Abstract
Microbial fuel cell (MFC) is used to remove organic pollutants while generating electricity. Biocathode plays as an efficient electrocatalyst for accelerating the Oxidation Reduction Reaction (ORR) of oxygen in MFC. This study integrated biocathode into a single-chamber microbial fuel cell (BSCMFC) to produce electricity from an organic substrate using aerobic activated sludge to gain more insights into anodic and cathodic biofilms. The maximum power density, current density, chemical oxygen demand (COD) removal, and coulombic efficiency were 0.593 W m−3, 2.6 A m−3, 83 ± 8.4%, and 22 ± 2.5%, respectively. Extracellular polymeric substances (EPS) produced by biofilm from the biocathode were higher than the bioanode. Infrared spectroscopy and Scanning Electron Microscope (SEM) examined confirmed the presence of biofilm by the adhesion on electrodes. The dominant phyla in bioanode were Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria, while the dominant phylum in the biocathode was Proteobacteria. Therefore, this study demonstrates the applicable use of BSCMFC for bioelectricity generation and pollution control.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Resolving power shortages and pollution are the top challenges facing world developments [1]. The wastewater treatment plants generate large amounts of activated sludge which contains organics compounds at a high concentration (66%) [2]. Activated sludge treatment and disposal have become difficult and expensive problems [3]. Therefore, new approaches are highly required. Among these technologies are microbial fuel cells (MFCs) which are considered as energy transducers consists of anode and cathode converting the stored chemical energy in a variety of biodegradable materials, ranging from pure compounds to multifaceted substrates present in wastewater into electrical energy by tiny electrogens as a biocatalyst to support redox reactions. In the anodic section, the electrogens can oxidize organic compounds anaerobically producing electrons and protons. Afterward, these electrons pass through an external circuit and combine with the transferred protons into the cathode by diffusion and a terminal electron acceptor usually oxygen producing water [4].
The efficiency of the electric current generation in MFCs is reduced due to overvoltage losses at the cathode electrode [5]. To eliminate this problem, MFC technology uses cathodes based on materials containing catalysts as platinum that actively remove excess electrons [6]. However, its use increases the technology cost. This leads to the search for modern, cheaper, and promising catalysts for cathodic processes [7]. One promising tool for cathodic MFC is the use of microorganisms as biocatalysts [8]. The aerobic biocathodes MFCs have the dominant range of bacterial species in a mixed population belonging to Alphaproteobacteria and Betaproteobacteria [9], Gammaproteobacteria [10], Bacteroidetes [11], and other less well-known groups.
Little studies illustrate the importance of extracellular polymeric substances (EPS) in the biocathode, which have many roles as cell attachment to solid surfaces, biofilm structure maturation, and protection from harsh environmental conditions [12]. EPS are typically made up of proteins, DNA, humic acids, polysaccharides, etc. that are secreted in pure and mixed cultures by microbes [12]. The redox properties of these EPS components could be used as an electron carrier in electrochemically active biofilms [13]. EPS are secreted by the bacteria when they form a biofilm on an electrode [14]. Because the EPS layer is not a solid barrier between cells and external electron donors/acceptors, these redox compounds can diffuse through the EPS and play a role in electron transfer between cells and electron donors/acceptors [15].
The wastewater treatment and energy production from artificial or real wastewater provide an overview of the MFC′s efficiency with a range of wastewater [16]. Microorganisms naturally grow on many complex compounds found in wastewater. It is necessary to know the microbiome and its biochemical properties, and metabolic activity to obtain successful wastewater bioremediation and electricity generation [17]. To expand the BSCMFC technology scope in the future, keeping in mind the enormous secret bacterial diversity, usually, electricigens in nature, isolation of potentially active ones is necessary. 16SrRNA sequencing was used to study the electroactive involved in the power generation in MFC systems [18]. It explains how the microbial population dynamics is interrelated with the bioreactor that works as a treatment system for wastewater [19].
To our knowledge, no previous studies have been performed comparing the responses of biocathode-isolated electrogenic bacteria to in vitro biofilm formation. Another aspect, there were a few studies in biocathode MFCs. This study aimed to use BSCMFC with aerobic activated sludge as a substrate to increase the rate of ORR and electroactive microbial activities along with the elimination of organic matter through wastewater treatment and electricity generation. The BSCMFC performance was studied by monitoring the power generation, columbic efficiency, and COD removal efficiency. The anode and cathode potentials were also evaluated. EPSs were extracted from the bioanode and biocathode and their protein and carbohydrate contents were analyzed. In addition, the chemical structure of each EPS fraction was characterized using FTIR. Moreover, microbial communities were analyzed using SEM and 16SrRNA. In addition, under in vitro conditions, the biofilm forming abilities of each anodic and cathodic culturable bacterial isolate were separately determined and their biochemical characteristics were performed.
Materials and method
Chemicals
All supplies and chemical materials were of analytical, and biochemical grade purity. All aqueous solutions were newly prepared throughout the experiments with de-ionized distilled water. Both carbon felt and carbon cloth were purchased from fuel cell store.
Configuration of BSCMFC
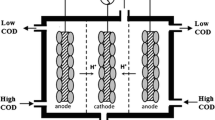
Performing experiments for BSCMFC were achieved in fed-batch mode. MFCs consisting of a cylindrical plexi-glass tube containing the anode and the cathode electrodes, placed on two opposite sides of a liquid chamber reactor (6.0 cm long, 4.6 cm in diameter, 100 mL effective working liquid volume), as shown in Scheme 1 [20]. The anode consisted of an unmodified sheet of three-dimensional carbon felt (not waterproof, project surface area of 0.005288 m2) glued to the top of the externally connected anode port. The anode was positioned on the opposite side of the chamber (perpendicularly to the cathode at a distance of 5 cm) and free from the membrane. The gas diffusion carbon cloth electrode was used as the backbone cathode electrode (6 × 6 cm each, cathode coated project area of 0.001663 m2). For ORR, the cathode electrode was left open to the air. The current collector (titanium wire) was placed on both sides of the electrodes; this elongated side was used for multimeter connection.
Microbial populations enumeration, incubational and operational conditions
The biocathode was inoculated with the adapted aerobic activated sludge. The sludge was collected from Zenien wastewater treatment plant located in the Bolaq Aldakrur, Giza, Egypt. This sludge was directly used as an anodic and cathodic inocula. The sample was conveyed to the laboratory in dry plastic water bottles and kept at 4 °C. The sludge was characterized chemically upon arrival as follows (mg L−1): total nitrogen, 145.57; total phosphorus, 25.761; total chlorides, 24.85; total solids, 701.6; total dissolved solids, 652.8; total suspended solids, 48.8; biochemical oxygen demand (BOD), 160; COD, 524.16.
