Abstract
Although regions of the sensorimotor cortex, insula, and anterior cingulate gyrus are reported to be activated during swallowing, findings concerning contributions of the cerebellum and basal ganglia have been contradictory. We investigated cerebellar and basal ganglionic activation using functional magnetic resonance imaging (fMRI). In 11 subjects, single-shot gradient-echo echoplanar image volumes sensitive to BOLD contrast were acquired in block design fashion using an oblique orientation covering both cerebrum and cerebellum. Using statistical parametric mapping, regional activation upon swallowing was observed in the sensorimotor cortex, insula, cerebellum, putamen, globus pallidus, thalamus, anterior cingulate gyrus, supplementary motor area, superior temporal gyrus, and substancia nigra. The cerebellum was activated bilaterally, especially on the left; activation of the putamen and globus pallidus was also found bilaterally. Thus, volitional swallowing involves the cerebellum and basal ganglia as well as cortical structures. The method used was well tolerated by normal subjects and should also be applicable to patients with dysphagia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recent studies using functional imaging of brain activity [functional magnetic resonance imaging (fMRI), as well as positron emission tomography (PET)] have indicated that central neural control mechanism, as well as control by the brainstem, are important for swallowing [1,2,3,4,5,6,7,8,9]. Regions activated on swallowing have been consistent in fMRI and PET studies irrespective of reflexive or volitional character of the swallowing, including the precentral gyrus, postcentral gyrus, insula, and anterior cingulate gyrus. Although several studies demonstrated activation of the cerebellum and basal ganglia [1,2,3,4,9], contributions of these structures have been less notable than other cortical regions such as the precentral and postcentral gyri. We investigated activation of cerebellum and basal ganglia using fMRI designed to image the entire brain, including the cerebellum, during swallowing.
Current studies have reported that event-related types of fMRI image acquisition are most successful in detecting neural activity related to swallowing [10,11]. However, Kern et al. [7] demonstrated that average activation maps of swallowing using block-trial and single-trial methods resemble each other. Therefore, we detected brain activity using conventional block design fMRI. We used less repetition of swallowing (1 swallow/10 s) than authors of previous reports, aiming to minimize motion artifact during swallowing [4].
Subjects and Methods
Subjects
We studied 11 right-handed volunteers with no history of swallowing problems (7 male, 4 female; age range=24–42 years). The study was approved by the ethics committee of Shiga University of Medical Science, and all subjects gave written informed consent for study participation.
Task Design, Apparatus, and Image Acquisition
Following orientation to the study and prior to fMRI, subjects practiced swallowing motion at one swallow/10 s. Subjects were scanned with eyes closed and the room light dimmed. Padding was placed behind the neck and around the head as needed to relax the cervical spine and to minimize subject movement.
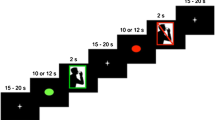
In each subject, functional (T2*–weighted) images followed by anatomic three-dimensional images of the entire brain [magnetization-prepared rapid-gradient echo (MP-RAGE)] were acquired with a 1.5-T MRI scanner (Magnetic Resonance Imaging Magnetom Vision Plus; Siemens, Erlangen, Germany). Functional images consisted of obliquely oriented single-shot gradient-echo echoplanar image (EPI) volumes that were sensitive to BOLD (blood oxygen level-dependent) contrast. Settings used were 90° flip angle, 60-ms echo time, 3-s repetition time, 20-cm field of view, 64 × 64-pixel matrix, and 3.13 × 3.13 × 7.2-mm voxel dimensions (slice thickness=6 mm; gap=1.2 mm). Using a midsagittal scout image, 16 oblique slices oriented parallel to the orbitomeatal line were obtained to include the entire brain volume, including cerebellum and cerebrum. During one hundred time points over the 5-min period, the 16 fMRI locations were scanned. Each EPI series began with 4 discarded volumes (12 s) to allow the magnetic resonance (MR) signal to reach equilibrium and 6 volumes (nonswallowing), followed by 90 volumes during which two comparison conditions (volitional swallowing and nonswallowing) were alternated every 30 s (Fig. 1). Volitional swallowing consisted of three swallowing motions completed over 30 s (1 swallow/10 s). Subjects were signaled to begin and stop swallowing their ambient saliva by brief auditory instructions (“start” and “stop”) that were given at a rate of once per 30 s. Each image series thus consisted of five task-alternation cycles.
