Abstract
Varying chemical oxygen demand (COD) and sulphate concentrations in substrate were used to determine reaction kinetics and mass balance of organic matter and sulphate transformation in a microbial fuel cell (MFC). MFC with anodic chamber volume of 1 L, fed with wastewater having COD of 500 mg/L and sulphate of 200 mg/L, could harvest power of 54.4 mW/m2, at a Coulombic efficiency of 14%, with respective COD and sulphate removals of 90 and 95%. Sulphide concentration, even up to 1500 mg/L, did not inhibit anodic biochemical reactions, due to instantaneous abiotic oxidation to sulphur, at high inlet sulphate. Experiments on abiotic oxidation of sulphide to sulphur revealed maximum oxidation taking place at an anodic potential of −200 mV. More than 99% sulphate removal could be achieved in a MFC with inlet COD/sulphate of 0.75, giving around 1.33 kg/m3 day COD removal. Bioelectrochemical conversion of sulphate facilitating sulphur recovery in a MFC makes it an interesting pollution abatement technique.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Domestic sewage and municipal wastewaters typically contain between 20 and 500 mg/L of sulphate. Several industrial wastewaters like paper and pulp, food processing, animal husbandry, and pharmaceutical industry contain much higher concentrations of sulphate, sulphite, or other sulphur compounds [1, 2]. Sulphate emissions are not a direct threat for the environment as sulphate is a chemically inert, non-volatile, and non-toxic compound. Current restrictions on sulphate emission in environmental legislation mainly aim to reduce the salt content of surface water and/or to minimize acid condensation in sewers [1]. Sulphate is anaerobically reduced to sulphide by sulphate reducing bacteria (SRB) [3]. Higher sulphide concentration above about 150 mg/L in wastewater is toxic to methanogenic bacteria, acetogenic bacteria, and SRB [1, 4]. However, this value is a gross approximation as inhibition due to sulphide depends on a wide variety of factors like sludge conditions, pH, type of bacteria present, and even on the chemical oxygen demand (COD)/sulphate ratio [1]. However, generation of sulphide during anaerobic treatment of sulphate rich wastewater can upset the entire system performance. Part of the sulphide also remains dissolved in the effluent of the anaerobic reactor. This results in a lowering of overall treatment efficiency of the system, as sulphide contributes to the effluent COD, since two moles of oxygen are required for complete oxidation of one mole of sulphide into sulphate. Moreover, sulphide can upset the treatment efficiency of the aerobic post treatment system by causing algal blooming in lagoons or activated sludge bulking [1]. Along with this, sulphide causes bad odours and can be corrosive to the pipelines and other appurtenances [4, 5]. With COD/sulphate lower than ten, process failure of anaerobic reactors has been reported in literature [1]. This necessitates simultaneous sulphide removal process during anaerobic treatment of sulphate rich wastewater.
Several methods have been used by researchers to remove sulphide; the most common being physico–chemical methods involving precipitation of sulphide using iron salts. However, major drawbacks of this method are the costs associated with iron dosage, clogging of the inlet pipes, and the accumulation of precipitated FeS in the reactor [1, 6]. Another common method for removal of sulphides is chemical oxidation, where several oxidants, such as molecular oxygen, hydrogen peroxide, hypochlorite, chlorine, and permanganate, are used. However, these treatments are unsatisfactory in terms of both chemicals used and disposal of the resulting hazardous sludge [5]. By comparison with the other processes, biological processes are cost-effective and operate at natural prevailing environmental conditions without any requirement for expensive chemicals and catalysts [4]. Some bacterial species like sulphide oxidizing bacteria (SOB) can oxidize sulfide to elemental sulphur [3, 7]. Partial biological oxidation of sulphide to S is a low-cost alternative for sulphur reclamation, as S is non-soluble and thus can be removed from the wastewater [1].