Microbiologically by the count plate method, the collected samples have comprised of the determination of the total number of culturable bacteria. The bacterial count was 5.4 × 107 CFU mL−1. The sludge was initially operated under fed-batch mode of operation (over 1201 h) at 30 ± 2 °C for a total of 50 days to allow a biofilm to form over the anode and the cathode surfaces [21]. The synthetic electrolyte contained CH3COONa, 2 g L−1; as the degradable organic substrate (electron donors) in a 50 mM phosphate buffer solution (PBS) amended with 12.5 mL micro metal solution and 12.5 mL vitamin solutions. The 50 mM PBS contained (g L−1): NaHCO3, 2.5; NH4Cl, 0.2; KH2PO4, 13.6; KCl, 0.33; NaCl, 0.3; K2HPO4, 17.4; CaCl2.2H2O, 0.15; MgCl2, 3.15; yeast extract, 1.0 resulting in an initial COD inlet of 1472 ± 17 mg L−1. The batch was replenished with fresh media when the MFC voltage was under 0.1 V to initiate a new cycle MFCs performance in terms of the power generation and substrate degradation was evaluated using the previously reported methods.
Analytics and computations of biocathode
During the MFCs experiments, the produced cell potentials data between the anode and the cathode were gathered via a multimeter (Lab jack U6-PRO) connected to a laptop by a personal data acquisition system. The multi-cycle technique was used to achieve maximum MFC power densities. The reactors were operated with an external circuit linked through 1000 Ω. The observed voltage was converted to current using the relationship I = V/R, where I is the current generated by an applied potential (V) calculated at a definite external resistance, R is 1000 Ω loaded to the cell. Accordingly, the polarization and power curves were plotted using the single cycle technique by recording the pseudo-steady-state voltage peak by reducing the applied external resistance (Rext) over a range from 10 MΩ to 330 Ω in reducing order stepwise after the MFC was operated stably. Power density (P, mW m−3) was normalized by the total liquid volume of the MFC.
After stabilization of the open circuit potential (OCP), the polarization was plotted at zero current and the power curves were calculated using Joule’s law. The internal resistance (Rint) was determined using a linear regression related to the ohmic zone on the linear part of the polarization curve [22]. COD concentrations of the influent and effluent were calculated and analyzed according to the APHA procedure. The organic substance concentration is measured as the COD removal efficiency (COD %) using the following equation: COD% = (CODinitial–CODfinal)/CODinitial × 100) [23]. The Coulombic efficiency (CE) was determined by incorporating the calculated current with the theoretical current based on COD consuming, CE (%) = (CP/CT) × 100. Where CT is the theoretical amount of coulombs production. CP is the actual current production collected by the anode during one batch cycle. Anode and cathode potentials were evaluated at room temperature (25 ± 1 °C) using the Voltamaster 6 potentiostat (PST006). Prior to measurements, MFCs was allowed to equilibrate until the open circuit voltage stabilized.
Biofilm composition analysis
Attachment assay
At the end of the MFC cycle, the anode and cathode biofilm electrodes were carefully removed from the BSCMFC configuration. To minimize the excess matters, the electrodes were gently washed with sterile distilled water, followed by washing with 0.1 M phosphate buffer saline (PBS, pH 7.0). Biocatalyst density on electrode surfaces was assessed by crystal violet assay. Electrodes were stained for a few seconds with a 1% (w/v) crystal violet stain and solubilized with 70% ethanol. This dye-ethanol solution was quantified at OD580. The generated OD value is directly proportional to biofilm thickness [24]. The fresh electrode was used as a cell attachment assay control.
EPS analysis
For proteins and carbohydrates quantification, the biofilm formed by the microbes on the electrodes was analyzed. Using a sterilized scalpel, the biofilm electrodes were cut into dimensions of 1 × 1 cm2. With 0.1 M PBS, the anode and cathode biofilms were washed twice and collected by vortexing then scraping in 1 mL 0.01 M KCl solution. Afterward, the biofilms were centrifuged for 20 min (3000 rpm, 4 °C) after sonication, and the supernatant was collected and filtered via a 0.22 µm membrane filter for EPS analysis. The phenol–sulfuric acid [25] and the Lowry methods [26] were used to quantify the amount of carbohydrate and protein, respectively.
Biofilm characterization
FTIR analysis
To confirm the formation of biofilms, samples were prepared by oven drying at 120 °C overnight to remove water on the anodic and cathodic electrodes, followed by grinding the scratched biofilm layer with dry KBr. The powders were analyzed in the Bruker Alpha 11 and recorded on an FTIR spectrophotometer in the region from 400 to 4000 cm−1.
SEM analysis
The anodic and the cathodic biofilm surfaces were characterized by SEM (JEOL, JXA-840A) for determining the biofilm formation after 72 days of microbial culture incubation in the BSCMFC. The electrodes were fixed with 2.5% glutaraldehyde, then washed with water three times and dehydrated for 10 min by successively immersing them in ethanol solutions (from 30 to 100%). The specimens were dried, mounted onto specimen stubs using graphite paste, and then covered with gold.
Analysis of microbial cultures
Isolation of electroactive
The biofilms were removed from the BSCMFC at the end of operation cycles to enumerate the electroactive bacterial species. The biofilms were washed with PBS, placed in tubes containing PBS, and the bacterial cells removal was performed by sonication. The PBS with the detached bacterial cells was serially diluted and the appropriate dilutions were poured plated on Luria–Bertani (LB) agar plates followed by incubation at 37 °C for 48 h. The plates from the anode and cathode were incubated anaerobically and aerobically, respectively. After incubation, morphologically dissimilar colonies were randomly selected from all plates and purified by streaking.
Assessment of biofilm formation
The isolated bacteria were tested for biofilm production according to the previously described method [27], in 96-well microtiter plates. The cell density per well was adjusted to be 1 × 106 CFU mL−1 in 200 μL LB medium. After incubation (37 °C, 48 h), the wells were emptied and filled with 200 μL of crystal violet solution (2% v/v). After 5 min, the plates were emptied, washed three times with distilled water and the dye was extracted by ethanol addition (300 μL). By a microplate reader, the OD600 was registered.
16SrRNA gene sequencing
Anodic and cathodic single colonies were grown in LB broth medium and incubated at 37 °C for 48 h. Afterward, the bacterial cultures were sent to the biotechnology Lab. (Fac. of Agriculture, Cairo University Research Park, FA-CURP, Egypt) for DNA extraction and PCR amplification. The DNA quantity and purity were measured spectrophotometrically (characterized by A260/A280 nm absorbance ratio). The universal primers used for the PCR amplification were 27 forward primers (5'AGAGTTTGATCCTGGCTCAG3') and 1492 reverse primers (5'GGYTACCTTGTTACGACTT3'). The amplified genes solution was subjected to agarose gel (1%) electrophoresis, purified and sequenced (MACROGEN sequencing company, Korea). PCR products were directly sequenced in one direction using the respective forward and reverse primers. The obtained nucleotide sequences were aligned using the Basic Local Alignment Search Tool (BLAST) and compared with the sequences in the NCBI database. A phylogenetic tree was constructed using the neighbor-joining model of the MEGA X program. The identified isolates were classified morphologically and biochemically using a standard procedures based on Bergey’s manual.