Data Analysis
Image processing and statistical analyses were carried out using SPM 99 software distributed by the Wellcome Department of Cognitive Neurology (University College, London, UK) and implemented in Matlab (Mathworks, Natick, MA) [12]. All volumes were realigned to the first volume, corrected for motion artifact, mean-adjusted by proportional scaling, coregistered with the subject's corresponding anatomic image, resliced, normalized to standard stereotactic space (template provided by the Montreal Neurological Institute, Canada) using nonlinear transformation, and smoothed using a 10-mm full-width-at-half-maximum Gaussian kernel. Activated voxels were detected using a general linear model approach for time-series data [12]. We defined a design matrix consisting of contrasts modeling the alternating periods of baseline and activation in each subject, with delayed boxcar fixed response as a reference waveform that included slow hemodynamic fluctuations unrelated to the tasks. A statistical parametric map of the t statistic was generated for each voxel, including removal of the global effect by proportional scaling, a high-pass filter (120-s cutoff period), and a low-pass Gaussian filter (4 s). Finally, activated voxels were analyzed for uncorrected height with the threshold at a p value of 0.001 in each subject. Conjunction analysis was used as the method for group analysis [12]. In the group analysis, voxels were judged to be activated when a height threshold corresponding to p<0.05 (corrected) was exceeded. Locations of significant signal increases in group analyses were recorded in terms of region name, Brodmann area (BA), stereotactic coordinates according to Talairach and Tournoux [13], t value, and Z value.
Results
Group Distribution Patterns of Activated Regions
All subjects tolerated scanning well, without excess body movements, and all succeeded in swallowing at a consistent rate during fMRI. Figure 2 shows the translation (x, y, and z axes) and rotation (pitch, roll, and yaw) of the images obtained from subject 3 using coordinates specified by Talairach and Tournoux [13]. The images in each subject were realigned for motion correction using SPM 99 software. The mean and maximum values of translation (x, y, and z axes) in all subjects were 0.25 and 0.80 mm, respectively. The mean and maximum values of rotation (pitch, roll, and yaw) were 0.27° and 0.80°, respectively. No subject noted any swallowing difficulties according to a questionnaire completed after the MRI session. Figure 3 shows the statistical parametric map obtained from subject 3. No marked motion artifact was found. To aid localization, activated regions were projected onto a T1-weighted anatomic image in Figures 3,4,5,6,7. Multiple regions of significant activation were detected of ambient saliva swallowing (Table 1 and Figs. 4,5,6,7). Across subjects, prominent and consistent activation foci were located within the precentral and postcentral gyrus (Brodmann areas or BA: 6, 4, 3, 40, 43, and 44; hereafter referred to as sensorimotor cortex), insular gyrus, cerebellum, putamen, and globus pallidus. Activation of these regions was observed in both hemispheres except for the cerebellum. Other regions of activation detected in the group analysis were located within the thalamus (right); anterior cingulate gyrus (bilateral; BA 24 and 32); bilateral supplementary motor area (BA 6; hereafter, SMA), superior temporal gyrus (BA 22 and 42) and subtantia nigra.
Three orthogonal projections of activated voxels of 11 subjects during volitional swallowing were obtained and subjected to group analysis (height threshold, p<0.05; corrected). Pre & Postcentral, precentral and postcentral gyri; ACG, anterior cingulate gyrus; Cer, cerebellum; Len & Th, putamen and globus pallidus (“lentiform nucleus”) and thalamus;A, anterior; R, right.
Individual Distribution Patterns of Activated Regions (Table 2)
Individual patterns of regional activation for each subject are shown in Table 2. From these data, the sensorimotor cortex, insular gyrus, and cerebellum clearly were activated in all subjects. While the sensorimotor cortex was activated bilaterally and symmetrically, insular activation was more prominent in the right hemisphere while cerebellar activation was more prominent in the left side. Strong activation in the thalamus, putamen, and globus pallidus was observed bilaterally, with no hemispheric predominance. Regions in the anterior cingulate gyrus, supplementary motor area (BA 6), and superior temporal gyrus (BA 22) were less consistently activated, as demonstrated in about three-quarters of the subjects in the individual analysis.
