Abstract
In this study, a novel laboratory-scale synchronous enhanced biological phosphorus removal and semi-nitritation (termed as EBPR-SN) combined with anammox process was put forward for achieving nutrient elimination from municipal wastewater at 27 ℃. This process consisted of two 10 L sequencing batch reactors (SBRs), i.e. EBPR-SN SBR followed by Anammox SBR. The EBPR-SN SBR was operated for 400 days with five periods and the Anammox SBR was operated starting on period IV. Eventually, for treating municipal wastewater containing low chemical oxygen demand/nitrogen (COD/N) of 3.2 (mg/mg), the EBPR-SN plus Anammox system performed advanced total inorganic nitrogen (TIN) and P removal, with TIN and P removal efficiencies of 81.4% and 94.3%, respectively. Further analysis suggested that the contributions of simultaneous partial nitrification denitrification, denitrification, and anammox to TIN removal were 15.0%, 45.0%, and 40.0%, respectively. The enriched phosphorus-accumulating organisms (PAOs) in the EBPR-SN SBR facilitated P removal. Besides, the EBPR-SN SBR achieved P removal and provided stable anammox substrates, suggesting a short sludge retention time (SRT 12 d) could achieve synergy between ammonia-oxidizing bacteria and PAOs. These results provided an alternative process for treating municipal wastewater with limited organics.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Insufficient carbon source in raw municipal wastewater and high aeration energy consumption are common problems in traditional biological nitrogen (N) and phosphorus (P) elimination processes. Consequently, novel process should be established to efficiently utilize the organics of raw municipal wastewater and reduce the demand for aeration. For N removal, anammox bacteria have been found to reduce ammonium using nitrite as electron acceptor, which provides a way to remove N from wastewater without using organic carbon sources [1, 2]. Except for limited organics being used by anammox bacteria for cell synthesis, the anammox process is expected to conserve nearly 100% of organics. Consequently, carbon sources are recycled in anammox process. Besides, low aeration costs and little sludge discharge are also two excellent advantages of anammox-based process [3]. Apart from N, P is another main contributor for eutrophication. Therefore, both N and P should be eliminated from wastewater. Nevertheless, most anammox-based processes do not combine with P elimination [4]. With regard to P elimination, enhanced biological P removal (EBPR) was considered to be the efficient process [5]. Phosphorus-accumulating organisms (PAOs) release stored polyphosphate and take up carbon sources to store as polyhydroxyalkanoates (PHAs) under anaerobic environment. In the following aerobic stage, PAOs utilize the PHAs as electron donor to perform excess P uptake [6]. Overall, on one hand, carbon sources are recycled through anammox process, and on the other hand, carbon sources are consumed through EBPR [7, 8]. Both N and P elimination is probable by combining anammox with EBPR to treat municipal wastewater using insufficient organics.
To date, some researches have been done for combining anammox with biological P elimination, such as EBPR followed by one-stage anammox treating municipal wastewater [9, 10], hydroxyapatite recovery in a single anammox reactor [11], and the combination of simultaneous nitritation, anammox, and denitrification with denitrifying P removal [12]. Nevertheless, these studies integrated nitritation and anammox in one reactor, resulting in negative effect on anammox bacteria growth in an aerobic environment. Anammox bacterial activity has been shown to be inhibited at low dissolved oxygen (DO) level (0.25–2% O2 saturation), even irreversibly with higher level (> 20% O2 saturation, approximate 1.5 mg/L at 35 °C) [13]. Furthermore, nitrite-oxidizing bacteria (NOB) will oxidize NO2− in single-stage anammox system [14], leading to the destruction of anammox. In view of this, the combination of nitritation/anammox with EBPR in two separate reactors might be an alternative process. Hence, this study attempted to combine EBPR with semi-nitritation (SN) in one reactor while leaving anammox as an independent anaerobic reaction, supposing the EBPR reactor achieved not only P removal but also SN, and the effluent NO2−/NH4+ was suitable for a subsequent anammox processes. In this system, anammox bacteria grew in an anaerobic environment, which was beneficial to maintain anammox activity. Besides, it is expected to save energy on aeration, due to the simultaneous achievement of SN and EBPR in one reactor.
The combination of nitritation/anammox and EBPR in two separate reactors have several advantages, while the application of integrating nitritation/anammox with EBPR in municipal wastewater treatment is still a challenge. The first challenge was how to achieve stable SN, as stable SN (or nitritation) being difficult to maintain in municipal wastewater due to its low ammonium concentration [15]. Previous studies have shown that low DO [16] and real-time control [17] are effective strategies for achieving nitritation. On one hand, the critical point of the real-time control strategy is to stop aeration before NH4+ being completely oxidized, preventing NO2− from being further oxidized due to lack of aeration. On the other hand, NH4+ will be residual from SN, as a result of real-time control concept. Therefore, low DO and residual NH4+ were expected to maintain SN. The second challenge was the contradiction in sludge retention time (SRT) between nitrifying bacteria and PAOs, i.e. PAOs require short SRT, while nitrifying bacteria need relatively long SRT (e.g. 13 ± 4 days for PAOs and 15 ~ 32 days for nitrifiers) [18, 19]. Indeed, the nitrifying bacteria are classified into ammonium-oxidizing bacteria (AOB) and NOB, and the SRT of NOB is longer than that of AOB (e.g. 15 days for AOB and 32 days for NOB at 29 ± 1 ℃) [19]. As a result, this study try to use a relative short SRT (12 ~ 15 days) to satisfy the growth of PAOs and AOB while washing out NOB. Besides, low DO (< 1.5 mg/L) was beneficial for maintaining AOB but unfavorable for NOB [20]. In addition, low DO is favorable for the growth of PAOs. In summary, this study attempted to use the combination of low DO, residual NH4+, and short SRT strategies to achieve simultaneous EBPR and SN in one reactor.