Statistical analysis
Using the Microsoft Excel 2016 version, statistical analysis was carried out. All parameters, calculations, means, and standard deviations were involved in data analysis.
Results and discussion
MFC performance during startup, acclimation, and bioelectricity generation
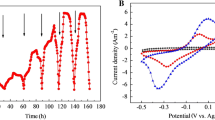
The open circuit voltage (OCV) changed with time when inoculated with aerobic activated sludge after 3 months’ long-term activity of continuous cultivation (Fig. 1a). At first, the maximum OCV was 363 mV (0–7.5 days), then followed by a rapid decrease in OCVs for 2 weeks to approximately 60 mV due to the microbial activity and an increase in substrate consumption. The slow speed of electricity generation can be associated with the lag time of bacterial cell formation on the anode surface at the initial stage of MFC activity [28]. Then, the MFC was inoculated with fresh media to regain the microbial activity for starting the 2nd cycle. These cycles took about (7.5–13 days) for increasing the voltage to 585 mV followed by decreasing the voltage gradually according to decreasing the acetate concentration in the media leading to start the third refreshment cycle. During the 3rd cycle (13–50 days), the average voltage output was 755 mV. This result can be due to the development of an electrode biofilm that facilitated the transport of electrons and improved voltage production. The BSCMFC was run for three consecutive rounds and fresh media were introduced to reactivate the microorganisms and promote the growth of anodic biofilm [29]. Therefore, the OCV cycles were indicative of the successful biofilm formation.
Figure 1b shows the effect of 1 kΩ on the MFC outputs at an initial sodium acetate concentration of 2 gL−1 under fed-batch mode after cultivating 3 months using the multi-cycle technique. The voltage was initially dropped from 755 mV in OCV to be 259 mV in closed circuit voltage (CCV) which dropped into 50 mV after 112 h. The voltage output of the BSCMFC exhibiting a relatively stable electricity production with the voltage output fluctuating around 282 ± 6.6 mV in each batch after 300 h. These results could be accomplished that the progression of mature microbial communities on both anodic and cathodic electrodes [30]. In addition, the generation of electricity from BSCMFC also indicated the successful reduction process of oxygen that carried out by the formed bioelectrocatalysts on the cathode [31]. Also, the voltage values can be due to the biofilm development on the electrode surfaces during all three operating cycles in both OCV and CCV modes, as well as the bacterial content capacity to generate and transfer the electrons to the anode surface without chemical mediators.
In this stage, the individual electrode potentials of the anode and cathode were kept at a constant level and collected to check the BSCMFC’s performance. At steady state, Fig. 1c recorded the biocathodic and bioanodic potentials, as well as the performance of the microbial fuel cell. In comparison to Ag/AgCl, the anode and cathode potentials were at 360 and 173 mV, respectively. As a result, the corresponding OCP vs. Ag/AgCl was around 533 mV, and the performance of the microbial fuel cell was greatly improved. The variations in the current generation were found to be due to variances in both cathode and anode potential, according to an analysis of the electrode potential curves. Thus, it was essential to develop bioanode and biocathode for efficient BSCMFC.
The COD removal in a medium of initial COD (1472 ± 17 mg L−1) increased in the 1st cycle to be 89% and reached to be 72% in the 3rd cycle. In the mature MFC during the long-term operation, the COD removal ratio was 83 ± 8.4%. The COD removal ratio was 83 ± 8.4% which was in line with previously recorded results [20]. They showed that at the end of the MFC operation period, the percentage of COD removal of acetate fed MFCS reached 96%. The difference in COD removal efficiency values could be explained by the various microbial sources (inoculum), the microbial population structures, electrode types, configuration of MFCs, and microbial activity.
Coulombic efficiency (CE), referred to the overall charge ratio transmitted to the anode over the maximum extractible charge after full oxidation of the electricity-converted substrate [32], was 22 ± 2.5% and decreased slowly to 13 ± 5% within 10d in the 4th cycle.
In the case of BSCMFC under fed-batch mode, the anodic biofilm reduced the organic substrate anaerobically into protons and electrons. The organic substrate was converted anaerobically to protons and electrons by the anodic biofilm. The anodic products are then transported into a biocathodic biofilm that may aerobically oxidize oxygen (electron acceptor) into water. After standard anaerobic anodic treatment (i.e. the excess COD from the anode is transferred into the biocathode, which led to significant growth of aerobic heterotrophs with the ability to remove slowly biodegradable COD in aerobic biocathodes), high COD removal efficiency occurred as a further aerobic cathodic step. [33]. According to Haung et al. [34], the optimum output of energy production is determined by the COD initial level concentration. When the substrate COD concentration was increased, metabolic reaction rates quickened, which could aid to improve MFC efficiency [35]. It can be concluded that the sludge was an effective substrate for electroactive. Furthermore, the bacterial composition in wastewater treatment would be responsible for biodegradability [36].
To assess the performances of the BSCMFC, the power curve and the polarization tests should be achieved after 60 days of stable phase at the end of the operation to evaluate the contribution of the biocathode performance (Fig. 2). The recorded results showed that the power density raised with lowering of the external resistance from 10 MΩ to 330 Ω; a maximum power density of 593 mW m−3 with the external resistance of 1 kΩ at a maximum current density of 26,154 mA m−3, and the corresponding cell voltage was 244 mV. The internal resistance of the BSCMFC could be determined to be 195 Ω from the linear fitting of polarization curves. The polarization curve showed that the power was generated by the system considers the principal objective of BSCMFC. In addition, Cai et al. [37] showed that lower external resistance was beneficial for electrogenic bacterial growth and improved MFC efficiency by investigating the impact of external resistance on biofilms. This study reported that the enrichment of microorganisms in the cathode electrode worked as a source of an electroactive catalyst layer during the ORR, so that the aerobic biocathode can help in the improvement of overall BSCMFC electricity output [38]. The findings were compared and indexed in Table 1 with other preceding researches. Xu et al. [39] reported that the power density of SCMFC was 1056 ± 110 mW m−2 when using a carbon brush that has a high surface area. On contrary, Yang and his colleagues produced a power density of 0.104 mW m−3 which was lower than our result [40]. The previous findings varied significantly according to the volume and reactor form, the microorganism's source, and the materials used.
