Abstract
Phenolic compounds could pose environmental problems if they are in excess, although they could be a renewable resource of substances with industrial interest. The novel strain Bacillus aryabhattai BA03 is able to produce high-added value metabolites from different phenolic compounds such as vanillin, 4-vinylguaiacol and 4-vinylphenol while inducing ligninolytic enzymes such as laccases (Lac) and lignin peroxidases (LiP). In comparison with the medium without inducers, the presence of 500 mg/L caffeic acid improved 9.1-fold times the expression of Lac (0.118 ± 0.004 U/mL) and 5.8-fold times the expression of LiP (2.300 ± 0.053 U/mL), just as these processes exhibited high global rates of biotransformation. When isoeugenol, ferulic acid or p-coumaric acid are in the media, the strain removed more than 90% of these compounds, secreting vanillin, 4-vinylguaiacol or 4-vinylphenol. Bacillus aryabhattai proved to be an appropriate tool for the removal of several phenolic compounds and the production of more valuable products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenolic compounds are a heterogeneous family, but their molecules contain, as a common feature, at least a benzene ring linked to one hydroxyl group. The rest of the derivatives are based on this structure but bonded to hydroxyl, methoxyl and/or carboxylic groups [1]. Although phenolic acids can be classified in different ways, in 1964 Harbone and Simmons proposed their division into groups based on the number of carbons in the molecule: benzoic acids (such as protocatechuic acids or p-hydroxybenzoic acid), cinnamic acids (including p-coumaric, ferulic, caffeic and sinapic acids) and other related compounds [1]. Phenolic compounds have been used in alcoholic drinks and foods due to their organoleptic properties; and as anti-allergenic, anti-inflammatory, anti-microbial and antioxidants because their biological and physiological functions are beneficial to human health [2, 3]. However, despite these beneficial effects, they can present deleterious effects on soil and water when they are widely available in the environment [4, 5], especially when they derive from oil, petrochemical, pharmaceutical or cosmetic industrial activities, among others [6].
Industrial wastewaters contain phenolic compounds in variable concentrations from 1 to 6000 mg/L [6]; and some strains are able to metabolize them, consequently being used in bioremediation processes such as natural attenuation, biostimulation, and bioaugmentation [7]. These microbial processes allow for the elimination of undesired substances, but sometimes the metabolic pathways involved can lead to intermediate or final products of biotechnological interest [8]. In this sense, phenolic compounds have been proposed as precursors of aromas and flavors, which have a variety of applications in the food, feed, cosmetic, chemical and pharmaceutical industries [9]. These types of bioactive compounds are present in nature but in small quantities, making their extraction and manufacture more expensive than their chemical synthesis. However, an ecofriendly way to obtain such bioactive compounds is based on the biotransformation of natural precursors by microbial cells or enzymes [10].
There is a potent group of oxidative enzymes widely studied in wood-rotting organisms, and particularly in white-rot basidiomycetes [11], whose substrates are the phenolic compounds. These ligninolytic enzymes have been classified into phenol oxidases such as laccases (EC 1.10.3.2) and heme-peroxidases, which includes lignin peroxidase (EC 1.11.1.14) and manganese peroxidase (EC 1.11.1.13), among others. Laccases (Lac) catalyze the oxidation of different phenolic and non-phenolic compounds using oxygen as an electron acceptor; meanwhile, peroxidases catalyze multistep oxidation and hydroxylation reactions using H2O2 as an electron acceptor. Specifically, lignin peroxidase (LiP) acts on lignin to oxidize it to phenolic and non-phenolic compounds along with semi-degraded lignin derivatives, whereas manganese peroxidase (MnP) oxidizes Mn(II) to Mn(III), further enhancing the degradation of phenolics [12, 13].
In this work, we focused on the induction of ligninolytic enzymes (LiP, MnP, and Lacs) of Bacillus aryabhattai BA03 by several phenolic compounds that could be found in effluents from industrial activities. At the same time, we sought to investigate their metabolism in order to promote this strain as a good candidate for bioremediation or biotransformation of effluents into high added-value products.
Materials and methods
Chemicals
The chemicals used in this study, including methanol for HPLC, were of analytical HPLC grade and acquired from Sigma–Aldrich.