The aim of this study was to investigate the feasibility of the process of combining synchronous EBPR and SN with anammox (EBPR-SN plus Anammox) to treat municipal wastewater with COD/N ratio of 3.2. The performance of N and P removal was evaluated through long-term (400 d) operation. Furthermore, P removal mechanisms were analyzed via detecting nutrient transformation stoichiometry in typical cycles, and the strategies of nitritation, as well as N elimination pathway, were stated. In addition, the potential application of the EBPR-SN plus Anammox process were analyzed.
Materials and methods
Experimental set-up and operation process
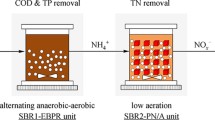
Figure 1 shows the operational mode and experimental device of the EBPR-SN plus Anammox system. Two laboratory-scale SBRs (effective capacity of 10 L for each one), which made up of methyl methacrylate, were used as the reactors. The EBPR-SN SBR applied anaerobic/aerobic operational mode. Per cycle of EBPR-SN reactor was 6 h, i.e. 2 h anaerobic (including 20 min feeding), 3 h aerobic, 30 min settling, 5 min discharging, and 25 min idle. The exchange ratio was 50% and 5 L domestic wastewater was pumped to reactor every cycle, with hydraulic retention time (HRT) of 10 h. pH and DO were real time detected using the 3420 Multi-Parameter Meter (WTW Company) during each cycle. In particular, residual NH4+ was maintained in the end of aeration by supplying low aeration rate (0.8 L/min) and fixed aeration time, with DO increasing from 0.3 to 1.5 mg/L in aerobic phase. More details about the EBPR-SN SBR are shown in Table 1. Anammox SBR started combining with EBPR-SN SBR on period IV (day 214), owing to stable SN being obtained in EBPR-SN reactor. Per cycle of Anammox SBR was also 6 h, i.e. 4 h anaerobic (including 9 min feeding), 1 h settling, 5 min discharging, and 55 min idle. Exchange ratio was 50% and 5 L of EBPR-SN effluent was pumped into Anammox reactor in each cycle, resulting in Anammox SBR HRT of 8 h. The water temperature was controlled at 27 ℃.
As shown in Table 1, the EBPR-SN SBR was operated for 400 d, dividing into five operation periods: nitritation start-up (period I, 1 ~ 47 d), SN achievement (period II, 48 ~ 176 d), nitritation deterioration and recovery (period III, 177 ~ 213 d), the combination of EBPR-SN and Anammox (period IV, 214 ~ 325 d), stable operation of EBPR-SN plus Anammox (period V, 326 ~ 400 d). In addition, for demonstrating robust performance, a starvation condition was carried out (day 287 ~ 313). In starvation period, the operation of two SBRs was all stopped.
Wastewater and seeding sludge
Municipal wastewater was the influent of EBPR-SN reactor, which was collected from a septic tank in the residential area of Beijing University of Technology (Beijing, China). The major nutrient concentrations were as follows: chemical oxygen demand (COD) 143 ~ 239 mg/L, NH4+ 42.8 ~ 86.1 mg N/L, NO2− < 1.0 mg N/L, NO3− < 1.0 mg N/L, TIN 43.3 ~ 86.4 mg N/L, COD/N ≤ 3.5, and PO43− 4.2 ~ 7.4 mg P/L. The effluent of EBPR-SN reactor was pumped into Anammox reactor through a middle tank. Additionally, in period IV, sodium acetate (58 mg/L COD) was added to the influent for enriching PAOs.
The seeding sludge of EBPR-SN reactor was complete nitrification flocculent sludge taken from an aeration tank in the Gaobeidian wastewater treatment plant (WWTP) (Fig. 2a). The mixed liquor suspended solids (MLSS) maintained 2576 ± 198 mg/L during the operation. The Anammox SBR granular sludge was taken from a reactor treating ammonium-rich wastewater (Fig. 2b). The MLSS of Anammox SBR was 1090 ± 39 mg/L.
Analytical methods
The water samples were filtered via the 0.45 mm filter papers for the determination of NH4+, NO2−, NO3−, PO43−, and chemical oxygen demand (COD), based on standard methods [21]. Freeze-dried biomass was used to detect polyhydroxyalkanoates (PHAs) and glycogen (Gly). PHAs were calculated by the sum of poly-b-hydroxybutyrate (PHB) and poly-b-hydroxyvalerate (PHV), which were measured using a gas chromatography (Agilent-6890 N) according to previous method [22] (detailed procedure available in supplementary material). Gly was determined according to previous method [23]. The microbial morphology of sludge was observed using digital imaging system (OLYMPUS CX31, Japan).
Calculations
Simultaneous partial nitrification denitrification (SPND) efficiency was the N loss during aeration in EBPR-SN SBR, based on the following calculation:
where Ae.NH4+, Ae.NO2−, and Ae.NO3− were N concentrations in aerobic ending (mg N/L). Ana.NH4+, Ana.NO2−, and Ana.NO3− were N concentrations in anaerobic ending (mg N/L).
In order to explore the N removal pathways, the contributions of anammox, SPND, and denitrification to N elimination were evaluated based on the following calculations, respectively:
where Ae.TIN was TIN concentration of EBPR-SN reactor at the end of aerobic (mg N/L). Ana.TIN was N concentration of EBPR-SN reactor at the end of anaerobic (mg N/L). Inf.TIN stands for TIN concentration of EBPR-SN reactor in influent (mg N/L). Eff.TIN was TIN concentration of Anammox SBR in effluent (mg N/L). AMX.Inf.TIN stands for TIN of Anammox SBR in influent (mg N/L).