EPS characterization of biofilms for anodic and cathodic electrodes
At the end of the BSCMFC operation (72 days), the absorbance of the bioanode and biocathode were evaluated to prove the significant growth of microbial biomass on the electrode surfaces. It could be observed that a higher biofilm thickness was observed on the cathode surface (1.422 OD) than that observed on the anode surface (0.952 OD). In addition, the content and composition of EPS are of great importance to the formation of these bacterial biofilms and related to their performance in BSCMFC, because it supports bacterial surface adhesion and cell aggregation. The polysaccharides and proteins contents in the EPS extracted from biocathodic and bioanodic biofilms were quantified. As shown in Fig. 3, The EPS protein content in the biocathode was 0.255 mg protein cm−2 and with polysaccharides content of 0.347 mg cm−2, while in the case of anodic biofilm EPS protein content was measured to be 0.176 mg protein cm−2 with polysaccharides content (0.229 mg cm−2). Thus, the EPS composition as determined from the polysaccharide/protein ratios to be 1.36 and 1.30 for cathodic and anodic biofilms, respectively. It was revealed that the EPS obtained from the aerobic enriched culture had a higher polysaccharide content than the protein level [49]. This implies the presence of an aerobic population on the cathode electrode's surface. This result is consistent with prior research using nitration reactors. According to Jemaat et al. [50], polysaccharides were the most abundant EPS constituent in the aerobic ammonia-oxidizing bacteria-enriched granules, with polysaccharide/protein ratios frequently more than two [50]. Furthermore, in contrast to earlier research, the EPS of the anaerobic-enriched culture included significantly more polysaccharide in the bioanode, Jiang et al. [51] reported high protein/polysaccharide ratios of EPS from anaerobic ammonia-oxidizing bacteria-dominated sludge. The different methods utilized to extract EPS and the varying protocols used to qualify polysaccharides and proteins could responsible for the inconsistent findings of several studies [52]. As a result, we can deduce that proteins are a prominent ingredient in anaerobic EPS and that proteins are likely to be responsible for the production of anodic aggregates; on the other hand, the polysaccharide fraction may be a crucial factor in aerobic cathode aggregates.
The biofilm includes polysaccharides, proteins, peptidoglycan, lipids, and nucleic acids. There was a link between the EPS concentration and the biofilm growth [53, 54]. The higher the secretion of EPS by the organism, the greater the biofilm density. More organism number is contained in a denser biofilm than in a lighter one. Therefore, the biofilm density increases relating to the cells age [55]. In conclusion, EPS has an important effect on the bioelectrochemical properties of the electrode surfaces and on biofilm adhesion the BSCMFC.
It is widely known that the protein content of microbial aggregates has a considerably stronger relationship with surface properties such as hydrophobicity and surface charge than the polysaccharide content [56]. While polysaccharide content might supply bacterial cells with a multitude of binding sites, it can also have a significant impact on cell aggregation. In the presence of multivalent ions, the impact of polysaccharides in cell aggregation can be considerably enhanced due to the availability of functional groups such as carboxylates [57].
FTIR analysis
The functional groups derived from proteins, polysaccharides, and amino acids that are involved in EPS were conducted presented using FTIR analysis spectra as indicated in Fig. 4. The 3900–400 cm−1 region provides the main information regarding the compositions and functionalities of the EPS constituents. The broad band's observed at 3280 and 3267 cm−1 are due to the functional groups of O–H and N–H, which can form hydrogen bonds with the carbonyl group of protein peptide bonds [58]. The peaks at 2925, 2919, and 2853 cm−1 can be attributed to the functional groups of membrane fatty acids and also to certain side chain amino acid vibrations, as the characteristic C–H stretching vibrations of the functional groups –CH3 and =CH2 dominate [59]. The observed band at 1632 cm−1 corresponds to the primary amide band (1630–1655 cm−1) that is associated with the C=O stretching vibrations of peptide groups in proteins Furthermore, the band observed in 1030 and 1009 cm−1 is attributable to the symmetric stretching vibration of PO2 groups in nucleic acid and to C–O–C and C–O–P stretching [60], likely this originating from the damage of bacterial cells during the extraction of EPS. Naumann [60] reported that the presence of carbohydrates and polysaccharides (1200 and 900 cm−1) in the cell wall influenced the nucleic acids. The peaks in 676 cm−1 indicated the glycosidic linkage exist between the glycosyl groups [61]. These findings showed the presence of EPS matrix on the electrode surfaces.
SEM analysis
Bioanode and biocathode surfaces were visualized to determine the relationship between the above-mentioned electrochemical analysis and biofilm coverage. A naked eye revealed that there is a dense rough layer of electroactive on carbon felt and carbon cloth surfaces. Figure 5a, b indicate the fresh structure of the electrodes at beginning of the experiment, while Fig. 4c, d depicts the SEM images of the bioanode and biocathode, respectively. It could be observed that the anodic biofilm had a partial and heterogeneous structure with two morphological distinct rod-shaped cells, which was similar to [62]. The morphology variation is assumed to be mainly caused due to the substrate feeding and electrogens, which can either acclimate and colonize with the anodic electrode by secreting matrix or connected together with thick layers of exopolysaccharide using pilus like structures [63]. While SEM image for the biocathode showed that the carbon cloth based biofilm loaded with the dense microbial consortium (Fig. 5d). It can be concluded that the biofilm was intended to consist of aerobes, which acted as a living membrane that prevented or minimized oxygen penetration into the anode. Hence, it can undergo the reduction process of O2 on the surface of cathode electrode with enhancement of BSCMFC [64]. Moreover, the carbon cloth had a fully accessible surface for microorganisms with a particular surface area and these colonized microorganisms effectively increased the COD reduction rate and the power production. Therefore, the microbial community in both electrodes supported cooperation between the anaerobic acetate oxidation process on the bioanode and aerobic oxygen reduction on the biocathode for electricity production [65].
Analysis of microbial cultures
The electroactive bacteria were isolated from the biocathode and bioanode in BSCMFC. The morphologically different isolates were obtained from the biofilm formed on electrodes. Twenty-one isolates were purified.
Biofilm ability
There was limited knowledge of the biofilm formation of the isolates and their significance in bioelectricity production. Table 2 shows that 90.48% and 9.52% of the isolates were strong and moderate biofilm formers, respectively. This result was contradictory with Zafar et al. [66], who reported that 5% of the anodic isolates were strong and 15% were moderate biofilm formers. With the EPS help, biofilm forming bacteria can highly organize themselves on the electrode surfaces and contribute to greater electricity generation in MFCs [67]. The isolated strains would offer the ability to degrade organic substances effectively and to generate electricity in the MFC system.