Culture media
The commercial medium Trypticasein Soy Broth (TSB) was used to grow the microorganism. Biotransformations and ligninolytic enzymes production were assayed in culture medium formulated with only 10 g/L yeast extract (YE). Both media (supplied by Pronadisa) were sterilized in autoclave (121 °C) for 15 min.
Microorganism and inoculum preparation
The strain under evaluation was B. aryabhattai BA03, which was originally isolated in our laboratory and deposited in the Spanish Type Culture Collection (CECT) with accession number CECT 9010. The sequence data in the EMBL and Genbank databases have the accession number LN824023.
The strain was reconstituted in 50 mL of TSB poured into Erlenmeyer flasks and incubated at 37 °C and 150 rpm. After 24 h, cells were recollected and cleaned twice with phosphate buffered saline containing 8 g/L of sodium chloride, 0.13 g/L of disodium hydrogen phosphate and 0.58 g/L sodium dihydrogen phosphate (pH 7.4). Biomass concentration was determined by optical density at 600 nm in a UV–Vis Spectrophotometer (Libra S60-Biochrom, Cambridge, UK).
Effect of phenolic compounds in the metabolism of Bacillus aryabhattai BA03
Ferulic acid, caffeic acid, vanillyl alcohol, 4-hydroxybenzoic acid, vanillin, p-coumaric and isoeugenol sterilized by filtration (0.20 μm membrane filters) were tested on YE medium at concentrations of 500 mg/L and 1000 mg/L. Experiments were carried out in 100 mL of culture medium placed in orbital shakers at 37 °C and 150 rpm. After pH adjustment with NaOH to 7.5, the inoculum was added at a final concentration of 500 mg/L. Experiments were performed in triplicate, including a control flask to measure the effects of the abiotic parameters.
Determination of ligninolytic enzymes
Samples of culture medium were withdrawn regularly to quantify the production of ligninolytic extracellular enzymes such as Lac, LiP and MnP. Cells were separated by centrifugation at 3420 g for 6 min and the supernatant was used to determine the enzymatic activity as follows.
Laccases activity was determined following the methodology proposed by Siroosi et al. [14] based on the increase of absorbance at 420 nm when the enzyme oxidizes the substrate 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS Ɛ = 36,000 M−1 cm−1). The reaction mixture contained 250 µL of 50 mM citrate–phosphate buffer (pH 5), 30 µL of 5 mM ABTS and 20 µL of supernatant. This activity was determined at 30 °C.
Lignin peroxidases activity was measured at 310 nm and 30 °C following the methodology proposed by Tien and Kirk [15]. The reaction mixture contained 200 µL of 0.25 M sodium tartrate buffer (pH 3.5), 40 µL veratryl alcohol 10 mM, 50 µL H2O2 4 mM and 710 µL of supernatant.
Manganese peroxidases activity was analyzed in two ways, either as manganese dependent or independent, following the methodology proposed by Kuwahara et al. [16]. The reaction mixture of MnP-dependent was carried out at 30 °C, pH 4.5 and contained 200 µL of 0.25 M sodium tartrate buffer, 50 µL of 2,6-dimethoxyphenol 20 mM, 50 µL of MnSO4 H2O 20 mM, 100 µL of H2O2 4 mM and 600 µL of supernatant. The reaction mixture for MnP-independent was the same, but without MnSO4 H2O.
All activities were done in triplicate and were defined as the amount of enzyme that oxidized one µmol of the substrate per minute (U/mL).
Determination of phenolic compounds
The concentration of initial phenolic compounds and metabolites formed were determined by liquid chromatography. Samples (1.5 mL) were taken at selected times (depending on the duration of the process), centrifuged at 3420 g for 6 min, and filtered through cellulose acetate membranes (0.2 µm pore). The liquid phase was used to analyze the concentration of compounds employing a reverse phase HPLC system (Agilent model 1200, Palo Alto, CA, USA) with an UV-diode array detector and a 4.6 × 150 mm Zorbax SB-Aq column (Agilent, Palo Alto, CA, USA) following the elution program described by Paz et al. [17]. Quantification was performed by extrapolating the peak areas using the corresponding equation of standard curves.
Statistical analysis
Production of ligninolytic enzymes with the different phenolic compounds was submitted to analysis of variance (ANOVA) by the Statistica Software 13.0. They were compared with the production in medium without inducers, using the Tukey’s test at significance level p < 0.05, and asterisks were used to label values with statistically significant differences among them.
