Abstract
Zinc oxide (ZnO) is considered as a potential antimicrobial agent. This work aims to investigate the properties of ZnO and Mn-doped ZnO (1% and 5%) fabricated using aqueous leaf extract of Melastoma malabathricum via green synthesis and its antibacterial activities. The synthesized ZnO and Mn-doped ZnO were characterized using different techniques such as powder X-ray diffraction, scanning electron microscopy, X-ray photoelectron spectroscopy and UV–Vis diffuse reflectance spectroscopy. The synthesized ZnO and Mn-doped ZnO were tested for its antibacterial properties on two Gram-negative bacteria: Escherichia coli and Pseudomonas aeruginosa, and two Gram-positive bacteria: Bacillus subtilis and Staphylococcus aureus. The results showed positive antibacterial effects for B. subtilis and S. aureus only. Among the three materials tested, 1% Mn-doped ZnO exhibited the highest antibacterial activity for B. subtilis with the minimum inhibitory concentration being 50 mg/mL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc oxide nanoparticles (ZnO NPs) are of interest as favorable antimicrobial agent due to its high resistance to heat denaturation, low decomposability and slightly longer usable lifespan in comparison to other organic antimicrobial agents [1]. ZnO has been shown to possess good antibacterial property against both Gram-positive and Gram-negative bacteria partially due to its reactive oxygen species (ROS) producing effect which had been researched to be cytotoxic towards bacterial cells [2, 3]. One of the properties of ZnO NPs which may be responsible for the high antimicrobial activity is the low-energy band gap [4]. This property allows for an above average production of reactive oxygen species when the ZnO interacts with the biomolecules [5].
The properties of ZnO can be slightly altered to a more favorable characteristic to produce ZnO powder that is more efficient against bacterial activity [6]. One of the ways this can be achieved is by doping with a different material. In the event that Mn-doped ZnO has a higher stability, the cytotoxic effect of the ZnO NPs may be lowered as the Mn doping may decrease the dissociation time of ZnO NPs. A more gradual dissociation of ZnO can result in a lower risk of an exponential increase of ROS and this phenomena favors the use of ZnO powder as an antiseptic cream on human skin [7]. Furthermore, the particle size of Mn-doped ZnO NP has been shown to be smaller in comparison to undoped ZnO NP, hence making it more effective in influencing the cell systems [8, 9].
Previous reports have also suggested that the crystalline structure and particle shape of the ZnO have less influence on the antimicrobial ability as compared to the particle size [10]. There are implications that negatively charged NPs are more effective for biological applications due to their higher adsorption rate; whereas, a positively charged NP would aid in reducing the toxicity of particle [11].
The green approach is where the biochemical compounds present in the organic extract can act as reducing agents in the synthesis or help the stabilization of the metal oxide NPs [12]. Biosynthesized nanoparticles also showed better antimicrobial action in comparison to its chemically synthesized counterpart as the green-synthesized ZnO has a stronger inhibitory effect [11]. The green-synthesized ZnO NPs showed relatively low toxicity as the toxic chemical that may appear in the chemical synthesis had been replaced by a non-toxic phytochemical [13,14,15,16].
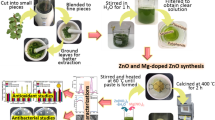
In this work, the green synthesis of ZnO employed the use of aqueous leaf extract from the plant, Melastoma malabathricum locally known in Brunei as ‘kuduk-kuduk’ (Fig. 1). This plant is native to Southeast Asia and belongs to the Melastomataceae family where it was previously reported to exhibit antiviral, cytotoxic and a high anti-oxidant activity [17, 18].
Herein, we report the first fabrication of ZnO and Mn-doped ZnO using the aqueous leaf extract of Melastoma malabathricum. The fabricated ZnO and Mn-doped ZnO NPs were characterized and tested for antibacterial activities against four bacterial strains.