Activation of the Cerebellum and the SMA (Fig. 5)
Cerebellar activation in the individual analysis was observed bilaterally except in subject 11. The cerebellar activation in the group analysis was located in the left posterior lobe. Local maxima of cerebellar activation in the group analysis were located at (x, y, z) = (−36, −56, −32) on the left. SMA area activation in BA 6 was observed in 73% of subjects. Activation of the SMA was detected bilaterally in the group analysis as well as in the individual analysis.
Activation of Putamen, Globus Pallidus, and Thalamus (Fig. 6)
Prominent, mostly bilateral activation was observed in putamen and globus pallidus in both group and individual analyses. In the group analysis, the maximal t value in the right putamen was 2.36 and was located at (x, y, z) = (32, −8, −2); the maximum t value in the left putamen was 1.05 and was located at (x, y, z) = (−24, −4, −4). Although thalamic activation appeared mostly bilateral in the individual analysis, preferential activation of the right thalamus was detected in the group analysis. Local maximum of thalamic activation in the group analysis was located at (x, y, z) = (8, −16, 0) on the right.
Insular Activation (Figs. 6 and 7)
Insular activation, located in BA 13, was detected in all 11 subjects. Insular activation was obtained bilaterally in 8 subjects and only on the right in 3 subjects (Table 2). In the group analysis, the maximal t value in the right insula was 1.72 and was located at (x, y, z) = (42, 6, −4); the maximum t value in the left insula was 1.28 and was located at (x, y, z) = (−42, 0, 0). Activated volumes were much larger in the right insula than in the left (Fig. 6).
Other Activated Regions
Weak activation of the superior temporal gyrus (BA 22) was observed mostly in the right hemisphere in the individual analysis and bilaterally in the group analysis. Activation in the anterior cingulate gyrus was observed bilaterally and symmetrically in both group and individual analyses. Activation in the subtantia nigra was also observed bilaterally.
Discussion
Most recent fMRI studies concerning swallowing have employed event-related paradigms to reduce motion artifact [3,5,6,7,8]. Birn et al. [11] demonstrated that a block-trial paradigm contained artifacts near edges of the brain in the individual analysis, using 15-s periods of repeated swallowing for this paradigm. As the degree-of-motion artifact varied among subjects, these investigators subsequently reported that activated regions obtained from block-trial and single-trial paradigms were similar to each other in the group analysis [7]. Block-trial paradigms for swallowing required subjects to swallow as quickly as possible [4,9,11]. In such a situation, motion related to swallowing would be larger than in usual volitional swallowing because of excessive movement of the tongue, jaw, and lip. Event-related paradigms convey the benefit of reduced motion artifact but require a long scan time to improve the signal-to-noise ratio [3,5,6,8]. Previous studies of swallowing using event-related acquisition usually employed a scan time between 8 and 9 min. In the clinical setting, patients with dysphagia cannot always tolerate long scanning times. Considering this practical problem, we used a block-trial paradigm to reduce scan time and set the repetition rate of swallowing at once every 10 s to minimize motion artifact. Individual statistical parametric maps in the present study showed less motion artifacts than previously reported [11]. The software (SPM 99) also may have helped to reduce motion artifacts because of its effectiveness in realigning images [14].
Swallowing involves numerous processes, such as oral motor control, intraoral sensory processing, salivation, and visceral regulation. The present study detected a multifocal cortical representation of swallowing in the precentral gyrus, postcentral gyrus, insula, and anterior cingulate gyrus, as previously reported using event-related task paradigms [3,5,6,7,8]. Although some of the activated areas may be related to self-pacing rather than swallowing, these findings suggest that multiple central regions are involved in control of volitional swallowing. However, Kern et al. [7] have cautioned that swallowing-related regional activation is not necessarily specific to deglutition, comparing swallowing with more isolated but related motor tasks such as lip pursing, tongue rolling, and jaw clenching. Further study is needed to determine swallowing-specific regions.