Results and discussion
Production of ligninolytic enzymes
Ligninolytic extracellular enzymes such as Lac, LiP and MnP are known for their high redox potential and their versatility in the degradation of compounds with both phenolic and non-phenolic constituents. Therefore, these enzymes have potential industrial applications in the sectors of biorefinery, textile, paper, beauty products, and dermatologic [11], but are also extremely valuable in bioremediation techniques [13, 18]. Accordingly, in order to understand the reactions involved in the metabolism of B. aryabhattai Ba03, we focused on the study of Lac, LiP and MnP. Figure 1 shows the production of Lac, LiP and MnP, both dependent and independent, in YE medium without supplementation of inducers, indicating maxima activities at 72 h for Lac (0.013 ± 0.000 U/mL) and 96 h for LiP (0.396 ± 0.002 U/mL); under these conditions neither of the two types of MnP were expressed.
Despite fungi laccases are the most studied, there are several references to bacterial laccases isolated from the genera Bacillus and Streptomyces, among other [19]. Bacteria show some significant advantages over fungi, including faster growth and a general tolerance of a broader range of habitats and conditions [20]. The drawback, though, is that bacterial enzymes are usually considered to be intracellular, thus raising the cost of their industrial implementation [21]. There are few studies on extracellular laccases produced by genus Bacillus, due to the low levels determined, although some authors found that these levels were enough to break bonds of the type C–C or C–O [22]. Thus, Chang et al. indicated that 0.003 U/mL of laccases promoted by Bacillus sp. are able to produce high ratios of lignin degradation [23], and Siroosi et al. used 0.01 U/mL to carry out decolorization processes [14].
Regarding LiP activity, there is some controversy in the literature about the presence of this enzyme in bacteria, since some authors argue that LiP belong exclusively to fungi, and in the case of bacteria being called dye-decolorizing peroxidases (DyPs, EC 1.11.1.19). Enzymes of this type are able to oxidize phenolic and non-phenolic compounds, and both have a high redox potential, LiP shows values of 1.26 ± 0.17 V, whereas DyPs estimated to range between 1.10 ± 0.02 and 1.20 ± 0.1 V [11, 19, 24].
The last type of peroxidases studied was the MnP activity, in spite of the fact that it was not detected when B. aryabhattai Ba03 grew in YE medium at 37 °C. Asina et al. [25] suggest that the carbon and nitrogen sources may induce the production of ligninolytic enzymes and MnP activity could be suppressed, in media with high nitrogen content for example.
Production of ligninolytic enzymes in the presence of inducers
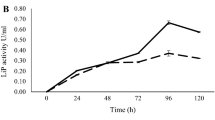
The induction of ligninolytic enzymes activity by phenolic compounds has been reported in many genera [26]. Hence, it has been hypothesized that the induction of laccases by aromatic compounds is a protective response to the toxicity of these compounds [27]. In this sense, Elisashvili et al. [28] suggested that enhanced laccases expression may function as a defense mechanism against chemical stress. Concerning LiP, the most studied producer has been the fungus Phanerochaete chrysosporium. Several authors tested different inducers, seeking to improve the production in submerged cultures of this microorganism. Ansari et al. [29] managed to increase the production of LiP to 0.22 U/mL after 15 days by the addition of glucose oxidase as compared to the control culture (without glucose oxidase). On the other hand, Zacchi et al. [30] obtained between 0.2 and 0.4 U/mL of LiP after 4 days using cellulose as a carbon source; a similar value (0.475 U/mL) was found by Kapich et al. [31] after 3 days using lignocellulosic substrates. In our case, the supplementation of YE culture media with phenolic compounds not only improved the production of Lac and LiP by B. aryabhattai Ba03 but also reduced the time needed to achieve maximal production. Results are presented in Fig. 2. It can be observed that in the presence of inducers, the maximal production time for Lac was reduced from 72 to 42 h (Fig. 2a, b), and LiP also was reduced from 96 to 56 h, indeed to 28 h in the presence of vanillyl alcohol (Fig. 2c, d). In addition, Table 1 shows the maxima values achieved for each tested compound. The positive effect of all the phenolic compounds assayed over Lac and LiP expressions can be observed. In comparison with the medium without inducers is notable the increment produced by the presence of 500 mg/L caffeic acid, which improved 9.1-fold times the expression of Lac (0.118 ± 0.004 U/mL) and 5.8-fold times the expression of LiP (2.300 ± 0.053 U/mL).