Materials and methods
Materials, techniques and methods used
Zn(NO3)2·6H2O (98%) and Mn(CH3CO2)2·4H2O were purchased from Sigma-Aldrich and used as received. Melastoma malabathricum (L.) leaves were obtained from Universiti Brunei Darussalam Botanical Research Centre (UBD BRC) and authenticated by a botanist from UBD BRC.
Powder X-ray diffraction (XRD, MiniflexII, Rigaku, Japan) analysis was carried out to determine the structural properties and phase purity of the fabricated ZnO and Mn-doped ZnO using Cu Kα radiation. The optical properties of the fabricated materials were examined using ultraviolet–visible (UV–Vis) diffuse reflectance spectroscopy (UV-3600 UV–Vis NIR Spectrophotometer, Shidmadzu, Japan). The chemical states and surface analysis of the fabricated ZnO and Mn-doped ZnO NPs was performed using X-ray photoelectron spectroscopy (XPS, JPS-90110MC, JEOL, Japan). The C 1 s (285 eV) was used as a reference to calibrate the peak positions of the elements. The morphology, particle size and composition were measured using scanning electron microscopy (SEM, JEOL, Japan). The fabricated ZnO and Mn-doped ZnO were also analyzed for surface charge (zeta potential) using Malvern Zetasizer Nano series.
Preparation of the aqueous leaf extract
The leaves of the Melastoma malabathricum (L.) were collected and washed to remove dust and residues. Leaves were air dried overnight before being finely cut and then ground into a thick paste. The thick paste weighing 5 g was then mixed with 100 mL of distilled water in a 250-mL beaker. The mixture was left to be stirred for 1 h before the leaf extract was obtained using gravity filtration.
Synthesis of undoped ZnO and (1% and 5%) Mn-doped ZnO
For the fabrication of undoped ZnO NPs, Zn(NO3)2·6H2O as a precursor was added into 30 mL of aqueous leaf extract. The solution mixture was preheated to 60 °C and then stirred at constant temperature until a paste was formed. The paste was transferred into a crucible and it was calcined at 400 °C for 2 h. The product was ground into fine powder and kept in a desiccator for further characterization and application.
For the synthesis of Mn-doped ZnO, the same procedure was followed but with an addition of Mn(CH3CO2)2·4H2O as a second precursor. The Mn(CH3CO2)2·4H2O was added immediately after the addition of Zn(NO3)2·6H2O into the extract solution. The same procedure was carried out throughout the experiment. The product obtained was ground into fine powder and kept in a desiccator for characterization and application.
Antibacterial activity screening
The microorganisms used in this study consisted of four bacterial strains, two Gram-negative bacteria: Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 27853) and two Gram-positive bacteria: Bacillus subtilis (ATCC 6633) and Staphylococcus aureus (ATCC 25923). The antibacterial activities of the green-synthesized ZnO were determined by disc diffusion method.
The samples were first screened for anti-microbial activity at the highest concentration (500 mg/mL). 10 µL of biosynthesized ZnO, 1% Mn-doped ZnO and 5% Mn-doped ZnO was applied onto a 6-mm Whatman No.1 filter paper disc and was left to dry overnight. They were then placed on a spread of bacterial lawn on a Mueller–Hinton agar (MHA) plate alongside one negative and two positive controls (Fig. 2a). The positive controls used consisted of 500 mg/mL commercial ZnO and 20 mg/mL Streptomycin. The negative control used was 10 mL of distilled water. The zone of inhibition was recorded after incubating the plates at 37 °C for 24 h. Each test was carried out in triplicates.
Minimum inhibitory concentration
The minimum inhibitory concentration (MIC) testing of the samples was carried out on the bacterial strains that have shown activity (inhibition zone of > 7 mm) from the previous antibacterial tests screening. Five concentrations of each sample were prepared at lower concentrations (25, 50, 100, 300 and 500 mg/mL) and 10 µL of each concentration of the sample was loaded onto paper disc to be placed onto Mueller–Hinton agar plate together with the positive and negative controls (Fig. 2b). The plate was incubated overnight at 37 °C and the zone of inhibition was measured by diameter comparison after 24 h. The experiments were performed in triplicates with fresh bacterial inoculum.
