.d. Rüsch Ultra-Tracheoflex had the shortest distance between cuff and flange. In addition, Young and Blunt [12], Oikkonen and Aromaa [11], and Asia and Shingu [10] did not specify an amount of time for infusion; ours was 30 s.
Data Analysis
The statistics and figures were computed using the statistical software packages SAS release 8.02 (SAS Institute, Cary, NC) and SPSS release 10.0.7 (SPSS Inc., Chicago, IL). We report two-tailed statistics throughout; the accepted type I error rate is chosen to be 0.05.
The aim of the study was to investigate the effect of the different experimental situations on the leakage of three different tracheostomy tubes of two different sizes. To take into account all three time points of the experiment (5, 10, and 15 min), we calculated the area under the leakage curve for each experiment. This area can take on values between 0.0 and 75.0. The main focus of the study was to analyze the four experimental situations: “1: Water/artificial ventilation,” “2: Water/no artificial ventilation,” “3: Saliva/artificial ventilation,” and “4: Saliva/no artificial ventilation.” To adjust for multiplicity, the significance value is set to 0.0125. A p value less than 0.0125 is considered significant.
We first analyzed the different experimental situations descriptively, showing boxplots and giving the mean ± standard deviation (minimum, median, and maximum) of the sample characteristics. In the confirmatory analysis, we analyzed the effect of the “manufacturer” by the nonparametric Kruskall–Wallis test. In case of a significant result, according to the closure test principle [14], pairwise comparisons between the different manufacturers were carried out by the nonparametric Mann–Whitney test. This procedure guarantees that the type I error rate is not inflated.
Finally, we conducted the following exploratory analyses and gave the resulting p values for informational purposes: The Kolmogoroff–Smirnow test indicated that neither the resulting areas nor suitable transformations of the areas were normally distributed. We considered a parametric analysis of variance with the factors “water (yes/no),” “artificial ventilation (yes/no),” “thickness (7mm/8mm),” and “manufacturer (Tracoe/Portex/Rüsch).” This analysis can be taken as a sensitivity analysis. We considered single effects and interactions between two factors at a time.
The thickness of the cannulas was not taken as a separate factor in the following investigations. Because of the high interaction effects between “water” and “artificial ventilation,” the analysis was carried out separately for the cannulas treated with water and those treated with saliva.
Results
The results of the four experimental situations—“1: Water/artificial ventilation,” “2: Water/no artificial ventilation,” “3: Saliva/artificial ventilation,” and “4: Saliva/no artificial ventilation”—are described. Figure 1 shows boxplots for cannula leakage by manufacturer. For the sample characteristics we give results for the first, second, and fourth situation; in situation “3: Saliva/artificial ventilation,” no leakage was observed.
In situation “1: Water/artificial ventilation,” leakage of the Tracoe cannulas was 44.36 ± 15.39 (10.02, 45.61,68.73), of the Rüsch cannulas it was 32.74 ± 21.48 (1.91, 35.15, 67.38), and of the Portex cannulas it was 13.65 ± 8.15 (2.68, 13.46, 26.20).
In situation “2: Water/no artificial ventilation,” leakage of the Tracoe cannulas was 65.74 ± 11.76 (31.65, 72.70, 75.00), of the Rüsch cannulas 53.88 ± 21.09 (9.85, 61.33, 74.35), and of the Portex cannulas 54.71 ± 20.72 (5.53, 60.45, 75.00).
Finally, in situation “4: Saliva/no artificial ventilation,” leakage of the Tracoe cannulas was 4.09 ± 5.20 (0.05, 3.04, 24.23), of the Rüsch cannulas it was 1.68 ± 2.22 (0.00, 0.78, 8.03), and of the Portex cannulas it was 0.11 ± 0.24 (0.00, 0.00, 0.93). For an overview, the mean ± standard deviation of the leakage by manufacturer are given in Table 1.
The nonparametric Kruskall–Wallis tests, performed to analyze the effect of the manufacturer on the area under the leakage curve, showed significant results for the two experimental situations “2: Water/no artificial ventilation” (p < 0.001) and “4: Saliva/no artificial ventilation” (p < 0.001), while in the situation “1: Water/artificial ventilation,” the result was not significant at the chosen level (p = 0.039). The p values of the pairwise comparisons between manufacturers are given in Table 2. Because the closure test principle does not allow a confirmatory analysis for the nonsignificant result in situation 1, the p values are given in parentheses. There are highly significant differences for all pairwise comparisons in situation “4: Saliva/no artificial ventilation”: p < 0.001 for Tracoe and Portex and for Rüsch and Portex and p = 0.009 for Tracoe and Rüsch. A borderline significance (p = 0.013) was found for the difference between Tracoe and Rüsch in situation “2: Water/no artificial ventilation.” The results confirm the impression gained from the descriptive analysis that the Portex cannulas leaked the least.