Course over time for production of Lac activity (a, b) and LiP activity (c, d) by Bacillus aryabhattai Ba03 in YE medium supplemented with inducers (500 mg/L): ferulic acid (dot and hyphen line), caffeic acid (squared dotted line), vanillyl alcohol (continuous line), vanillin (dashed line), p-coumaric (dotted line) and isoeugenol (long dashed line)
Metabolism of different phenolic compounds
The aim of the current study was to explore the production of ligninolytic enzymes in the presence of phenolic compounds that could be found in industrial effluents. This approach could be useful to explain how the strain could work in real wastewaters with a combination of these phenolic compounds. For example, in oil industries the extraction of olive or palm oil generates high amounts of waste, with a high phytotoxicity and impact on land and water environments [8]. These activities produce huge volumes of effluents with high amounts of phenolic compounds such as caffeic acid, p-coumaric acid, ferulic acid or vanillin. In addition, these wastewaters also present high levels of organic content, toxicity, dark brown color, and antimicrobial properties [4]. Therefore, the metabolism of different phenolic compounds was assayed in YE medium, based on previous work [17, 32] which reported that this strain was unable to perform biotransformation in sugar-containing media.
All compounds were prepared at an initial concentration of 500 mg/L due to this concentration was the minimum inhibitory concentration found by Lemos et al. [33] for Bacillus cereus. Additionally, when the strain biotransformed the phenolic compounds into valuable products, the concentration was increased until 1000 mg/L. Independently on the concentration, it was observed that the concentration of phenolic compounds in the control experiment remained constant, thus confirming that all the biotransformation processes were caused by the strain.
Table 2 shows the compounds studied as precursors (previously assayed as inducers of enzymes), as well as the maxima concentration of extracellular metabolites released by B. aryabhattai. Figure 3 in turn shows the course of time as well as the chemical structure of the products released by decarboxylation or oxidation of the biotransformation processes. Bacillus aryabhattai transformed phenolic compounds into other substrates with the exception of caffeic acid, from which no other metabolite was detected by HPLC. The remaining compounds were metabolized between 61.9 and 93.9% and transformed into other valuable metabolites such as vanillin, vanillyl alcohol, 4-vinyl guaiacol (4VG) and 4-vinylphenol.
Isoeugenol (4-hydroxy-3-methoxy-1-propenyl-benzene) is a phenylpropanoid easily available from the industry associated with the essential oil of the clove tree (Syzygium aromaticum), which requires specific management [34]. Despite some authors reporting the toxic effect of isoeugenol on the cells when it was added before the strain growth [35, 36], in our experiments isoeugenol was directly supplied at the beginning of the biotransformation, without waiting for the stationary phase. Figure 3a shows that 68.0% of isoeugenol was degraded by B. aryabhattai BA03 in the first 24 h at a rate of 28.346 mg/L h. After that, the degradation rate slowed down drastically, achieving a maximal production of vanillin (149.461 ± 1.571 mg/L) at 312 h. Finally, after 17 days, the concentration of isoeugenol was reduced to only 16.790 ± 0.721 mg/L, whereas vanillin was slightly decreased (10.8%) without producing other metabolites. Some authors have postulated that vanillin is an intermediate of reaction, rather than a final product, that can be metabolized over prolonged periods into vanillyl alcohol and vanillic acid [9, 37]. For this, recombinant cells and systems that prevent the degradation of produced vanillin, and systems that allow for vanillin recovery, are being developed. Recently, Zhao et al. [38], using a sol–gel chitosan membrane and a recombinant strain of Escherichia coli, were able to obtain approximately 4500 mg/L of vanillin from 6000 mg/L of isoeugenol. In our case, B. aryabhattai BA03 only produced vanillin as a metabolite from isoeugenol, and it was not metabolized again. However, when 1000 mg/L vanillin was used as a substrate (Fig. 3b), 76.4% of vanillin was converted into 690.3 ± 0.00 mg/L of vanillyl alcohol after just 24 h. The different behavior in these two cases could be related to the different concentrations assayed (149.5 ± 1.57 mg/L in Fig. 3a and 930.7 ± 1.08 mg/L in Fig. 3b), which suggests that a minimal threshold is necessary to provoke the response of the microorganism against the toxicity of vanillin, or to produce the necessary enzymes necessary to be transformed into another metabolite (vanillyl alcohol). In addition, the biotransformation of vanillyl alcohol into vanillin by B. aryabhattai BA03 (Table 2) was almost entirely irreversible, since starting with 485.6 ± 18.65 mg/L of vanillyl alcohol, despite 61.9% of the initial concentration being degraded, only a minimal amount of vanillin was released (26.3 ± 0.85 mg/L), the product yield being only 0.087 mg/mg. These findings contradict the metabolic pathway of 1-propenylbenzene isoeugenol proposed by Xu et al. [9], where isoeugenol is transformed into vanillin through an epoxide–diol pathway and then further converted to vanillic acid and protocatechuic acid.