Results and discussion
Using green and phytogenic approach, we report that undoped ZnO, 1% and 5% Mn-doped ZnO were successfully fabricated using the aqueous leaf extract of Melastoma malabathricum (L.). The advantage of this approach is that it is simple, environmentally friendly, and it does not require any harmful chemicals, high-energy inputs, capping or reducing agents and lengthy treatments. The reaction medium for this protocol was water and the fabrication process took place at low temperature, 30–60 °C, except calcination of the paste at 400 °C.
Powder X-ray diffraction
The powder XRD analysis was conducted to determine the purity, structure and crystallite size of the ZnO and Mn-doped ZnO. Figure 3a shows the XRD patterns obtained from the undoped ZnO, Mn-doped ZnO and commercial ZnO (ZnO–C) samples. The diffraction peaks correspond to (100), (002), (101), (102), (110) and (103) crystal planes of the wurtzite crystal structure of ZnO NPs (JCPDS-36–1451), and no additional features from impurities and residues were detected, suggesting that the fabricated ZnO NPs are of single-phase ZnO without impurities. These results confirmed that the undoped ZnO and Mn-doped ZnO have been successfully synthesized. The absence of Mn oxide peaks suggests that Mn has been inserted into the lattice and the samples are pure. The mean crystallite size of the nanoparticles was calculated using Scherrer’s equation and was found to be 4.75 nm, 4.36 nm and 4.62 nm for undoped ZnO, 1% Mn-doped ZnO and 5% Mn-doped ZnO, respectively.
UV–Vis DRS studies
To examine the effects of Mn-doping on the optical properties, the undoped ZnO, 1% and 5% Mn-doped ZnO were analyzed using UV–Vis DRS spectroscopy within the wavelength range of 200–800 nm. Figure 3b presents the UV–Vis DRS absorbance spectra of ZnO–C and undoped ZnO as well as 1% and 5% Mn-doped ZnO NPs. From the spectra, the absorbance of ZnO–C was observed at ~ 390 nm. Whereas absorbance for undoped ZnO, 1% and 5% Mn-doped ZnO showed a red shift. In comparison to the ZnO–C, the biosynthesized ZnO also showed high absorbance in the range 500–800 nm. The approximate band gap energy was calculated by inputting the absorption peak values (\(\lambda )\) in the following equation:
where h is Planks constant (6.626 × 10–34 Js), c is the speed of light (3.0 × 108 ms−1) and λ is wavelength (nm). The approximate band gap energy was found to be 3.06 eV for ZnO, 2.66 eV for 1% Mn-doped ZnO and 2.56 eV for 5% Mn-doped ZnO. The synthesized ZnO and Mn-doped ZnO samples have lower band gap energy values in comparison to the ZnO–C (3.18 eV). The 5% Mn-doped ZnO has the lowest band gap energy compared to the other two synthesized ZnO.
Surface charge of nanoparticles is an important factor that influences cell membrane interaction. In this work, the zeta potential of the fabricated ZnO surface charge was investigated using Malvern Zetasizer Nano series. The zeta potential for the undoped ZnO NP, 1% Mn-doped ZnO and 5% Mn-doped ZnO was found to be − 0.15 mV, 2.32 mV and 2.22 mV, respectively (Table 1). This indicates that there is a net positive surface charge for 1% Mn-doped ZnO and 5% Mn-doped ZnO; whilst, the undoped ZnO had a negative surface charge. This suggests that the Mn doping can affect the surface charge of ZnO, although the values obtained for the 1% and 5% Mn were similar to each other.