Results and discussion
N removal performance
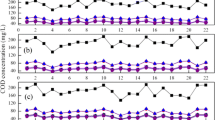
The purpose of period I (1 ~ 47 d) was to achieve nitritation from full nitrification in EBPR-SN SBR. Throughout period I, influent concentrations of NH4+ and chemical oxygen demand (COD) were 62.1 mg N/L and 184 mg/L, respectively (Fig. 3a). Effluent nitrite accumulation ratio (NAR) ((NAR = △NO2− /(△NO2− + △NO3−), where △NO2− and △NO3− were the increased concentrations during the aerobic phase) gradually increased from 12 to 100%, indicating that nitritation was achieved in a short time. NAR was maintained at an average of 97.2% (Fig. 3a) in period II (48 ~ 176 d), illustrating that stable nitritation was obtained. Stable nitritation was achieved by combining the following strategies: (1) Low DO (0.4 ~ 1.0 mg/L). It was reported that AOB obtained a higher oxygen affinity compared to NOB under low DO condition [24]. The strategy has been successfully applied in previous studies [16]; (2) Short SRT (15 d). Due to the shorter SRT of AOB compared to NOB, a relative short SRT was beneficial to flush out NOB [25]; (3) Maintenance of residual NH4+. For the purpose of achieving SN, at the end of each aeration cycle, residual NH4+ was maintained by controlling 3 h of aeration (Fig. 3a). Due to the presence of residual NH4+ reducing the risk of excessive aeration, maintaining stable nitritation was more reliable through SN rather than full nitritation.
Nutrient removal in EBPR-SN SBR. a N variation, including influent (Inf.), effluent (Eff.), effluent NO2−/NH4+, NAR. b COD removal performance, including COD in influent, end of anaerobic, effluent (Inf.COD, An.COD, and Eff.COD), and COD removal efficiency (Re.COD). c P removal performance, including P in influent, end of anaerobic, effluent (Inf.P, An.P, and Eff.P), and P removal efficiency (Re.P)
However, in period III (177 ~ 213 d), the effluent nitrate increased to 1.1 mg/L on day 177. Over the next 14 days (177–191 d), NAR dropped to 7.9% and nitritation stopped completely (Fig. 3a). It was observed that temperature decreased from 27 ℃ to 19℃, resulting in DO increasing from 0.4 ~ 1.0 mg/L to 2.0 ~ 3.4 mg/L with a consistent aerobic rate (Fig. S1). The reason for increased DO was due to the fact that microbial activities decreased with the decrease of temperature, resulting in the decrease of DO requirement for microorganisms, in turn causing the increase of DO in liquid. Under high DO condition, NOB activity was higher than AOB and the nitritation performance was gradually destroyed. In order to quickly re-establish nitritation, DO was adjusted to 0.2 ~ 0.7 mg/L by decreasing aeration rate to 0.4 L/min. Over the next 17 d, NAR gradually increased to 100%, indicating that nitritation could be recovered by reducing DO. The results suggested that stable nitritation relied on low DO, and the EBPR-SN SBR was heated to 27 ℃ in the next periods to avoid the increase of DO.
The NO2−/NH4+ ratio in EBPR-SN SBR effluent was stable during period IV (214 ~ 325 d), with an average of 1.13 (Fig. 3a), being close to the theoretic ratio (1.32) for anammox reaction [26]. A high NAR (99.39%) was also maintained in the reactor. The effluent was fed into the following Anammox reactor through a middle tank. The final effluent TIN was 13.0 mg N/L, which mainly consisted of NO3− (Fig. 4a), with TIN removal efficiency of 79.4% (Fig. 4b). The generated NO3− to reactive NH4+ ratio (0.58) was higher than the theoretical value (0.26) produced by anammox bacteria (Fig. 4a), resulting in excess nitrate in the Anammox SBR effluent. The excess nitrate was generated by NOB, because NOB could compete for NO2− with anammox bacteria, and DO used for NOB could be obtained from the air when decanting the EBPR-SN SBR. Previous studies also indicated that NOB can compete with anammox bacteria for NO2− when substrates (NH4+ and NO2−) become limiting [27].
a N removal in the EBPR-SN SBR coupled with Anammox SBR, including influent TIN (Inf.TIN), the EBPR-SN SBR effluent TIN (EBPR-SN Eff.TIN). Anammox SBR influent NH4+, NO2−, and NO3− (AMX.Inf.); effluent TIN, NH4+, NO2−, and NO3− (Eff.). The generated NO3− to reactive NH4+ ratio of Anammox SBR (NO3−/NH4+). b The contributions of SPND (SPND.Re/TIN.Re), denitrification (DN.Re/TIN.Re), and anammox (AMX.Re/TIN.Re) to TIN removal
In period V (326 ~ 400 d), the influent of EBPR-SN SBR was municipal wastewater, with COD and TIN concentrations of 218 mg/L and 68.1 ± 5.9 mg N/L, respectively, with COD/N ratio of 3.2. Stable nitritation was obtained with a NAR of 98.4% (Fig. 3a). The NO2−/NH4+ in EBPR-SN SBR effluent was relatively stable, with NO2−/NH4+ of 1.09 (Fig. 3a), providing appropriate substrates for anammox bacteria. The Anammox SBR effluent TIN and NH4+ were 12.6 ± 3.4 and 2.6 ± 1.6 mg N/L (Fig. 4a), respectively. Taken together, TIN removal efficiency of EBPR-SN plus Anammox process was 81.4% (Fig. 4b). The 83.0% of TIN in the Anammox SBR effluent was NO3−. The reason for excess NO3− was same to period IV. The EBPR-SN plus Anammox process still had potential to further enhance the N removal efficiency by getting the observed concentration of NO3− closer to the theoretical value that the anammox reaction could produce.
The contributions of EBPR-SN SBR and Anammox SBR to TIN removal
To better understand the fate of N during the process, the contributions of different pathways to TIN removal were calculated.