Isoeugenol can also be used as a lignin model compound. Lignin has a highly heterogeneous structure, and compounds with similar linkages and functionality are used to represent the most common substructure of lignin [39]. Peroxidases and lacasses are able to cleave the lignin structure, although the metabolites produced from cleavage of linkages β-aryl ether are not always observed. In this sense, several authors have explained the pathway through vanillic acid to lignin depolymerization [40, 41]. For example, Rhodococcus jostii RHA1 is a bacterium producer of peroxidase DypB that effects such a metabolic route; but neither vanillin nor p-hydroxybenzaldehyde were observed in fermentations using a wild-type strain. Only when some deletion of genes was carried out these compounds were detected [41]. In agreement with these authors, our results showed that vanillin was generated as metabolite, but in our case without performing genetic modifications of the strain.
Finally, ferulic acid (FA) and p-coumaric acid (p-CA) were also studied, since they are two of the most important hydroxycinnamic acids available in the environment, and their increase has adverse effects on soil [5]. Ferulic acid results compiled in a previous study [17] showed that the catabolic pathway included 4-vinyl guaiacol (4VG) as an intermediary metabolite, with a global volumetric productivity of 35.621 mg/L h and a product yield of 0.51 mg/mg. Also of note here is that 97.5% of the initial FA was degraded in this short period. 4-vinyl guaiacol was a transitory peak that gradually decreased; at the same time vanillin appeared, producing a maximal threshold of 45.8 ± 0.69 mg/L after 192 h. Similarly, 96.2% of p-CA was also metabolized by B. aryabhattai in only 22 h to produce 851.4 ± 11.17 mg/L of 4-vinilphenol (4VP) (see Fig. 3c). P-coumaric acid is also a hidroxycinnamic acid derived from plant cell walls as a secondary metabolite, and it is found covalently linked to polysaccharides and lignin by ester bonds and/or ether bonds [42]. Meanwhile, the metabolite produced (4VP) has several applications as a flavor and fragrance in the perfume, food and beverages industries, but also as an antifungal agent [43].
Consequently, all the compounds tested in this study, with the exception of caffeic acid, can be removed, simultaneously releasing another high-added metabolite (Table 1). Molecular structure was seen to transform one compound into another, requiring oxidation reactions. Based on data in the literature and observing that all enzymatic activities increased when inducers are present in the medium, we determined that there is a correlation between them, and we postulated B. aryabhattai as a microorganism with laccase and peroxidase activities inducible by phenolic compounds.
Conclusions
Bacillus aryabhattai BA03 shows interesting capabilities in the degradation of recalcitrant compounds and the production of some value-added compounds. The metabolism of phenolic compounds not only exhibit a high rate also shows a strong influence of them on the production of ligninolytic enzymes. Consequently, B. aryabhattai BA03 can be a useful tool for cleaning effluents with a high content of phenolic compounds, and at the same time the enzymes produced (Lac and LiP) could be recollected and reused with bioremediation aims in mind.