Scanning electron microscopy and energy-dispersive X-ray spectroscopy
The SEM images suggested that the fabricated ZnO showed a roughly spherical shape. Figure 4a illustrates an agglomerated spherical shape of the synthesized nanoparticles [19]. The morphology of the 1% Mn-doped ZnO was spherical-shaped NPs as illustrated in Fig. 4b. Based on Fig. 4, the sizes of the NPs were calculated to be approximately 272 nm, 705 nm and 222 nm for ZnO, 1% Mn-doped ZnO and 5% Mn-doped ZnO, respectively. The inset in each figure shows the respective EDX spectra that confirmed the presence of zinc, oxygen in all three samples and manganese in Mn-doped ZnO samples as presented in Fig. 4b, c. Few other low-intensity peaks were also observed in the EDX spectra. These peaks indicate the presence of elements, such as carbon and potassium which could be originated from the leaf extract. It was reported that these phytochemicals from leaf extract are involved in the reduction and capping of the ZnO [20].
X-ray photoelectron spectroscopy analysis
XPS was used to quantitatively analyze and examine the bonding, chemical states and surface chemical composition of the undoped ZnOs. Figure 5a shows the survey spectrum of the fabricated undoped ZnO that displays all the expected elements which correspond to Zn 2p, O 1 s and C 1 s. This suggests that there were no impurities in the undoped ZnO. Figure 5b shows the C 1 s photoelectron peak at a binding energy (BE) of 285 eV, which was attributed to the surface carbon impurities. The carbon element represents the phytochemical compounds of the leaf extract present on the surface of the samples. The chemical states of O were examined, and XP spectrum of O 1 s of the undoped ZnO NPs are shown in Fig. 5c. The O 1 s spectrum of the ZnO was fitted with three peaks which may be present in different oxygen environments, the presence of loosely bound oxygen on the surface of ZnO, O2− ions in the wurtzite structure of hexagonal Zn2+ ions and O2− in the oxygen-deficient regions within the ZnO matrix [16]. Figure 5d shows Zn 2p spectrum resulted in two peaks at binding energies of 1021 eV and 1044 eV which corresponds to the Zn2+ 2p3/2 and Zn2+ 2p1/2, respectively, indicating that Zn in the undoped ZnO is in the form of Zn2+ [16].
Figure 6 shows XP spectra of the 5% Mn-doped ZnO. Figure 6a shows the survey spectrum of the fabricated 5% Mn-doped ZnO that displays all the expected elements which correspond to Zn 2p, Mn 2p, O 1 s and C 1 s. Figure 6b shows the C 1 s photoelectron peak at a binding energy (BE) of 285 eV, which was attributed to the surface carbon impurities. The carbon element represents the phytochemical compounds of the leaf extract present on the surface of the samples. Figure 6c shows the Zn 2p spectrum in which two peaks at binding energies of 1021 eV and 1044 eV correspond to the photo-splitting electrons, Zn2+ 2p3/2 and Zn2+ 2p1/2, respectively, indicating that Zn in the sample is in the form of Zn2+. Figure 6d shows the Mn 2p spectrum with two peaks at binding energy 642 eV and 653 eV which corresponds to the Mn2+ 2p3/2 and Mn2+ 2p1/2 respectively. To determine the binding states of oxygen in 5% Mn-doped ZnO, the O 1 s XPS peak was fitted to three peaks centered at BE of 530.52, 532.22 and 528.06 eV. Figure 6e shows the chemical states of O in the 5% Mn-doped ZnO. The O 1 s spectrum of the ZnO can be ideally thought of as being comprised of three factors: the presence of loosely bound oxygen on the surface of ZnO, O2− ions in the wurtzite structure of hexagonal Zn2+ ions and O2− in the oxygen-deficient regions within the ZnO matrix [16].