Molecular analysis
The 16SrRNA gene sequences for the 13 isolates that had high biofilm formers were performed to classify the compositional difference between the anodic and cathodic bacterial populations. The absorbance ratio of the extracted DNA samples was equal or above 1.8 (Table 2). The agarose gel showed the reproducible PCR bands were considered as PCR positive, while isolates of negative PCR findings were regarded as PCR negative. Furthermore, eight isolates with very faint bands were overlooked (Fig. 6a), but the others with reproducible PCR products were amplified and sequenced.
The bioanodic diversity was dominated by Proteobacteria phylum (42.86%), accompanied by phyla Firmicutes (28.57%), Bacteroidetes (14.29%), and Actinobacteria (14.29%) (Fig. 6b) with seven strains: Kocuria polaris, Comamonas nitrativorans, Alcaligenes faecalis, Flavobacterium lindanitolerans, Bacillus wiedmannii, Bacillus safensis, and Pseudomonas pseudoalcaligenes (Table 3). The majority of these species were facultative anaerobes; this may be consistent with the fact that biofilm strains are limited to species capable of transporting electrons [68]. While cathodic diversity was dominant only by one phylum known as Proteobacteria with six strains: Pseudomonas putida, 2 Pseudomonas monteilii, Pseudomonas plecoglossicida, Achromobacter aegrifaciens, and Castellaniella denitrificans. Table 3 demonstrates the electroactive identification and their accession numbers (MN538880 to MN538892). Some identified percentages of bacteria were found to be 96.74, 96.41, 96.25, 96.14, 95.56, 93.89, 92.99, and 90.82%, suggesting that the electroactives were novel in our MFC. A phylogenetic dendrogram was constructed to clarify the genetic relationship between the electroactives based on 16SrRNA (Fig. 7).
The microbial composition demonstrated the synergistic activity of various functional groups in the BSCMFC to convert acetate into bioelectricity. Bio-electroactives play a role in the electrons transfer by outer membrane cytochromes, pili conductivity, and extracellular excretion between the biofilms and the anolyte electrode [69]. In previous studies, some of our species were known as electroactive. Bacillus sp. known to transmit the electrons into the anode through soluble redox-active mediators [70] could be used for bioremediation of sugar industry wastewater and electricity production [71]. Clearly, no MFC had been recorded for Kocuria, but Comamonas was previously reported [72]. The microbial electrochemical systems had been previously associated with members of the Flavobacterium genus, but their role remains unknown for its large metabolic ability in current production [73].
Alcaligenes genus isolated from the mixed consortium in an MFC [74] participates in the heavy metal intracellular decrease in the MFC cathode chamber [75]. These strains have the unique ability to metabolize ammonium and have nanowires for the possible delivery of electrons to electrode surfaces externally [76]. Pseudomonas is another identified bioanode, which can also produce redox mediators which not only enhance its capacity for electrical generation but also provide electron transfer mediators for other microorganisms [72]. In the cathode, Pseudomonas can electrochemically reduce oxygen [77]. P. putida could be treated the oil refinery wastewater and generate an electric current in an air–cathode chamber [78]. Various species, such as P. aeruginosa, P. fluorescens, B. subtilis, Shewanella putrefaciens, and other various species had been documented for cathodic oxygen reduction to water [79]. Further study opens the way for understanding the role of Castellaniella and Achromobacter in current electrosynthesis production.
Over the numerous mixed-species biocathode, several electrotrophs with different metabolic and respiratory capacities remained to be identified [80]. Thus, the exoelectrogens abundance and certain synergetic bacteria could enhance the anodic biofilm oxidation and the oxygen reduction by biocathode without the aid of artificial redox mediators or other catalysts coated on the cathode surface. Based on the 16SrRNA sequence, some genera were novel that requiring further identification and application in MFC. Biofilms can help us unveiling new electrogenic microorganisms and improve our understanding of their role in the functioning of BSCMFC.
Biochemical characterization
Table 4 shows the microscopic characteristics, biochemical and sugar fermentation tests for the electroactive. Among these, ten strains were gram negative rods while, the other three strains were gram positive rods and cocci. All electroactive were non-spore former except B. wiedmannii and B. safensis. The biochemical features revealed significant changes in metabolic capacities. All strains tested for catalase were positive meaning that the electroactives were strict aerobes or facultative anaerobes and most of them were motile. All strains except P. pseudoalcaligenes were indole negative. Using the methyl red test, B. wiedmannii demonstrated positive acid fermentation. A. faecalis, B. wiedmannii, and B. safensis were Voges Proskauer positive. Eight strains were positive for simmons citrate, while the remaining five strains showed no change.
Furthermore, P. putida and A. aegrifaciens were H2S positive and demonstrated that they could minimize their sulfur compound to sulfides, whereas the others were H2S negative. In addition, six strains were nitrate positive indicated that these strains could remove nitrogen compounds from wastewater and the others could not reduce NO3. A. aegrifaciens was urease positive that could degrade the urea and cleaned up waste products into the ammonia. This strain could, therefore, also be used for wastewater treatment with high ammonia content. Additionally, only B. wiedmannii and B. safensis was able to use the extracellular development of gelatinase enzymes in polypeptides and amino acids. Based on the lipid hydrolysis, two strains had shown positive lipase enzyme, which could be used in bioremediation as an indication of lipolytic activity.
K. polaris, A. faecalis, and B. wiedmannii were positive for starch hydrolysis. Table 4 reveals that four strains had sugar fermentation capacities. It implied that these electroactive did not specifically participate in the pathway of electrode reduction and they were rather breaking down complex sources of carbon that supplied simpler substrates with non-fermenting bacteria. Our results were consistent with Vejarano et al. [81] and Kim et al. [82]; they showed that electrochemically active bacteria had been restricted to simple organic acids as electron donors and based on fermentation products.