The TIN was removed by Anammox SBR and EBPR-SN SBR (Fig. 4). The TIN was removed through the anammox pathway in the Anammox SBR. As in the EBPR-SN SBR, TIN was removed through two pathways: On one hand, the NOX− that remained from the last cycle was removed through denitrification (DN) at the beginning of the anaerobic phase, using influent organic matters as electron acceptors. On the other hand, TIN loss was observed in the aerobic phase of EBPR-SN SBR. The average TIN loss was 9.2 mg/L in period IV and period V (Fig. S2), which exceeded the need of N assimilation [28]. This indicated that not only nitrification occurred in the reactor but also DN occurred in the aerobic phase. Generally, the occurrence of TIN removal during aeration was simultaneous nitrification denitrification (SND). In this study, nitritation was maintained, which resulted in SND being achieved through the nitrite pathway (using NO2− as electron acceptor), suggesting that this should be defined as simultaneous partial nitrification denitrification (SPND). More details about SPND are shown in Fig. 5a (TIN loss) and Fig. S2. The typical cycle of EBPR-SN SBR also showed that TIN was lost during the aerobic phase (Fig. 5a). Due to the occurrence of SPND, carbon sources were metabolized efficiently and improved the TIN removal efficiency of the system. The SPND in the EBPR process merits further study.
Therefore, the TIN was removed through DN, SPND, and anammox pathways. In period IV, 34.7% of the TIN was removed by anammox (Fig. 4b). The contributions of SPND and DN were 17.4% and 47.9%, respectively (Fig. 4b). In period V, SPND.Re/TIN.Re showed a decreasing trend due to the decrease of influent COD/N ratio, with an average value of 15.0% (Fig. 4b), which was consistent with previous study that the amount of available carbon was a crucial factor for SND efficiency [29]. The contribution of DN to TIN elimination was stable, with average value of 45.0% (Fig. 4b). The contribution of anammox was 40.0% (Fig. 4b). The TIN removal efficiency of this process could be enhanced by promoting the contribution of anammox. Contribution of anammox to N elimination could be further improved by decreasing the contributions of SPND and DN. The contribution of SPND would be reduced with a decreasing influent COD/N ratio, and the contribution of DN would be reduced with the increase of exchange ratio of EBPR-SN SBR. The anammox pathway would play a more important role in TIN removal under the aforementioned conditions. Therefore, the EBPR-SN plus Anammox system possessed potential for treating low-COD/N ratio (< 3.2) municipal wastewater.
P removal performance
The COD/N was 3.0 during period I (1 ~ 47 d) (Table 1). In the first 15 d, P removal efficiency was 92.0%. In the next 32 d, P removal efficiency fluctuated between 10.2% and 98.0% (Fig. 3c). The residual NOx− from the last cycle increased from 10.2 to 14.0 mg N/L over 32 d (Fig. 3a). More carbon sources were required to metabolize the residual NOx− by ordinary heterotrophic organisms (OHO). As a result, the carbon sources were reduced for the anaerobic phase so that the synthesis of PHAs was reduced [30], then affecting the P removal. P removal efficiency further decreased from 37.6 to 6.2% over the course of period II (48 ~ 176 d) (Fig. 3c). This was for the same reason as in period I. In period III (177 ~ 213 d), P removal efficiency slightly increased from 6.9 to 26.5%, since the production of NOx− decreased along with decreasing DO concentration. The carbon sources used for denitrifying the residual NOX− reduced. Therefore, carbon used for PAOs synthesis PHAs increased.
In period IV (214 ~ 325 d), the EBPR-SN SBR began to enrich PAOs and enhance P removal. According to previous study, PAOs were successfully enriched when carbon sources were added [29]. Consequently, from day 214 to 249, the influent COD gradually increased from 200 to 250 mg/L by adding sodium acetate to the raw municipal wastewater (Fig. 3b), with P removal efficiency increasing from 11.4% to 71.4%. From day 250 to day 325, the influent COD and TIN were 257 mg/L and 68.9 mg N/L, respectively, with COD/N ratio of 3.7. From day 250−272, P removal efficiency exceeded 90.0% (Fig. 3c). But in the next 5 d, the P removal efficiency dropped to 68.7% (Fig. 3c). Meanwhile, the sludge concentration increased from 2571 mg/L on day 269 to 2840 mg/L on day 275, indicating that the sludge (mainly consisting of heterotrophic bacteria, such as PAOs) grew faster with higher influent COD. Therefore, the SRT was adjusted from 15 to 12 d starting on day 278. In the remaining 48 d (4 SRTs) of period IV, the average removal efficiency was 94%, and PO43− concentration averaged at 0.37 mg P/L in effluent (Fig. 3c). P removal performance was stable and efficient, indicating that PAOs were enriched and EBPR was occurring. Although the microbial community of this study was not analyzed, previous study has demonstrated that PAOs were enriched successfully using a similar method [29]. The results demonstrated that adequate carbon sources and short SRT (12 d) could facilitate the achievement of EBPR in a SN reactor.
After the enrichment of PAOs in period IV, the influent was municipal wastewater without adding carbon source in period V (326 ~ 400 d), with COD/N of 3.2. As shown in Fig. 3c, in EBPR-SN SBR, PO43− in influent and effluent were 5.6 ± 0.9 and 0.3 ± 0.2 mg P/L, respectively, with removal efficiency of 94.3%, indicating an advanced P removal performance. The advanced P elimination performance was attributed to the enrichment of PAOs in last period; then PAOs promoted the utilization of carbon sources for synthesizing endogenous carbon sources in anaerobic phase. The COD was 58 mg/L in anaerobic ending, which was close to the COD concentration in aerobic ending (37 mg/L), indicating that high utilization efficiency of influent COD (73.2%) was reached in the anaerobic phase.
The mechanism of P removal
To reveal the reasons for advanced P elimination, nutrient transformation stoichiometry during the typical cycle of day 279 was calculated for the mechanism analysis (Fig. 5a).
At anaerobic stage, P release amount to PHAs production ratio and Gly consumption to PHAs production ratio were 0.58 mmol P/ mmol C and 0.34 mmol C/ mmol C, respectively, which were consistent with PAOs models (0.19 ~ 0.625 mmol P/mmol C and 0.385 mmol C/ mmol C, respectively [31]). But the Gly consumption/PHAs production was much less than Gly-accumulating organisms (GAOs) (0.5 mmol C/ mmol C [32], 0.628 mmol C/ mmol C [23]), illustrating that PAOs were responsible for PHA transformation, whereas the contribution of GAOs to the PHAs synthesis was limited. At aerobic stage, Gly production/PHAs consumption and P uptake amount (PUA)/Gly production were 0.73 mmol C/mmol C and 0.90 mmol P/ mmol C, respectively, which were predicted by PAO model (0.68 ~ 2.13 mmol C/ mmol C and 0.96 mmol P/ mmol C, respectively [33, 34]). PUA/CPHAs (0.65 mmol P/mmol C) exceeded model value (0.41 mmol P/ mmol C [33]), indicating that per mmol PHAs could consume extra PO43−. This indirectly explained that excess P absorption was achieved using merely 1.41 mmol C/L of PHAs.