In the present study, activation was observed in the cerebellum and basal ganglia. Activation of the cerebellum during swallowing has been reported in a few studies using PET and fMRI [1,2,4,9]. Zald and Pardo [2] reported that left cerebellar activation by volitional swallowing differed from cerebellar foci activated in tongue movement. Cerebellar activation was bilateral in the present study, with the left cerebellar focus appearing more prominent than the right, a finding consistent with previous reports [1,2]. Several previous fMRI studies have focused on the cerebrum [3,5,7]. Although Mosier et al. [4,9] reported cerebellar activation during volitional swallowing, several fMRI studies did not show any cerebellar activation [6,8]. The causes of these disparate findings among studies concerning cerebellar activation are not clear. Studies that showed cerebellar activation employed self-paced repeated swallowing as the task. We used self-paced swallowing every 10 s as the task and subjects were trained in the task just before scanning. From investigations concerning cerebellar contribution to motor tasks, the cerebellum may work to control adaptive coordination, sequencing, timing, learning, and memory of motion [15,16,17]. Recently, Mosier and Bereznaya [9] proposed that parallel cortical networks exist for volitional control of swallowing. They detected cerebellar activation during volitional swallowing and documented that the cerebellum has inhibitory connections to the primary motor cortex, primary sensory cortex, supplementary motor area, and cingulate cortex. As the above regions of activation also were detected in the present study, the cerebellum may regulate a neural network related to swallowing.
Prominent activation also was observed in the putamen and globus pallidus. The basal ganglia functionally connect the cerebral cortex and the thalamus, with one likely function being gating of sensory input to achieve motor control [18]. Activation of the putamen and globus pallidus, as in the present study, has shown conflicting results in previous studies [1,2,3,4,5,6,7,8,9]. Using principal component analysis, Mosier and Bereznaya [9] found that the basal ganglia and thalamus have positive correlations with primary motor cortex, primary sensory cortex, and supplementary motor cortex but negative correlations with the cerebellum. According to their results, the putaminal and pallidal activations detected in the present study may modulate cerebellar function based on input from the sensory and motor cortices. Subjects in the present study were requested to swallow in a precise manner. Such voluntary swallowing may require a high level of control via the basal ganglia and cerebellum. The SMA, which showed activation in the present study, is thought to be important in planning sequential movement [19]. This activation also may aid in motor control of ingestive behaviors in concert with the cerebellum and basal ganglia. Using PET, Hamdy et al. [1] detected cerebellar activation but no activation in the putamen. These authors steadily infused water into the oral cavity at rates of 30, 60, or 90 ml/min as an ongoing swallowing task. Subsequently, they detected activation in the putamen using fMRI [3] with volitional swallowing every 30 s. Accordingly, rate and mode of swallowing may influence activation of the basal ganglia.
The present study outlines various brain regions, including cerebellum and basal ganglia, that appear related to volitional swallowing using conventional fMRI. Further study is needed to clarify the role of these regions in volitional swallowing in hopes of improving treatment of dysphagia.
References
S Hamdy JC Rothwell DJ Brooks D Bailey Q Aziz DG Thompson (1999) ArticleTitleIdentification of the cerebral loci processing human swallowing with H2 15O PET activation. J Neurophysiol 81 1917–1926 Occurrence Handle1:STN:280:DyaK1M3hvVaitA%3D%3D Occurrence Handle10200226
DH Zald JV Pardo (1999) ArticleTitleThe functional neuroanatomy of voluntary swallowing. Ann Neurol 46 281–286 Occurrence Handle10.1002/1531-8249(199909)46:3<281::AID-ANA2>3.3.CO;2-C Occurrence Handle1:STN:280:DyaK1MvgvF2rsQ%3D%3D Occurrence Handle10482257