In the exploratory analysis, the parametric four-factorial analysis of variance indicated effects for the factors “water (yes/no),” “artificial ventilation (yes/no),” and “manufacturer” (Tracoe/Portex/Rüsch) (p < 0.001 for all three factors), while the factor “thickness (7mm/8mm)” (p = 0.892) did not explain the variation in the areas under the leakage curve. On the other hand, high interactions existed between “water” and “artificial ventilation” (p < 0.001) and between “artificial ventilation” and “manufacturer” (p = 0.0079). There also seems to be an interaction between “water” and “manufacturer” (p = 0.057). Therefore, we analyzed the cannulas treated with water and those treated with saliva separately in another step.
Figure 2 shows that under treatment with water, leakage is higher without artificial ventilation. This effect is also illustrated in Figure 3 for the cannulas treated with saliva, although the absolute values of leakage were much smaller. For all the cannulas of all three manufacturers, the sample characteristics for treatment with water without ventilation are 58.11 ± 18.86 (5.53, 64.54, 75.00); for water with ventilation, 30.25 ± 20.03 (1.83, 24.65, 68.73); for saliva without ventilation, 1.96 ± 3.61 (0.00, 0.64, 24.23); and for saliva with ventilation, 0.00 ± 0.00 (0.00, 0.00, 0.00).
Discussion
This study compared the ability of three commonly used tracheostomy tube cuffs to prevent the leakage of water and artificial saliva past the cuff. Those cuffs are used to protect the lower airway from contamination by material leaking from the subglottis. Past studies of Young and Blunt [12], Oikkonen and Aromaa [11], and Asia and Shingu [10] evaluated the leakage of only water past the cuffs of tracheostomy tubes. This study is the first to test the leakage of saliva past tracheostomy tubes. A cuffed tracheostomy tube has to prevent the continuous aspiration of saliva into the lungs. We were interested in whether there are differences in protecting the lower airway against leaked water versus leaked artificial saliva. Does viscosity play an important role?
The rate of water leakage between the cuff and tracheal wall was surprisingly rapid in most of the tested tracheostomy tubes. This finding is consistent with previous studies [10–12]. We detected that under treatment with water, leakage is higher without artificial ventilation. Our laboratory findings suggest that the tested tracheostomy tubes will also not guard against the aspiration of artificial saliva in the lower airway, but, in contrast to the water experiments, leakage was significantly less. There were significant interactions between “water” and “artificial saliva,” which means that fluids with more viscous consistencies such as saliva result in less leakage. No leakage was measured in the saliva and artificial ventilation treatment.
The study shows that the Portex Blue Line Ultra tracheostomy tubes effectively prevent leakage of subglottic fluid into the lungs. Only in situation “2: Water/no artificial ventilation” (p = 0.883) did we find no significant differences between Rüsch and Portex tubes. There was a borderline significant difference between Tracoe and Rüsch tubes in situation “1: Water/artificial ventilation” (p = 0.068). We found that the TRACOE vario tracheostomy tubes leaked more than the Rüsch Ultra-Tracheoflex and the Portex Blue Line Ultra tracheostomy tubes in the water experiments with or without artificial ventilation and in the saliva experiments without artificial ventilation.
The study by Dullenkopf et al. [15] showed that both cuff material and cuff membrane thickness have an important impact on leakage of fluid around tracheal tubes with cuff. They found out that the Microcuff endotracheal tube HVLP ICU (Microcuff, Weinheim, Germany) prevented fluid leakage most effectively compared with four commonly used cuffed endotracheal tubes (Mallinckrodt HiLo, Portex Profile Soft Seal, Rüschelit Supoer Safety Clear, and Sheridan CF). The Microcuff consists of an ultrathin 7-μm polyurethane cuff membrane. Currently, the manufacture produces only endotracheal tube cuffs made of polyurethane, but not tracheal tube cuffs because the material is still very expensive. In contrast with the study of Dullenkopf et al. [15], our results show that the tracheostomy tubes with the thinnest cuff membrane had the least leakage. We decided that the leakage of fluid is associated with the thickness of the cuff wall membrane. Research will show that ultrathin polyurethane will be the cuff material of the future.