According to the results of mechanism, the reasons of ideal P elimination were as below: (1) the storage of PHAs was mostly conducted by PAOs rather than GAOs; (2) requirement of PHAs for PO43− absorption was lower than model value.
Overall, the results of nutrient removal performance and mechanisms suggested that the TIN removal mainly relied on anammox and DN, and the SPND also played a limited role. The contributions of anammox, DN, and SPND to TIN removal were 40.0%, 45%, and 15%, respectively. Besides, advanced P removal performance was attributed to the storage of PHAs which was mostly conducted by PAOs rather than GAOs. According to mechanisms and typical cycles, COD, N, and P removal schematic diagram and mass balance are described in Fig. 6.
It was worth mentioning that there was 27 days’ starvation period (287 ~ 313 d) during period IV. The results showed that both EBPR-SN SBR and Anammox SBR could recover removal performance in the first day of restart operation, indicating robustness of the process.
Nutrient variations in typical cycles
In order to deeply investigate the process before and after enrichment of PAOs, typical cycles in period II and period IV were selected for analysis. The typical cycles of day 169 and day 279 were chosen.
On day 167, the P concentration remained constant during both anaerobic and aerobic phases, with concentration of approximate 6 mg P/L (Fig. S3a), suggesting PAOs being inhibited. On day 279, the variation in P concentration was quite different than that on day 167. P released rapidly and peaked at 29.26 mg P/L, with PHAs being synthesized and Gly being consumed (Fig. 5a). In the subsequent aerobic phase, P was almost completely consumed. The effluent P concentration was 0.4 mg P/L. Meanwhile, PHAs decreased from 1.58 to 0.17 mmol/L. It was also found that merely 1.8 mg N/L nitrite was produced in first hour, NO2− mainly being produced in last 2 h. The concentration of generated NO2− and remaining NH4+ were 17.9 and 14.4 mg N/L, respectively, with NAR being 97.8%. Then, effluent was fed into Anammox reactor. Figure 5b shows the typical cycle of Anammox SBR. At the end of feeding, NH4+, NO2−, and NO3− were 5.2, 7.1, and 6.6 mg N/L, respectively. At the end of reaction, NH4+, NO2−, and NO3− were 0.9, 0, and 9.3 mg N/L, respectively. This illustrating that NH4+ and NO2− decreased by 4.3 and 7.1 mg N/L, respectively, while NO3− increased by 2.7 mg N/L. Based on the theoretic value of anammox reaction, consumption of 4.3 mg N/L NH4+ should produce 1.1 mg N/L NO3− and consume 5.6 mg N/L NO2−. But the results showed that consumed NO2− was 7.1 mg N/L. An additional 1.5 mg N/L of NO2− was consumed which could be oxidized to NO3−. This meant that 2.6 mg N/L of NO3− would be expected, which was very approximate to the measured NO3− values (2.7 mg N/L), suggesting that additional 1.5 mg N/L NO2− was exactly oxidized to NO3− by NOB rather than anammox bacteria. The result of typical cycle was consistent with long-term performance.
Effect of free nitrite acid (FNA) on aerobic P uptake
With the increase of NO2− in typical cycle, FNA also increased. Previous studies have found complete inhibition of aerobic P uptake with FNA concentration of 1.5 × 10–3 mg/L (equivalent to 20.0 mg NO2−–N/L at 20–22 °C and pH 7.0) [35] and half inhibition at FNA concentration of 0.52 × 10–3 mg/L (equivalent to 6.9 mg NO2−–N/L at 20–22 °C and pH 7.0) [36]. It was to be questioned whether FNA would inhibit aerobic P uptake in this process. Therefore, FNA concentration was analyzed in typical cycle. Figure 5a shows that the concentration of FNA increased from 0.03 × 10–3 to 2.6 × 10–3 mg/L (NO2−–N increasing from 0.2 to18.0 mg/L) during aerobic phase. P uptake mainly occurred in the first hour and the corresponding FNA was only 0.1 × 10–3 mg/L. In the next 2 h, P was almost completely removed before FNA increasing to 1.5 × 10–3 mg/L. Owing to aerobic P uptake being prior to nitritation, FNA would not have a significant effect on aerobic P uptake, and it was a reason for EBPR and SN which were able to occur in a single reactor. It was due to a large number of heterotrophic PAOs that were better able to outcompete nitrifying autotrophs for the limited DO [37].
Real-time indication of pH in EBPR-SN SBR
Fig. S3b shows the change of pH during a typical cycle in EBPR-SN SBR. On day 167, pH increased in the first 40 min and then declined in the anaerobic phase. The increased pH was due to the denitrification of the remaining NO2− from the last cycle. The decline in pH might be due to the production of short-chain fatty acids during the anaerobic phase [38]. During the subsequent aerobic phase, pH increased slightly and then dropped. The increase of pH was due to a small amounts of COD being degraded by heterotrophic bacteria; then aeration blew off CO2 causing the pH to increase. The next decline in pH was due to the production of acid in nitritation [17]. On day 279, pH also increased in the first 10 min and then decreased throughout the anaerobic phase, and the decline in pH was greater than that on day 167. The greater decline in pH was due to H+ being generated with P release (Eq. 5) [39]. In the subsequent aerobic phase, pH increased rapidly and then decreased, and the increase was also greater than that of day 167. The larger increase in pH was due to aerobic P uptake (Eq. 6) [39]. After P uptake being completed, the pH decreased with the generated H+ from nitritation (Eq. 7). The variation of pH could indicate the variation of P and N. According to variation of pH, it was expected to establish real-time control strategy in the future.