Conclusion
This study demonstrated that the located biofilm on the surface of the cathode enhanced the current response (2615 mA m−3) of the BSCMFC and power generation (593 mW m−3) with the aid of aerobic exoelectrogens. These exoelectrogens can reduce oxygen without the aid of exogenous mediators. Also, most the electrogenic bacteria were strongly biofilm former in vitro. The 16SrRNA sequencing technology is successfully applied to analyze the microbial community for both bioanode and biocathode. Biofilms can help us unveiling new electrogenic microorganisms and improve our understanding of their role in the functioning of BSCMFC.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Xu H, Wang L, Lin C et al (2020) Improved simultaneous decolorization and power generation in a microbial fuel cell with the sponge anode modified by polyaniline and chitosan. Appl Biochem Biotechnol 192:698–718. https://doi.org/10.1007/s12010-020-03346-2
Xiao B, Yang F, Liu J (2011) Enhancing simultaneous electricity production and reduction of sewage sludge in two-chamber MFC by aerobic sludge digestion and sludge pretreatments. J Hazard Mater 189:444–449. https://doi.org/10.1016/j.jhazmat.2011.02.058
Xin X, Chen B-Y, Hong J (2019) Unraveling interactive characteristics of microbial community associated with bioelectric energy production in sludge fermentation fluid-fed microbial fuel cells. Bioresour Technol 289:121652. https://doi.org/10.1016/j.biortech.2019.121652
Lovley DR, Nevin KP (2011) A shift in the current: new applications and concepts for microbe-electrode electron exchange. Curr Opin Biotechnol 22:441–448. https://doi.org/10.1016/j.copbio.2011.01.009
Chandrasekhar K (2019) Effective and nonprecious cathode catalysts for oxygen reduction reaction in microbial fuel cells. In: Microbial Electrochemical Technology, p 485–501. https://doi.org/10.1016/B978-0-444-64052-9.00019-4
Kakaei K, Esrafili MD, Ehsani A (2019) Oxygen reduction reaction. In: Interface Science and Technology, vol 27. p 203–252. https://doi.org/10.1016/B978-0-12-814523-4.00006-X
Song H-L, Zhu Y, Li J (2019) Electron transfer mechanisms, characteristics and applications of biological cathode microbial fuel cells–a mini review. Arab J Chem 12:2236–2243. https://doi.org/10.1016/j.arabjc.2015.01.008
Milner EM, Popescu D, Curtis T et al (2016) Microbial fuel cells with highly active aerobic biocathodes. J Power Sour 324:8–16. https://doi.org/10.1016/j.jpowsour.2016.05.055
Du Y, Feng Y, Dong Y, Qu Y, Liu J, ZX& RN, (2014) Coupling interaction of cathodic reduction and microbial metabolism in aerobic biocathode of microbial fuel cell. RSC Adv 4:34350–34355. https://doi.org/10.1039/c4ra03441d
Rothballer M, Picot M, Sieper T et al (2015) Monophyletic group of unclassified $γ$-Proteobacteria dominates in mixed culture biofilm of high-performing oxygen reducing biocathode. Bioelectrochemistry 106:167–176. https://doi.org/10.1016/j.bioelechem.2015.04.004
Wang Z, Zheng Y, Xiao Y et al (2013) Analysis of oxygen reduction and microbial community of air-diffusion biocathode in microbial fuel cells. Bioresour Technol 144:74–79. https://doi.org/10.1016/j.biortech.2013.06.093
Zakaria BS, Dhar BR (2020) Changes in syntrophic microbial communities, EPS matrix, and gene-expression patterns in biofilm anode in response to silver nanoparticles exposure. Sci Total Environ 734:139395. https://doi.org/10.1016/j.scitotenv.2020.139395
Tan B, Zhou S, Wang Y et al (2019) Molecular insight into electron transfer properties of extracellular polymeric substances of electroactive bacteria by surface-enhanced Raman spectroscopy. Sci China Technol Sci 62:1679–1687. https://doi.org/10.1007/s11431-018-9437-0
Stöckl M, Teubner NC, Holtmann D et al (2019) Extracellular polymeric substances from Geobacter sulfurreducens biofilms in microbial fuel cells. ACS Appl Mater Interfaces 11:8961–8968. https://doi.org/10.1021/acsami.8b14340
Xiao Y, Zhao F (2017) Electrochemical roles of extracellular polymeric substances in biofilms. Curr Opin Electrochem 4:206–211. https://doi.org/10.1016/j.coelec.2017.09.016
Pandey P, Shinde VN, Deopurkar RL et al (2016) Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl Energy 168:706–723. https://doi.org/10.1016/j.apenergy.2016.01.056
Vilajeliu-Pons A, Puig S, Pous N et al (2015) Microbiome characterization of MFCs used for the treatment of swine manure. J Hazard Mater 288:60–68. https://doi.org/10.1016/j.jhazmat.2015.02.014
Rittman BE, Krajmalnik-Brown R, Halden RU (2008) Pregenomic, genomic and post-genomic study of microbial communities involved in bioenergy. Nat Rev Microbiol 6:604–612. https://doi.org/10.1038/nrmicro1939
Wittebolle L, Vooren NV, Verstraete W, Boon N (2009) High reproducibility of ammonia-oxidizing bacterial communities in parallel sequential batch reactors. J Appl Microbiol 107:385–394
Khater DZ, El-Khatib KM, Hassan HM (2017) Microbial diversity structure in acetate single chamber microbial fuel cell for electricity generation. J Genet Eng Biotechnol. https://doi.org/10.1016/j.jgeb.2017.01.008
Lee K, Zhang L, Lui H et al (2009) Oxygen reduction reaction (ORR) catalyzed by carbon-supported cobalt polypyrrole (Co-PPy/C) electrocatalysts. Electrochim Acta 54:4704–4711. https://doi.org/10.1016/j.electacta.2009.03.081
Picoreanu C, Head IM, Katuri KP et al (2007) Computational model for biofim-based microbial fuel cells. Water Res 41:2921–2940. https://doi.org/10.1016/j.watres.2007.04.009
Andrew DE, Clescenri LS, Breenberg AE (1995) Standard method for the examination of water and wastewater, 19th edn. APHA, AWW, WEF , Washington
Reguera G, McCarthy KD, Mehta T et al (2005) Extracellular electron transfer via microbial nanowires. Nature 435:1098–1101. https://doi.org/10.1038/nature03661
Dubois M, Gilles KA, Hamilton JK et al (1956) Phenol sulphuric acid method for total carbohydrate. Anal Chem 8:350–356. https://doi.org/10.1021/ac60111a017
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Borges S, Silva J, Teixeira P (2012) Survival and biofilm formation by Group B streptococci in simulated vaginal fluid at different pHs. Antonie Van Leeuwenhoek 101:677–682. https://doi.org/10.1007/s10482-011-9666-y
Richter H, McCarthy K, Nevin KP et al (2008) Electricity generation by Geobacter sulfurreducens attached to gold electrodes. Langmuir 24:4376–4379. https://doi.org/10.1021/la703469y
Kamel MS, Abd-Alla MH, Abdul-Raouf UM et al (2019) Characterization of anodic biofilm bacterial communities and performance evaluation of a mediator-free microbial fuel cell. Environ Eng Res 25:862–870. https://doi.org/10.4491/eer.2019.412