S Hamdy DJ Mikulis A Crawley S Xue H Lau S Henry NE Diamant (1999) ArticleTitleCortical activation during human volitional swallowing: an event-related fMRI study. Am J physiol Gastrointest Liver Physiol 277 G219–G225 Occurrence Handle1:CAS:528:DyaK1MXkslKnsbs%3D
K Mosier R Patel WC Liu A Kalnin J Maldjian S Baredes (1999) ArticleTitleCortical representation of swallowing in normal adults: functional implications. Laryngoscope 109 1417–1423 Occurrence Handle1:STN:280:DyaK1MvivVygtw%3D%3D Occurrence Handle10499047
RE Martin BG Goodyear JS Gati RS Menon (2001) ArticleTitleCerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol 85 938–950 Occurrence Handle1:STN:280:DC%2BD3M3htlyntA%3D%3D Occurrence Handle11160524
MK Kern S Jaradeh RC Arndorfer R Shaker (2001) ArticleTitleCerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol 280 G345–G360
M Kern R Birn S Jaradeh A Jesmanowiz R Cox J Hyde R Shaker (2001) ArticleTitleSwallow-related cerebral cortical activity maps are not specific to deglutition. Am J Physiol Gastrointest Liver Physiol 280 G531–G538 Occurrence Handle1:CAS:528:DC%2BD3MXjtVyqtrw%3D Occurrence Handle11254478
CJ Hartnick C Rudolph JP Willging SK Holland (2001) ArticleTitleFunctional magnetic resonance imaging of the pediatric swallow: imaging the cortex and the brainstem. Laryngoscope 111 1183–1191 Occurrence Handle1:STN:280:DC%2BD3Mrit1yjsA%3D%3D Occurrence Handle11568539
K Mosier I Bereznaya (2001) ArticleTitleParallel cortical networks for volitional control of swallowing in humans. Exp Brain Res 140 280–289 Occurrence Handle10.1007/s002210100813 Occurrence Handle1:STN:280:DC%2BD3Mrntl2ntQ%3D%3D Occurrence Handle11681303
RM Birn PA Bandettini RW Cox A Jesmanowicz R Shaker (1998) ArticleTitleMagnetic field changes in the human brain due to swallowing or speaking. Magn Reson Med 40 55–60 Occurrence Handle1:STN:280:DyaK1czitF2qsg%3D%3D Occurrence Handle9660553
RM Birn PA Bandettini RW Cox R Shaker (1999) ArticleTitleEvent-related fMRI of tasks involving brief motion. Hum Brain Mapp 7 106–114 Occurrence Handle10.1002/(SICI)1097-0193(1999)7:2<106::AID-HBM4>3.3.CO;2-F Occurrence Handle1:STN:280:DyaK1M7jtlGltQ%3D%3D Occurrence Handle9950068
KJ Friston AP Holmes CJ Price C Buchel KJ Worsley (1999) ArticleTitleMultisubject fMRI studies and conjunction analyses. Neuroimage 10 385–396 Occurrence Handle10.1006/nimg.1999.0484 Occurrence Handle1:STN:280:DyaK1MvisFeqsw%3D%3D Occurrence Handle10493897
J Talairach P Tournoux (1988) Cerebral structures in three-dimensional space. J Talairach P Tournoux (Eds) Co-planar stereotaxic atlas of the human brain. Thieme New York 9–18
JL Andersson C Hutton J Ashburner R Turner K Friston (2001) ArticleTitleModeling geometric deformations in EPI time series. Neuroimage 13 903–919 Occurrence Handle10.1006/nimg.2001.0746 Occurrence Handle1:STN:280:DC%2BD3MzpsFejtw%3D%3D Occurrence Handle11304086
SW Keele R Ivry (1990) ArticleTitleDoes the cerebellum provide a common computation for diverse tasks? A timing hypothesis. Ann NY Acad Sci 608 179–211 Occurrence Handle1:STN:280:By6C28nhvFU%3D Occurrence Handle2075953
WT Thach HP Goodkin JG Keating (1992) ArticleTitleThe cerebellum and the adaptive coordination of movement. Annu Rev Neurosci 15 403–442 Occurrence Handle1575449
O Hikosaka H Nakahara MK Rand S Katsuyuki X Lu K Nakamura S Miyachi K Doya (1999) ArticleTitleParallel neural networks for learning sequential procedures. Trends Neurosci 22 464–471 Occurrence Handle1:CAS:528:DyaK1MXmsVWhtrg%3D Occurrence Handle10481194
R Kaji (2001) ArticleTitleBasal ganglia as a sensory gating device for motor control. J Med Invest 48 142–146 Occurrence Handle1:STN:280:DC%2BD3MnktlGhtw%3D%3D Occurrence Handle11694953
J Tanji (1994) ArticleTitleThe supplementary motor area in the cerebral cortex. Neurosci Res 19 251–268 Occurrence Handle1:STN:280:ByuA3sngvVU%3D Occurrence Handle8058203
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by a grant-in-aid for exploratory research (13877281) awarded to M. Suzuki.
Rights and permissions
About this article
Cite this article
Suzuki, M., Asada, Y., Ito, J. et al. Activation of Cerebellum and Basal Ganglia on Volitional Swallowing Detected by Functional Magnetic Resonance Imaging . Dysphagia 18, 71–77 (2003). https://doi.org/10.1007/s00455-002-0088-x
Issue Date:
DOI: https://doi.org/10.1007/s00455-002-0088-x