Antibacterial activity screening
The in vitro antimicrobial activity of the ZnO, 1% Mn-doped ZnO and 5% Mn-doped ZnO fabricated using Melastoma malabathricum aqueous leaf extract was evaluated against two Gram-negative bacteria (E. coli and P. aeruginosa) and two Gram-positive bacteria (B. subtilis and S. aureus). The antibacterial screening shows that P. aeruginosa and E. coli tests did not exhibit any zone of inhibitions at the maximum concentration of 500 mg/mL tested (Table 2). This could be due to the bacteria having a metallostasis mechanism that can enable it to thrive in Zn-excess and Zn-depleted condition. The mechanism in P. aeruginosa involves a zinc uptake regulator (ZUR) protein which regulates the Zn content in the cell [21]. Furthermore, P. aeruginosa has protein pumps such as the CzcCBA efflux pump which plays an essential role in the resistance to heavy metal and lowering the risk of metal intoxication. Hence, the overall effect of Zn2+ ions toxicity mechanism suggests that it is nullified in these bacteria.
However, all the green-synthesised ZnO reported here showed antibacterial activity against B. subtilis and S. aureus at the highest concentration tested at 500 mg/mL. No inhibition zone was detected for the negative control in all the tests.
Minimum inhibitory concentration
The MIC was performed on ZnO, which upon screening, was found to have a medium to high zone of inhibition of at least 7 mm. The results for the MIC tests of B. subtilis and S. aureus with the undoped and Mn-doped ZnO samples are shown in Figs. 7 and 8. There was no zone of inhibition observed for the negative control. The zone of inhibition for the positive control, streptomycin was higher when compared to the ZnO, 1% Mn-doped ZnO and 5% Mn-doped ZnO.
The B. subtilis showed the highest zone of inhibition against 1% Mn-doped ZnO at 50 mg/mL. This was followed by 100 mg/ml of 1% Mn-doped ZnO, 25 mg/mL of 1% Mn-doped ZnO and 25 mg/mL of 5% Mn-doped ZnO where both had the same zone of inhibition diameters (Table 3). For S. aureus, ZnO recorded the highest zone of inhibition at 50 mg/mL followed by 100 mg/mL of 1% Mn-doped ZnO. The 5% Mn-doped ZnO recorded the lowest value of the zone of inhibition between 8.5 and 9.7 mm for the range of concentrations tested.
As seen in Figs. 9 and 10, the toxicity of the green and phytogenic fabricated ZnO was comparatively low in comparison to the positive control. This difference between the biosynthesized ZnO and the positive control, streptomycin was significantly different with p < 0.01 (Tables 4 and 5).
The lower toxicity of the green-synthesized ZnO could be due to the agglomeration and aggregation of the particles which prevents the NPs from penetrating inside the bacteria effectively due to the submicron size and the lack of homogeneity [22]. This aggregation of ZnO could have raised due to the ZnO being nearly insoluble in water and the high polarity of the water which causes the ZnO to agglomerate [23].
The 1% Mn-doped ZnO was found to have a higher antibacterial activity for B. subtilis compared to the undoped ZnO (p < 0.05) (Table 4) in contrast to S. aureus (Table 5). This supports the theory by Mishra et al. [11] which suggests that the lower the band gap energy, the higher the antibacterial effect [4]. In addition to this, the 1% Mn-doped ZnO had the most positive zeta potential charge in comparison to the other biosynthesized ZnO. The higher positive zeta potential of 1% Mn-doped ZnO is suggested to have interacted with the negative surface potential of bacterial membrane which leads to shifting of potential towards surface charged neutrality that altered the membrane permeability or disrupted the membrane structure and results in bacterial inhibition [24].
Conclusion
A green and phytogenic method was successfully used to fabricate undoped ZnO, 1% and 5% Mn-doped ZnO using aqueous leaf extract of M. malabathricum plant which was confirmed by powder XRD, XPS, SEM, and EDX. Powder XRD and XPS confirmed the wurtzite structure of the materials and the oxidation state of both Zn and Mn was found to be + 2. The zeta potential measurements showed that undoped ZnO has a net negative surface charge; whereas, the 1% and 5% Mn-doped ZnO have a net positive surface charge. The biosynthesized undoped ZnO, 1% and 5% Mn-doped ZnO showed no bacterial activity on the Gram-negative bacteria P. aeruginosa and E. coli. However, these fabricated NPs showed an antibacterial effect towards Gram-positive bacteria S. aureus and B. subtilis. The 1% Mn-doped ZnO was the most effective on B. subtilis with the minimum inhibitory concentration being 50 mg/mL.