References
Vermerris W, Nicholson R (2006) Phenolic compound biochemistry. Springer, Netherlands
Araújo M, Pimentel FB, Alves RC, Oliveira MBPP (2015) Phenolic compounds from olive mill wastes: health effects, analytical approach and application as food antioxidants. Trends Food Sci Technol 45:200–211
Costa DC, Costa HS, Albuquerque TG et al (2015) Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci Technol 45:336–354
Kietkwanboot A, Tran HTM, Suttinun O (2015) Simultaneous dephenolization and decolorization of treated palm oil mill effluent by oil palm fiber-immobilized Trametes hirsuta strain AK 04. Water Air Soil Pollut 226:345
Gauri SS, Mandal SM, Dey S, Pati BR (2012) Biotransformation of p-coumaric acid and 2,4-dichlorophenoxy acetic acid by Azotobacter sp. strain SSB81. Bioresour Technol 126:350–353
Saha S, Badhe N, Pal S et al (2017) Carbon and nutrient-limiting conditions stimulate biodegradation of low concentration of phenol. Biochem Eng J 126:40–49
Dzionek A, Wojcieszynska D, Guzik U (2016) Natural carriers in bioremediation: a review. Electron J Biotechnol 23:28–36
Dermeche S, Nadour M, Larroche C et al (2013) Olive mill wastes: biochemical characterizations and valorization strategies. Process Biochem 48:1532–1552
Xu P, Hua D, Ma C (2007) Microbial transformation of propenylbenzenes for natural flavour production. Trends Biotechnol 25:571–576
Berger RG (2015) Biotechnology as a source of natural volatile flavours. Curr Opin Food Sci 1:38–43
Falade AO, Nwodo UU, Iweriebor BC et al (2017) Lignin peroxidase functionalities and prospective applications. Microbiologyopen 6:1–14
Asgher M, Shahid M, Kamal S, Iqbal HMN (2014) Recent trends and valorization of immobilization strategies and ligninolytic enzymes by industrial biotechnology. J Mol Catal B Enzym 101:56–66
Paramjeet S, Manasa P, Korrapati N (2018) Biofuels: production of fungal-mediated ligninolytic enzymes and the modes of bioprocesses utilizing agro-based residues. Biocatal Agric Biotechnol 14:57–71
Siroosi M, Amoozegar MA, Khajeh K (2016) Purification and characterization of an alkaline chloride-tolerant laccase from a halotolerant bacterium, Bacillus sp. strain WT. J Mol Catal B Enzym 134:89–97
Tien M, Kirk TK (1988) Lignin peroxidase of Phanerochaete chrysosporium. Methods Enzymol 161:238–249
Kuwahara M, Glenn JK, Morgan MA, Gold MH (1984) Separation and characterization of two extracelluar H2O2-dependent oxidases from ligninolytic cultures of Phanerochaete chrysosporium. FEBS Lett 169:247–250
Paz A, Outeiriño D, de Souza Pinheiro, Oliveira R, Domínguez JM (2018) Fed-batch production of vanillin by Bacillus aryabhattai BA03. N Biotechnol 40:186–191
Sharma B, Dangi AK, Shukla P (2018) Contemporary enzyme based technologies for bioremediation: a review. J Environ Manage 210:10–22
de Gonzalo G, Colpa DI, Habib MHM, Fraaije MW (2016) Bacterial enzymes involved in lignin degradation. J Biotechnol 236:110–119
Margot J, Bennati-Granier C, Maillard J et al (2013) Bacterial versus fungal laccase: potential for micropollutant degradation. AMB Express 3:63
Fernandes Rigamonte Alves T, Batista Da Silveira W, Lopes Passos FM, Zucchi TD (2014) Laccases from actinobacteria—What we have and What to expect. Adv Microbiol 4:285–296
Catherine H, Penninckx M, Frédéric D (2016) Product formation from phenolic compounds removal by laccases: a review. Environ Technol Innov 5:250–266
Chang YC, Choi DB, Takamizawa K, Kikuchi S (2014) Isolation of Bacillus sp. strains capable of decomposing alkali lignin and their application in combination with lactic acid bacteria for enhancing cellulase performance. Bioresour Technol 152:429–436
Tian JH, Pourcher AM, Klingelschmitt F et al (2016) Class P dye-decolorizing peroxidase gene: degenerated primers design and phylogenetic analysis. J Microbiol Methods 130:148–153
Asina FNU, Brzonova I, Kozliak E et al (2017) Microbial treatment of industrial lignin: successes, problems and challenges. Renew Sustain Energy Rev 77:1179–1205
Chandra R, Chowdhary P (2015) Properties of bacterial laccases and their application in bioremediation of industrial wastes. Environ Sci Process Impacts 17:326–342