Zhang G, Zhang H, Zhang C et al (2013) Simultaneous nitrogen and carbon removal in a single chamber microbial fuel cell with a rotating biocathode. Process Biochem 48:893–900. https://doi.org/10.1016/j.procbio.2013.03.008
Bergel A, Féron D, Mollica A (2005) Catalysis of oxygen reduction in PEM fuel cell by seawater biofilm. Electrochem commun 7:900–904. https://doi.org/10.1016/j.elecom.2005.06.006
Sharma Y, Li B (2010) Optimizing energy harvest in wastewater treatment by combining anaerobic hydrogen producing biofermentor (HPB) and microbial fuel cell (MFC). Int J Hydrogen Energy 35:3789–3797. https://doi.org/10.1016/j.ijhydene.2010.01.042
Freguia S, Rabaey K, Yuan Z, Keller J (2008) Sequential anode–cathode configuration improves cathodic oxygen reduction and effluent quality of microbial fuel cells. Water Res 42:1387–1396. https://doi.org/10.1016/j.watres.2007.10.007
Huang L, Logan BE (2008) Electricity generation and treatment of paper recycling wastewater using a microbial fuel cell. Appl Microbiol Biotechnol 80:349–355. https://doi.org/10.1007/s00253-008-1546-7
Samsudeen N, Radhakrishnan TK, Matheswaran M (2015) Bioelectricity production from microbial fuel cell using mixed bacterial culture isolated from distillery wastewater. Bioresour Technol 195:242–247. https://doi.org/10.1016/j.biortech.2015.07.023
Ibarbalz FM, Figuerola ELM, Erijman L (2013) Industrial activated sludge exhibit unique bacterial community composition at high taxonomic ranks. Water Res 47:3854–3864. https://doi.org/10.1016/j.watres.2013.04.010
Cai W-F, Geng J-F, Pu K-B et al (2018) Investigation of a two-dimensional model on microbial fuel cell with different biofilm porosities and external resistances. Chem Eng J 333:572–582. https://doi.org/10.1016/j.cej.2017.09.189
Sun J, Bi Z, Hou B et al (2010) Further treatment of decolorization liquid of azo dye coupled with increased power production using microbial fuel cell equipped with an aerobic biocathode. Water Res 45:283–291. https://doi.org/10.1016/j.watres.2010.07.059
Xu G, Zheng X, Lu Y et al (2019) Development of microbial community within the cathodic biofilm of single-chamber air-cathode microbial fuel cell. Sci Total Environ 665:641–648. https://doi.org/10.1016/j.scitotenv.2019.02.175
Yang N, Zhan G, Wu T et al (2018) Effect of air-exposed biocathode on the performance of a Thauera-dominated membraneless single-chamber microbial fuel cell (SCMFC). J Environ Sci 66:216–224. https://doi.org/10.1016/j.jes.2017.05.013
Xu G, Zheng X, Lu Y et al (2019) Development of microbial community within the cathodic bio fi lm of single-chamber air-cathode microbial fuel cell. Sci Total Environ 665:641–648. https://doi.org/10.1016/j.scitotenv.2019.02.175
Gao C, Wang A, Wu W et al (2014) Enrichment of anodic biofilm inoculated with anaerobic or aerobic sludge in single chambered air-cathode microbial fuel cells. Bioresour Technol 167:124–132. https://doi.org/10.1016/j.biortech.2014.05.120
Kalathil S, Lee J, Cho MH (2012) Efficient decolorization of real dye wastewater and bioelectricity generation using a novel single chamber biocathode-microbial fuel cell. Bioresour Technol 119:22–27. https://doi.org/10.1016/j.biortech.2012.05.059
Yan H, Saito T, Regan JM (2012) Nitrogen removal in a single-chamber microbial fuel cell with nitrifying biofilm enriched at the air cathode. Water Res 46:2215–2224. https://doi.org/10.1016/j.watres.2012.01.050
Zhang G, Zhao Q, Jiao Y et al (2012) Biocathode microbial fuel cell for efficient electricity recovery from dairy manure. Biosens Bioelectron 31:537–543. https://doi.org/10.1016/j.bios.2011.11.036
Zhang G, Zhao Q, Jiao Y et al (2012) Efficient electricity generation from sewage sludge using biocathode microbial fuel cell. Water Res 46:43–52. https://doi.org/10.1016/j.watres.2011.10.036
Chen G, Choi S, Lee T et al (2008) Application of biocathode in microbial fuel cells : cell performance and microbial community. Appl Microbiol Biotechnol 79:379–388. https://doi.org/10.1007/s00253-008-1451-0
Zhang G, Zhao Q, Jiao Y et al (2011) Improved performance of microbial fuel cell using combination biocathode of graphite fiber brush and graphite granules. J Power Sour 196:6036–6041. https://doi.org/10.1016/j.jpowsour.2011.03.096
Shen L, Yao Y, Meng F (2014) Reactor performance and microbial ecology of a nitritation membrane bioreactor. J Memb Sci 462:139–146. https://doi.org/10.1016/j.memsci.2014.03.034
Jemaat Z, Suárez-Ojeda ME, Pérez J, Carrera J (2014) Partial nitritation and o-cresol removal with aerobic granular biomass in a continuous airlift reactor. Water Res 48:354–362. https://doi.org/10.1016/j.watres.2013.09.048
Jiang T, Zhang H, Qiang H et al (2013) Start-up of the anammox process and membrane fouling analysis in a novel rotating membrane bioreactor. Desalination 311:46–53. https://doi.org/10.1016/j.desal.2012.10.031
Chen Y-P, Li C, Guo J-S et al (2013) Extraction and characterization of extracellular polymeric substances in biofilm and sludge via completely autotrophic nitrogen removal over nitrite system. Appl Biochem Biotechnol 169:526–538. https://doi.org/10.1007/s12010-012-9996-x
Ahimou F, Semmens MJ, Haugstad G, Novak PJ (2007) Effect of protein, polysaccharide, and oxygen concentration profiles on biofilm cohesiveness. Appl Environ Microbiol 73:2905–2910. https://doi.org/10.1128/AEM.02420-06
Yuvraj C, Aranganathan V (2017) MFC—An Approach in Enhancing Electricity Generation Using Electroactive Biofilm of Dissimilatory Iron-Reducing (DIR) Bacteria. Arab J Sci Eng 42:2341–2347. https://doi.org/10.1007/s13369-017-2529-8
Sun D, Cheng S, Wang A et al (2015) Temporal-spatial changes in viabilities and electrochemical properties of anode biofilms. Environ Sci Technol 49:5227–5235. https://doi.org/10.1021/acs.est.5b00175
Jorand F, Boue-Bigne F, Block JC, Urbain V (1998) Hydrophobic/hydrophilic properties of activated sludge exopolymeric substances. Water Sci Technol 37:307–315. https://doi.org/10.1016/S0273-1223(98)00123-1
Yuan S-J, Sun M, Sheng G-P et al (2011) Identification of key constituents and structure of the extracellular polymeric substances excreted by Bacillus megaterium TF10 for their flocculation capacity. Environ Sci Technol 45:1152–1157. https://doi.org/10.1021/es1030905
Comte S, Guibaud G, Baudu M (2006) Biosorption properties of extracellular polymeric substances (EPS) resulting from activated sludge according to their type: soluble or bound. Process Biochem 41:815–823. https://doi.org/10.1016/j.procbio.2005.10.014