References
Demirel R, Suvacı E, Şahin İ, Dağ S, Kılıç V (2018) Antimicrobial activity of designed undoped and doped MicNo-ZnO particles. J Drug Deliv Sci Technol 47:309–321. https://doi.org/10.1016/j.jddst.2018.07.024
Dobrucka R, Długaszewska J (2016) Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J Biol Sci 23:517–523. https://doi.org/10.1016/j.sjbs.2015.05.016
Bui V, Park D, Lee Y-C (2017) Chitosan combined with ZnO, TiO2 and Ag nanoparticles for antimicrobial wound healing applications: a mini review of the research trends. Polymers (Basel) 9:21. https://doi.org/10.3390/polym9010021
Kundu D, Hazra C, Chatterjee A, Chaudhari A, Mishra S (2014) Extracellular biosynthesis of zinc oxide nanoparticles using Rhodococcus pyridinivorans NT2: Multifunctional textile finishing, biosafety evaluation and in vitro drug delivery in colon carcinoma. J Photochem Photobiol B Biol 140:194–204. https://doi.org/10.1016/j.jphotobiol.2014.08.001
Arakha M, Roy J, Nayak PS, Mallick B, Jha S (2017) Zinc oxide nanoparticle energy band gap reduction triggers the oxidative stress resulting into autophagy-mediated apoptotic cell death. Free Radic Biol Med 110:42–53. https://doi.org/10.1016/j.freeradbiomed.2017.05.015
Zhang Y, Nguyen KC, Caldwell D, Fine JH, Lefebvre DE, Tayabali AF (2017) Immune responses during single and repeated murine endotracheal exposures of zinc oxide nanoparticles. NanoImpact 7:54–65. https://doi.org/10.1016/j.impact.2017.06.003
Ahtzaz S, Nasir M, Shahzadi L, Amir W, Anjum A, Arshad R, Iqbal F, Chaudhry AA, Yar M, ur Rehman I (2017) A study on the effect of zinc oxide and zinc peroxide nanoparticles to enhance angiogenesis-pro-angiogenic grafts for tissue regeneration applications. Mater Des 132:409–418. https://doi.org/10.1016/j.matdes.2017.07.023
Moontragoon P, Pinitsoontorn S, Thongbai P (2013) Mn-doped ZnO nanoparticles: preparation, characterization, and calculation of electronic and magnetic properties. Microelectron Eng 108:158–162. https://doi.org/10.1016/j.mee.2013.01.061
Zare E, Pourseyedi S, Khatami M, Darezereshki E (2017) Simple biosynthesis of zinc oxide nanoparticles using nature’s source, and it’s in vitro bio-activity. J Mol Struct 1146:96–103. https://doi.org/10.1016/j.molstruc.2017.05.118
Dumbrava A, Berger D, Matei C, Radu MD, Gheorghe E (2018) Characterization and applications of a new composite material obtained by green synthesis, through deposition of zinc oxide onto calcium carbonate precipitated in green seaweeds extract. Ceram Int 44:4931–4936. https://doi.org/10.1016/j.ceramint.2017.12.084
Mishra PK, Mishra H, Ekielski A, Talegaonkar S, Vaidya B (2017) Zinc oxide nanoparticles: a promising nanomaterial for biomedical applications. Drug Discov Today 22:1825–1834. https://doi.org/10.1016/j.drudis.2017.08.006
Ehsan S, Sajjad M (2017) Bioinspired synthesis of zinc oxide nanoparticle and its combined efficacy with different antibiotics against multidrug resistant bacteria. J Biomater Nanobiotechnol 08:159–175. https://doi.org/10.4236/jbnb.2017.82011