De Souza CGM, Tychanowicz GK, De Souza DF, Peralta RM (2004) Production of laccase isoforms by Pleurotus pulmonarius in response to presence of phenolic and aromatic compounds. J Basic Microbiol 44:129–136
Elisashvili V, Kachlishvili E, Khardziani T, Agathos SN (2010) Effect of aromatic compounds on the production of laccase and manganese peroxidase by white-rot basidiomycetes. J Ind Microbiol Biotechnol 37:1091–1096
Ansari Z, Karimi A, Ebrahimi S, Emami E (2016) Improvement in ligninolytic activity of Phanerochaete chrysosporium cultures by glucose oxidase. Biochem Eng J 105:332–338
Zacchi L, Burla G, Zuolong D, Harvey PJ (2000) Metabolism of cellulose by Phanerochaete chrysosporium in continuously agitated culture is associated with enhanced production of lignin peroxidase. J Biotechnol 78:185–192
Kapich AN, Prior BA, Botha A et al (2004) Effect of lignocellulose-containing substrates on production of ligninolytic peroxidases in submerged cultures of Phanerochaete chrysosporium ME-446. Enzyme Microb Technol 34:187–195
Paz A, Carballo J, Pérez MJ, Domínguez JM (2016) Bacillus aryabhattai BA03: a novel approach to the production of natural value-added compounds. World J Microbiol Biotechnol 32:159
Lemos M, Borges A, Teodósio J et al (2014) The effects of ferulic and salicylic acids on Bacillus cereus and Pseudomonas fluorescens single- and dual-species biofilms. Int Biodeterior Biodegradation 86:42–51
Ashengroph M, Nahvi I, Zarkesh-Esfahani H, Momenbeik F (2012) Conversion of isoeugenol to vanillin by Psychrobacter sp. Strain CSW4. Appl Biochem Biotechnol 166:1–12
Zhang Y, Xu P, Han S et al (2006) Metabolism of isoeugenol via isoeugenol-diol by a newly isolated strain of Bacillus subtilis HS8. Appl Microbiol Biotechnol 73:771–779
Zhao LQ, Sun ZH, Zheng P, He JY (2006) Biotransformation of isoeugenol to vanillin by Bacillus fusiformis CGMCC1347 with the addition of resin HD-8. Process Biochem 41:1673–1676
Hua D, Ma C, Lin S et al (2007) Biotransformation of isoeugenol to vanillin by a newly isolated Bacillus pumilus strain: identification of major metabolites. J Biotechnol 130:463–470
Zhao L, Xie Y, Chen L et al (2018) Efficient biotransformation of isoeugenol to vanillin in recombinant strains of Escherichia coli by using engineered isoeugenol monooxygenase and sol–gel chitosan membrane. Process Biochem 71:76–81
Patil ND, Tanguy NR, Yan N (2015) Lignin interunit linkages and model compounds. In: Lignin in Polymer Composites. William Andrew Publishing, Elsevier, pp 27–47
Chen Z, Wan C (2017) Biological valorization strategies for converting lignin into fuels and chemicals. Renew Sustain Energy Rev 73:610–621
Sainsbury PD, Hardiman EM, Ahmad M et al (2013) Breaking down lignin to high-value chemicals: the conversion of lignocellulose to vanillin in a gene deletion mutant of Rhodococcus jostii RHA1. ACS Chem Biol 8:2151–2156
Max B, Torrado AM, Moldes AB et al (2009) Ferulic acid and p-coumaric acid solubilization by alkaline hydrolysis of the solid residue obtained after acid prehydrolysis of vine shoot prunings: effect of the hydroxide and pH. Biochem Eng J 43:129–134
Salgado JM, Rodríguez-Solana R, Curiel JA et al (2014) Bioproduction of 4-vinylphenol from corn cob alkaline hydrolyzate in two-phase extractive fermentation using free or immobilized recombinant E. coli expressing pad gene. Enzyme Microb Technol 58–59:22–28
Acknowledgements
The authors are grateful to the Spanish Ministry of Economy and Competitiveness for financial support of this research (project CTQ2015-71436-C2-1-R), which has partial funding from the FEDER funds of the European Union; and to the Xunta de Galicia (Consellería de Cultura, Educación e Ordenación Universitaria), for Alicia Pérez Paz’s postdoctoral fellowship ED481B 2018/073. This study forms part of the activities of the Group with Potential for Growth (ED431B 2018/54-GPC), the Xunta de Galicia (Spain).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paz, A., Costa-Trigo, I., Tugores, F. et al. Biotransformation of phenolic compounds by Bacillus aryabhattai. Bioprocess Biosyst Eng 42, 1671–1679 (2019). https://doi.org/10.1007/s00449-019-02163-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02163-0