In above equations, C2H4O2 = PHAs, HPO3 = poly-P, and C2H4N2 = stored organics.
Application of the EBPR-SN plus Anammox process for treating municipal wastewater with low COD/N
This research established a novel EBPR-SN plus Anammox system for achieving nutrient elimination from low COD/N (3.2) municipal wastewater. The TIN and P removal efficiencies of the EBPR-SN plus Anammox process (81.4% and 94.3%, respectively) were higher than the related processes (52.0% and 71%, [40]; 43.8 ~ 70.0% and 51.6 ~ 90.2%, [41]; 74.6% and 94.0%, [39]; 77.7% and 94.0%, [29]) which even possessed higher COD/N in influent (Table 2). In particular, the EBPR-SN plus Anammox process only contained 3 h of aerobic time per cycle with low DO (0.3 ~ 1.5 mg/L) (Fig. S3b), which provided a considerable saving in energy consumption compared with other works that remove N and P with high DO or long aerobic times (240 min aerobic time with DO of 2 ~ 3 mg/L, [40]; 330 min aerobic time with DO of 2 ~ 5 mg/L, [42]; 180 ~ 430 min aerobic time with DO of 3 ~ 4, [41]; 120 min aerobic time with DO of 3 mg/L, [39]; and 150 min aerobic time with DO of 1 ± 0.3 mg/L, [29]) (Table 2). The carbon sources being mainly stored as PHAs by PAOs and the contribution of anammox to TIN removal were the main reasons for advanced nutrient elimination performance of EBPR-SN plus Anammox process. Additionally, in comparison to other systems that use anammox processes combined with P removal [9, 12], the EBPR-SN plus Anammox system excelled because it maintained anammox bacteria activity. Furthermore, comparing with the reported simultaneous N and P removal processes containing many middle tanks and pumps or requiring recycling [39], the EBPR-SN plus Anammox process used much less equipment, simplifying the setup and reducing cost. The EBPR-SN plus Anammox system not only played a role in autotrophic N removal but also made full use of influent carbon sources for achieving nutrient removal. Owing to synchronous N and P removal, this system possessed great advantages compared with the processes which only achieved N removal from municipal wastewater [43,44,45]. Besides, it is known that excessive aeration can destabilize nitritation [46]; instead, this study was able to prevent nitritation from excess aeration by achieving SN instead of full nitritation. Therefore, more stable nitritation could be obtained by residual NH4+ protection against excess aeration. The EBPR-SN plus anammox process has potential to scale up to pilot-scale and then full-scale application for the low COD/N of influent between 3 and 12 in WWTPs [47]. The organics available in influent of WWTPs are sufficient for advanced N and P removal when the EBPR-SN plus anammox process is applied. Based on the results of this study, the scaled-up process would perform well to treat wastewater with low COD/N ratio. Due to fluctuating COD, TIN, and P concentrations, the application of online control strategy will be required to ensue effluent quality. The result of typical cycle demonstrated that COD and P removal were prior to nitritation, and therefore, controlling the NO2−/NH4+ ratio of 1.32 in EBPR-SN SBR effluent via online NO2− and NH4+ probes will not only ensue proper ratio of anammox substrates but also achieve high COD and P removal and high TIN removal in the subsequent anammox SBR. In scaled-up systems, the organics required by PAOs can be obtained from sludge fermentation products in WWTPs [48, 49] rather than acetate in this laboratory study.
Compared with conventional nitrification/denitrification N removal processes, the EBPR-SN plus anammox process saved 60% aeration energy due to N removal via nitritation/anammox pathway [4]. Furthermore, the aeration cost for P uptake would reduce due to simultaneous achievement of aerobic P uptake and nitritation in one shared aeration condition. Therefore, the potential energy saving of this process would be larger. Overall, this study provided a novel and economical way for advanced nutrient elimination from municipal wastewater with low COD/N. However, for practical application of the process, many issues need to be investigated further, such as microbial communities and influence factors.
Conclusions
The EBPR-SN plus Anammox process was established for N and P removal from municipal wastewater. The EBPR-SN SBR was started up and stably operated at 27 °C under low DO (0.3 ~ 1.5 mg/L) and short SRT (12 days), followed by combining with anammox SBR. Finally, with low COD/N of 3.2, the effluent concentrations of TIN and P were 12.6 ± 3.4 mg N/L and 0.3 ± 0.2 mg P/L, respectively, resulting in TIN and P removal efficiencies of 81.4% and 94.3%, respectively. The contributions of SPND, DN, and anammox to TIN removal were 15.0%, 45.0%, and 40.0%, respectively. Advanced P removal performance was attributed to the storage of PHAs being mostly conducted by PAOs rather than GAOs. EBPR and SN were simultaneously achieved in one SBR, suggesting that the synergy of AOB and PAOs could be achieved with SRT of 12 d. Stable nitritation resulted from the combination of low DO, short SRT, and residual NH4+. Notably, the aerobic time was only 3 h per cycle with low DO aeration during the whole process, offering a considerable saving in energy consumption.