The observation period was restricted to 15 min with three controls of the cuff pressure. Although the intracuff pressure was initially set at 25 cmH2O, after 15 min it had dropped to 18.76–1990 cmH2O (Tracoe), 19.54–20.64 cmH2O (Rüsch) and 19.07–20.49 cmH2O (Portex). The cuff pressure should be recorded regularly on the patient chart. Finger palpation of the pilot balloon has been shown to be an unreliable and insufficient method of cuff pressure estimation [16]; thus, objective measurement of cuff pressure by manometry is required to avoid overinflation. Exact pressure ratio is required because decreased pressure may increase the rate of aspiration and extremely high pressure may lead to tracheal stenosis or a tracheoesophageal fistula. In clinical practice, endotracheal tube pressures are routinely high and should be measured by manometry [17], but regular measurement of tracheal cuff pressure is unusual [18].
In conclusion, the present study shows that an inflated tracheostomy tube cuff cannot prevent aspiration, it only slows the already aspirated bolus movement in the lungs [1]. When water runs into the subglottic space above the inflated cuff, it almost completely disappears down the longitudinal channels caused by folds in the cuff wall material. Because of viscosity, the leakage of saliva was significantly reduced compared with water.
Thus, it appears that an inflated cuff does not prevent aspiration in patients who aspirate when they eat. It should be determined early whether a patient with a tracheostomy aspirate or not to find whether the patient needs a tracheostomy tube with or without cuff and whether he/she is able to take food orally or needs to be nourished with a PEG tube. The earlier the swallowing examination, the earlier the patient achieves the best tracheal tube management.
References
Logemann J: Evaluation and Treatment of Swallowing Disorders, 2nd ed. Austin, TX: Pro-Ed, 1998
Dikeman K, Kazandjian M: Communication and Swallowing Management of Tracheotomized and Ventilator-Dependent Adults, 3rd printing ed. San Diego, CA: Singular Publishing Group, 1997
Dettelbach M, Gross R, Mahlmann J, Eibling D: Effects of the Passy-Muir valve on aspiration in patients with tracheostomy. Head Neck 17:297–300, 1995
Tolep K, Getch C, Criner G: Swallowing dysfunction in patients receiving prolonged mechanical ventilation. Chest 109:167–172, 1996
Cameron J, Reynolds J, Zuidema G: Aspiration in patients with tracheotomies. Surg Gynecol Obstet 136:68–70, 1973
Elpern E, Jacobs E, Bone R: Incidence of aspiration in tracheally intubated adults. Heart Lung 16:527–531, 1987
Bach J, Alba A: Tracheostomy ventilation: a study of efficacy with deflated cuffs and cuffless tubes. Chest 19:679–683, 1990
Seegobin R, Van Hasselt G: Aspiration beyond endotracheal cuffs. Can Anaesth Soc J 33:273–279, 1986
Young P, Rollinson M, Downward G, Henderson S: Leakage of fluid past the tracheal tube cuff in a benchtop model. Br J Anaesth 78:557–562, 1997
Asai T, Shingu K: Leakage of fluid around high-volume, low-pressure cuffs. Anaesthesia 56:38–42, 2001
Oikkonen M, Aromaa U: Leakage of fluid around low-pressure tracheal tube cuffs. Anaesthesia 52:567–569, 1997
Young P, Blunt C: Compliance characteristics of the Portex soft seal cuff improves seal against leakage of fluid in a pig trachea model. Crit Care 3:123–126, 1999
Breatnach E, Abbott G, Fraser R: Dimensions of the normal human trachea. AJR Am J Roentgenol 142:903–906, 1984
Marcus R, Peritz E, Gabriel K: On closed testing procedures with special reference to ordered analysis of variance. Biometrica 63:655–660, 1976
Dullenkopf A, Gerber A, Weiss M: Fluid leakage past tracheal tube cuffs: evaluation of the new microcuff endotracheal tube. Intensive Care Med 29:1849–1853, 2003
Fernandez R, Blanch L, Mancebo J, Bonsoms N: Endotracheal tube cuff pressure assessment: pitfalls of finger estimation and need for objective measurement. Crit Care Med 18:1423–1426, 1990
Satishkumar S, Young P: Tracheal cuff pressure - a survey of clinical practice. Br J Anaesth 88:456, 2002
Spittle C, Beavis S: Do you measure tracheal cuff pressure? a survey of clinical practice. Br J Anaesth 87:344P–345P, 2001
Acknowledgments
The authors thank TRACOE Medical GmbH, Rüsch GmbH, and Smiths Medical for supplying the tracheostomy tubes. The author (U. Winklmaier) was a scholarship recipient of the Hanns-Seidel-Foundation, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Winklmaier, U., Wüst, K., Schiller, S. et al. Leakage of Fluid in Different Types of Tracheal Tubes. Dysphagia 21, 237–242 (2006). https://doi.org/10.1007/s00455-006-9047-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-006-9047-2