Nagajyothi PC, Cha SJ, Yang IJ, Sreekanth TVM, Kim KJ, Shin HM (2015) Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J Photochem Photobiol B Biol 146:10–17. https://doi.org/10.1016/j.jphotobiol.2015.02.008
Khan MM, Saadah NH, Khan ME, Harunsani MH, Tan AL, Cho MH (2019) Potentials of Costus woodsonii leaf extract in producing narrow band gap ZnO nanoparticles. Mater Sci Semicond Process 91:194–200. https://doi.org/10.1016/j.mssp.2018.11.030
Khan MM, Saadah NH, Khan ME, Harunsani MH, Tan AL, Cho MH (2019) Phytogenic synthesis of band gap-narrowed ZnO nanoparticles using the bulb extract of Costus woodsonii. Bionanoscience 9:334–344. https://doi.org/10.1007/s12668-019-00616-0
Ansari SA, Khan MM, Kalathil S, Nisar A, Lee J, Cho MH (2013) Oxygen vacancy induced band gap narrowing of ZnO nanostructures by an electrochemically active biofilm. Nanoscale 5:9238. https://doi.org/10.1039/c3nr02678g
Joffry SM, Yob NJ, Rofiee MS, Affandi MMRMM, Suhaili Z, Othman F, Akim AM, Desa MNM, Zakaria ZA (2012) Melastoma malabathricum (L.) smith ethnomedicinal uses, chemical constituents, and pharmacological properties: a review, evidence-based complement. Altern Med 2012:1–48. https://doi.org/10.1155/2012/258434
Lohézic-Le Dévéhat F, Bakhtiar A, Bézivin C, Amoros M, Boustie J (2002) Antiviral and cytotoxic activities of some Indonesian plants. Fitoterapia 73:400–405. https://doi.org/10.1016/S0367-326X(02)00125-9
Raj LFA, Jayalaskhmy E (2015) Biosynthesis and characterization of zinc oxide nanoparticles using root extract of Zingiber Officinale. Orient J Chem 31:51–56. https://doi.org/10.13005/ojc/310105
Moezzi A, McDonagh AM, Cortie MB (2012) Zinc oxide particles: synthesis, properties and applications. Chem Eng J 185–186:1–22. https://doi.org/10.1016/j.cej.2012.01.076
Gonzalez MR, Ducret V, Leoni S, Perron K (2019) Pseudomonas aeruginosa zinc homeostasis: key issues for an opportunistic pathogen. Biochim Biophys Acta Gene Regul Mech 1862:722–733. https://doi.org/10.1016/j.bbagrm.2018.01.018
Saravanan M, Gopinath V, Chaurasia MK, Syed A, Ameen F, Purushothaman N (2018) Green synthesis of anisotropic zinc oxide nanoparticles with antibacterial and cytofriendly properties. Microb Pathog 115:57–63. https://doi.org/10.1016/j.micpath.2017.12.039
Dimapilis EAS, Hsu CS, Mendoza RMO, Lu MC (2018) Zinc oxide nanoparticles for water disinfection. Sustain Environ Res 28:47–56. https://doi.org/10.1016/j.serj.2017.10.001
Arakha M, Saleem M, Mallick BC, Jha S (2015) The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle. Sci Rep 5:9578. https://doi.org/10.1038/srep09578
Acknowledgements
Authors would like to acknowledge the FIC block grant UBD/RSCH/1.4/FICBF(b)/2018/012 received from Universiti Brunei Darussalam, Brunei Darussalam. Authors would also like to thanks CAMES, UBD for extending their facilities for some analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, M.M., Harunsani, M.H., Tan, A.L. et al. Antibacterial activities of zinc oxide and Mn-doped zinc oxide synthesized using Melastoma malabathricum (L.) leaf extract. Bioprocess Biosyst Eng 43, 1499–1508 (2020). https://doi.org/10.1007/s00449-020-02343-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02343-3