Abbreviations
- AOB:
-
Ammonia-oxidizing bacteria
- C/N:
-
Carbon/nitrogen
- COD:
-
Chemical oxygen demand
- DN:
-
Denitrification
- DO:
-
Dissolved oxygen
- EBPR-SN:
-
Enhanced biological phosphorus removal and semi-nitritation
- FNA:
-
Free nitrite acid
- GAOs:
-
Glycogen-accumulating organisms
- Gly:
-
Glycogen
- MLSS:
-
Mixed liquor suspended solids
- N:
-
Nitrogen
- NAR:
-
Nitrite accumulation ratio
- NOB:
-
Nitrite-oxidizing bacteria
- OHO:
-
Ordinary heterotrophic organisms
- P:
-
Phosphorus
- PAOs:
-
Phosphorus-accumulating organisms
- PHAs:
-
Polyhydroxyalkanoates
- PHB:
-
Poly-b-hydroxybutyrate
- PHV:
-
Poly-b-hydroxyvalerate
- PRA:
-
Phosphorus release amount
- PUA:
-
Phosphorus uptake amount
- SBR:
-
Sequencing batch reactor
- SPND:
-
Simultaneous partial nitrification denitrification
- SRT:
-
Sludge retention time
- TIN:
-
Total inorganic nitrogen
- WWTP:
-
Wastewater treatment plants
References
Li J, Li J, Gao R et al (2018) A critical review of one-stage anammox processes for treating industrial wastewater: optimization strategies based on key functional microorganisms. Bioresource Technol 265:498–505
Li J, Peng Y, Zhang L et al (2019) Quantify the contribution of anammox for enhanced nitrogen removal through metagenomic analysis and mass balance in an anoxic moving bed biofilm reactor. Water Res 160:178–187
Mulder A, Vandegraaf AA, Robertson LA, Kuenen JG (1995) Anaerobic ammonium oxidation discovered in a denitrifying fluidized-bed reactor. Fems Microbiol Ecol 16(3):177–183
Ma B, Wang S, Cao S et al (2016) Biological nitrogen removal from sewage via anammox: Recent advances. Bioresour Technol 200:981–990
Oehmen A, Lemos PC, Carvalho G et al (2007) Advances in enhanced biological phosphorus removal: From micro to macro scale. Water Res 41(11):2271–2300
Tuszynska A, Kaszubowska M, Kowal P, Ciesielski S, Makinia J (2019) The metabolic activity of denitrifying microorganisms accumulating polyphosphate in response to addition of fusel oil. Bioproc Biosyst Eng 42(1):143–155
Miao Y, Peng Y, Zhang L et al (2018) Partial nitrification-anammox (PNA) treating sewage with intermittent aeration mode: effect of influent C/N ratios. Chem Eng J 334:664–672
Mino T, Van Loosdrecht M, Heijnen JJ (1998) Microbiology and biochemistry of the enhanced biological phosphate removal process. Water Res 32(11):3193–3207
Yang Y, Zhang L, Shao H, Zhang S, Gu P, Peng Y (2017) Enhanced nutrients removal from municipal wastewater through biological phosphorus removal followed by partial nitritation/anammox. Front Env Sci Eng 11:82
Cao Y, Kwok BH, van Loosdrecht MCM et al (2017) The occurrence of enhanced biological phosphorus removal in a 200,000 m(3)/day partial nitration and Anammox activated sludge process at the Changi water reclamation plant. Singapore Water Sci Technol 75(3):741–751
Ma H, Xue Y, Zhang Y, Kobayashi T, Kubota K, Li Y (2020) Simultaneous nitrogen removal and phosphorus recovery using an anammox expanded reactor operated at 25 °C. Water Res 172:115510
Wen X, Zhou J, Li Y, Qing X, He Q (2016) A novel process combining simultaneous partial nitrification, anammox and denitrification (SNAD) with denitrifying phosphorus removal (DPR) to treat sewage. Bioresour Technol 222:309–316
Yan Y, Wang Y, Wang W, Zhou S, Wang J, Guo J (2019) Comparison of short-term dosing ferrous ion and nanoscale zero-valent iron for rapid recovery of anammox activity from dissolved oxygen inhibition. Water Res 153:284–294
Zhang F, Peng Y, Miao L, Wang Z, Wang S, Li B (2017) A novel simultaneous partial nitrification Anammox and denitrification (SNAD) with intermittent aeration for cost-effective nitrogen removal from mature landfill leachate. Chem Eng J 313:619–628
Ali M, Okabe S (2015) Anammox-based technologies for nitrogen removal: advances in process start-up and remaining issues. Chemosphere 141:144–153
Ma Y, Peng Y, Wang S, Yuan Z, Wang X (2009) Achieving nitrogen removal via nitrite in a pilot-scale continuous pre-denitrification plant. Water Res 43(3):563–572
Yang Q, Peng Y, Liu X, Zeng W, Mino T, Satoh H (2007) Nitrogen removal via nitrite from municipal wastewater at low temperatures using real-time control to optimize nitrifying communities. Environ Sci Technol 41(23):8159–8164
Winkler MKH, Kleerebezem R, Khunjar WO, de Bruin B, van Loosdrecht MCM (2012) Evaluating the solid retention time of bacteria in flocculent and granular sludge. Water Res 46(16):4973–4980
Liu W, Yang Q, Ma B et al (2017) Rapid achievement of nitritation using aerobic starvation. Environ Sci Technol 51(7):4001–4008
Ge S, Wang S, Yang X, Qiu S, Li B, Peng Y (2015) Detection of nitrifiers and evaluation of partial nitrification for wastewater treatment: a review. Chemosphere 140:85–98
APHAs (1998) Standard methods for the examination of water and wastewater. American Public Health Association, Washington
Oehmen A, Zeng RJ, Saunders AM, Blackall LL, Keller J, Yuan Z (2006) Anaerobic and aerobic metabolism of glycogen-accumulating organisms selected with propionate as the sole carbon source. Microbiol-Sgm 152(9):2767–2778
Zeng RJ, van Loosdrecht M, Yuan ZG, Keller J (2003) Metabolic model for glycogen-accumulating organisms in anaerobic/aerobic activated sludge systems. Biotechnol Bioeng 81(1):92–105
Bernet N, Dangcong P, Delgenes JP, Moletta R (2001) Nitrification at low oxygen concentration in biofilm reactor. J Environ Eng 127(3):266–271
Wu J, He C, van Loosdrecht MCM, Perez J (2016) Selection of ammonium oxidizing bacteria, (AOB) over nitrite oxidizing bacteria (NOB) based on conversion rates. Chem Eng J 304:953–961