Nanda PK, Krishna Rao K, Nayak PL (2007) Biodegradable polymers. XI. Spectral, thermal, morphological, and biodegradability properties of environment-friendly green plastics of soy protein modified with thiosemicarbazide. J Appl Polym Sci 103:3134–3142. https://doi.org/10.1002/app.24590
Naumann D (2000) Encyclopedia of analytical chemistry. Wiley, Chichester, pp 102–131
Saif FAAA, Sakr EAE (2020) Characterization and bioactivities of exopolysaccharide produced from probiotic Lactobacillus plantarum 47FE and Lactobacillus pentosus 68FE. Bioact Carbohydr Diet Fibre. https://doi.org/10.1016/j.bcdf.2020.100231
Guo H, Xie S, Deng H et al (2020) Electricity production and bacterial communities of microbial fuel cell supplied with oily sludge. Environ Prog Sustain Energy. https://doi.org/10.1002/ep.13409
Abbas SZ, Rafatullah M, Ismail N, Shakoori FR (2018) Electrochemistry and microbiology of microbial fuel cells treating marine sediments polluted with heavy metals. RSC Adv 8:18800–18813. https://doi.org/10.1039/C8RA01711E
Chouler J, Bentley I, Vaz F et al (2017) Exploring the use of cost-effective membrane materials for Microbial Fuel Cell based sensors. Electrochim Acta 231:319–326. https://doi.org/10.1016/j.electacta.2017.01.195
Lincy MJA, Kumar BA, Vasantha VS, Varalakshmi P (2015) Microbial fuel cells: a promising alternative energy source. Oppor Chall. https://doi.org/10.1201/b18718-6
Zafar Z, Ayaz K, Sharafat I et al (2018) Enrichment of electricigenic biofilm for synchronized generation of electric current and waste water treatment in microbial fuel cells. Int J Electrochem Sci 13:4424–4437
Angelaalincy MJ, Navanietha Krishnaraj R, Shakambari G et al (2018) Biofilm engineering approaches for improving the performance of microbial fuel cells and bioelectrochemical systems. Front Energy Res 6:63. https://doi.org/10.3389/fenrg.2018.00063
Chae K-J, Choi M-J, Lee J-W et al (2009) Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour Technol 100:3518–3525. https://doi.org/10.1016/j.biortech.2009.02.065
Richter H, Nevin KP, Jia H et al (2009) Cyclic voltammetry of biofilms of wild type and mutant Geobacter sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type IV pili, and protons in extracellular electron transfer. Energy Environ Sci 2:506–516. https://doi.org/10.1039/B816647A
Nimje VR, Chen C-Y, Chen C-C et al (2009) Stable and high energy generation by a strain of Bacillus subtilis in a microbial fuel cell. J Power Sour 190:258–263. https://doi.org/10.1016/j.jpowsour.2009.01.019
Córdova-Bautista Y, Ramirez-Morales E, Perez-Hernandez B et al (2020) Electricity production and bioremediation from synthetic sugar industry wastewater by using microbial isolate in microbial fuel cell. Sugar Tech 22:820–829. https://doi.org/10.1007/s12355-020-00830-1
Rabaey K, Boon N, Hofte M, Verstraete W (2005) Microbial phenazine production enhances electron transfer in biofuel cells. Env Sci Technol 39:3401–3408. https://doi.org/10.1021/es048563o
Uria N, Ferrera I, Mas J (2017) Electrochemical performance and microbial community profiles in microbial fuel cells in relation to electron transfer mechanisms. BMC Microbiol 17(208):1–12. https://doi.org/10.1186/s12866-017-1115-2
Rabaey K, Boon N, Siciliano SD, Verhaege M, Vestraete W (2004) Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl Env Microbiol 70:5373–5382. https://doi.org/10.1128/AEM.70.9.5373-5382.2004
Thatoi H, Das S, Mishra J et al (2014) Bacterial chromate reductase, a potential enzyme for bioremediation of hexavalent chromium: a review. J Environ Manage 146:383–399. https://doi.org/10.1016/j.jenvman.2014.07.014
Wang X, Yu P, Zeng C et al (2015) Enhanced denitrification rate of Alcaligenes faecalis with electrode as electron donor. Appl Environ Microbiol. https://doi.org/10.1128/AEM.00683-15
Cournet A, Délia M-L, Bergel A et al (2010) Electrochemical reduction of oxygen catalyzed by a wide range of bacteria including Gram-positive. Electrochem commun 12:505–508. https://doi.org/10.1016/j.elecom.2010.01.026
Majumder D, Maity JP, Tseng M-J et al (2014) Electricity generation and wastewater treatment of oil refinery in microbial fuel cells using Pseudomonas putida. Int J Mol Sci 15:16772–16786. https://doi.org/10.3390/ijms150916772
Rosenbaum M, Aulenta F, Villano M, Angenent LT (2011) Cathodes as electron donors for microbial metabolism: which extracellular electron transfer mechanisms are involved? Bioresour Technol 102:324–333. https://doi.org/10.1016/j.biortech.2010.07.008
Cristiani P, Franzetti A, Gandolfi I et al (2013) Bacterial DGGE fingerprints of biofilms on electrodes of membraneless microbial fuel cells. Int Biodeterior Biodegrad 84:211–219. https://doi.org/10.1016/j.ibiod.2012.05.040
Vejarano F, Benitez-Campo N, Bravo E et al (2018) Electrochemical monitoring and microbial characterization of a domestic wastewater-fed microbial fuel cell inoculated with anaerobic sludge. Rev Ciencias 22:13–32
Kim IS, Chae K-J, Choi M-J, Verstraete W (2008) Microbial fuel cells: recent advances, bacterial communities and application beyond electricity generation. Environ Eng Res 13:51–65. https://doi.org/10.4491/eer.2008.13.2.051
Acknowledgments
The authors thank the financial support provided by the Public Administration of Environmental Projects—Applied research committee, Community Service and Environmental Development Sector, Ain shams university. The authors would like to thank the participants of the research theme from National Research Centre (NRC) for the fruitful discussions, research facilities, and their support.
Funding
The financial support provided by the Public Administration of Environmental Projects—Applied research committee, Community Service and Environmental Development Sector, Ain shams university.
Author information
Authors and Affiliations
Contributions
EAES, DZK, KME-k conceived and designed the study ES and DK performed the experiments. All the authors wrote the original draft preparation, validation, reviewing, and editing, read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Yes.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sakr, E.A.E., Khater, D.Z. & El-khatib, K.M. Anodic and cathodic biofilms coupled with electricity generation in single-chamber microbial fuel cell using activated sludge. Bioprocess Biosyst Eng 44, 2627–2643 (2021). https://doi.org/10.1007/s00449-021-02632-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02632-5