Du R, Cao S, Li B, Niu M, Wang S, Peng Y (2017) Performance and microbial community analysis of a novel DEAMOX based on partial-denitrification and anammox treating ammonia and nitrate wastewaters. Water Res 108:46–56
Fuessel J, Lam P, Lavik G et al (2012) Nitrite oxidation in the namibian oxygen minimum zone. Isme J 6(6):1200–1209
Veuger B, Pitcher A, Schouten S, Damste JSS, Middelburg JJ (2013) Nitrification and growth of autotrophic nitrifying bacteria and Thaumarchaeota in the coastal North Sea. Biogeosciences 10(3):1775–1785
Wang X, Wang S, Xue T, Li B, Dai X, Peng Y (2015) Treating low carbon/nitrogen (C/N) wastewater in simultaneous nitrification-endogenous denitrification and phosphorous removal (SNDPR) systems by strengthening anaerobic intracellular carbon storage. Water Res 77:191–200
Carucci A, Kuhni M, Brun R et al (1999) Microbial competition for the organic substrates and its impact on EBPR systems under conditions of changing carbon feed. Water Sci Technol 39(1):75–85
Smolders G, Vandermeij J, Vanloosdrecht M, Heijnen JJ (1994) Model of the anaerobic metabolism of the biological phosphorus removal process-stoichiometry and pH Influence. Biotechnol Bioeng 43(6):461–470
Filipe C, Daigger GT, Grady C (2001) A metabolic model for acetate uptake under anaerobic conditions by glycogen accumulating organisms: Stoichiometry, kinetics, and the effect of pH. Biotechnol Bioeng 76(1):17–31
Smolders G, Vandermeij J, Vanloosdrecht M, Heijnen JJ (1994) Stoichiometric model of the aerobic metabolism of the biological phosphorus removal process. Biotechnol Bioeng 44(7):837–848
Coats ER, Brinkman CK, Lee S (2017) Characterizing and contrasting the microbial ecology of laboratory and full-scale EBPR systems cultured on synthetic and real wastewaters. Water Res 108:124–136
Saito T, Brdjanovic D, van Loosdrecht M (2004) Effect of nitrite on phosphate uptake by phosphate accumulating organisms. Water Res 38(17):3760–3768
Pijuan M, Ye L, Yuan Z (2010) Free nitrous acid inhibition on the aerobic metabolism of poly-phosphate accumulating organisms. Water Res 44(20):6063–6072
Zeng W, Yang Y, Li L, Wang X, Peng Y (2011) Effect of nitrite from nitritation on biological phosphorus removal in a sequencing batch reactor treating domestic wastewater. Bioresour Technol 102(12):6657–6664
Yuan Y, Peng Y, Liu Y, Jin B, Wang B, Wang S (2014) Change of pH during excess sludge fermentation under alkaline, acidic and neutral conditions. Bioresour Technol 174:1–5
Zhao W, Zhang Y, Lv P, Wang M, Peng Y, Li B (2016) Advanced nitrogen and phosphorus removal in the pre-denitrification anaerobic/anoxic/aerobic nitrification sequence batch reactor (pre-A(2)NSBR) treating low carbon/nitrogen (C/N) wastewater. Chem Eng J 302:296–304
Wang F, Lu S, Wei Y, Ji M (2009) Characteristics of aerobic granule and nitrogen and phosphorus removal in a SBR. J Hazard Mater 164(2–3):1223–1227
Rahimi Y, Torabian A, Mehrdadi N, Shahmoradi B (2011) Simultaneous nitrification-denitrification and phosphorus removal in a fixed bed sequencing batch reactor (FBSBR). J Hazard Mater 185(2–3):852–857
Lo IW, Lo KV, Mavinic DS, Shiskowski D, Ramey W (2010) Contributions of biofilm and suspended sludge to nitrogen transformation and nitrous oxide emission in hybrid sequencing batch system. J Environ Sci-China 22(7):953–960
Du R, Peng Y, Ji J, Shi L, Gao R, Li X (2019) Partial denitrification providing nitrite: Opportunities of extending application for anammox. Environ Int 131:105001
Du R, Cao S, Li B, Zhang H, Wang S, Peng Y (2019) Synergy of partial-denitrification and anammox in continuously fed upflow sludge blanket reactor for simultaneous nitrate and ammonia removal at room temperature. Bioresour Technol 274:386–394
Ji J, Peng Y, Li X, Zhang Q, Liu X (2020) A novel partial nitrification-synchronous anammox and endogenous partial denitrification (PN-SAEPD) process for advanced nitrogen removal from municipal wastewater at ambient temperatures. Water Res 175:115690
Gao D, Peng Y, Li B, Liang H (2008) Shortcut nitrification-denitrification by real-time control strategies. J Biotechnol 136S:S652
Ji J, Peng Y, Wang B, Li X, Zhang Q (2020) A novel SNPR process for advanced nitrogen and phosphorus removal from mainstream wastewater based on anammox, endogenous partial-denitrification and denitrifying dephosphatation. Water Res 170:115363
Liu J, Yuan Y, Li B et al (2017) Enhanced nitrogen and phosphorus removal from municipal wastewater in an anaerobic-aerobic-anoxic sequencing batch reactor with sludge fermentation products as carbon source. Bioresour Technol 244:1158–1165
Jin B, Niu J, Zhang J et al (2020) Response of extracellular polymeric substances and enzymatic activity to salinity for the waste activated sludge anaerobic fermentation process. Bioproc Biosyst Eng 43(4):737–745
Acknowledgements
This research was supported by National Key Research and Development Program of China (2016YFC0401103), National Natural Science Foundation of China (21806006), Beijing Municipal Science & Technology Project (D171100001017002), and the Funding Projects of Beijing Municipal Commission of Education.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yuan, C., Peng, Y., Ji, J. et al. Advanced nitrogen and phosphorus removal from municipal wastewater via simultaneous enhanced biological phosphorus removal and semi-nitritation (EBPR-SN) combined with anammox. Bioprocess Biosyst Eng 43, 2039–2052 (2020). https://doi.org/10.1007/s00449-020-02392-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02392-8