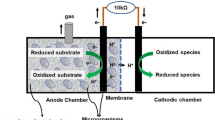
In a microbial fuel cell (MFC), organic compounds are oxidized by microorganisms, and the electrons generated from this oxidation can be used to produce energy and other value-added products [8]. Although MFCs have been established for effective removal of organic matter, however, treatment of wastewater in MFC with high sulphate concentration still remains a challenge [8, 9]. Ghangrekar, Murthy [10] studied the effect of COD and sulphate ratio in a microbial fuel cell and observed an increase in COD removal and power production with decrease in COD/sulphate up to 0.8 in the wastewater. Conversion of these compounds leads to the release of sulphides, which are toxic and odorous as mentioned earlier. Sulphides can be oxidized to various sulphur species. Depending on the redox potential and specific reaction conditions, over 30 different species can be produced [11]. Sulphide is oxidized under standard conditions to elemental sulphur at potentials at least higher than −0.274 V versus standard hydrogen electrode (SHE). Increasing the potential can further oxidize elemental sulphur. At more positive redox potentials, more oxidized forms of sulphur, such as sulphite and sulphate or polysulphide, will be the reaction products [11]. Reduction of sulphate to sulphide in anaerobic reactors causes substrate loss, because substrate is used to form sulphide instead of methane, where methane production is the core process of the reactor, as well as it needs additional treatment cost to abate the emission of sulphurous compounds in the biogas. A MFC could partially recover the energy comprised in the sulphide through its re-oxidation to elemental sulphur at the anode. While treating wastewater with high sulphate concentration, the energy loss due to lower methane gas production can be compensated by electricity generation from oxidation of sulphide in a MFC [11]. However, relatively lower power density of MFCs restricts its practical applications. Hence, sulphate removal studies in MFCs should also focus on improving the power generated by the system to make a significant contribution to the knowledge base.
Power production in a MFC is limited by the overpotential of oxygen reduction reaction (ORR) at the cathode [12]. To reduce ORR overpotential, catalysts are often used on the cathode. Platinum is the most expensive and frequently used catalyst [12]. To reduce cost of wastewater treatment in MFCs, recent research has focussed on development of low-cost cathode catalysts. Recently, cobalt tetroxide was developed as a low-cost catalyst for use in Lithium-ion batteries [13, 14]. In this study, sulphate and organic matter removal at different inlet COD/sulphate ratios was explored in MFCs using cobalt tetraoxide (Co3O4) nano-octahedron as cathode catalyst. The performance characteristics of the MFC system, including power output, sulphate removal, and organic matter degradation, were monitored, and kinetics and mass balance of reactions were worked out. Unique bioelectrochemical conversion of sulphate to facilitate sulphur recovery from sulphate rich wastewaters in a MFC marks the novelty of the approach and also makes the process an interesting pollution abatement technique.
Materials and methods
Synthesis of catalyst
The Co3O4 nano-octahedron catalyst material was synthesized through a modified hydrothermal process reported earlier [15]. In a typical procedure, 0.2 mol of Co(NO3)2·6H2O and 0.05 mol of NaOH were dissolved in 40 mL deionized water. The solution was heated at 180 °C in a Teflon lined autoclave for about 5 h and was allowed to cool to room temperature. The product obtained was washed five times with ethanol and distilled water and subsequently dried at 60 °C. Finally, the resultant material was calcinated at 500 °C in a muffle furnace for a period of 3 h to obtain Co3O4 nano-octahedron particles.
Construction of the MFCs
Four cylindrical dual-chamber MFCs (MFC-1, MFC-2, MFC-3, and MFC-4) were constructed by boring in acrylic sheets of thickness 2.5 cm, making an effective anodic and cathodic chamber volume of 50 mL to treat synthetic wastewater spiked with sulphate. Carbon felt anode of projected surface area 16 cm2 was placed in the middle of the anode chamber. Cathode made of stainless steel (SS) mesh of projected surface area 16 cm2 was used in MFC-1 and MFC-2 without catalyst. In MFC-3 and MFC-4, similar SS mesh coated with 0.5 mg/cm2 of Co3O4 nano-octahedrons was used as cathode. A solution of the catalyst Co3O4 was prepared with acetone as the solvent and Nafion as binder, which was sprayed using a spray gun on the SS mesh for MFC-3 and MFC-4 cathodes. Nafion membrane was used as the proton exchange membrane, which was hot-pressed manually at a temperature of 60 °C to the stainless steel cathode. The electrodes were connected to an external resistance of 100 Ω using concealed copper wires. All four MFCs were, later on, also used for kinetics and mass balance studies for COD and sulphate removal, using catalysed cathodes.
A cylindrical clayware dual-chamber MFC with anodic chamber volume 900 mL was used to treat raw sewage spiked with sulphate to investigate scaling up options of MFC treating real wastewater. Carbon felt anode of projected surface area 300 cm2 was placed in the middle of the anode chamber. The cathode made of carbon felt of projected surface area 400 cm2 coated with 0.5 mg/cm2 of Co3O4 nano-octahedrons was used as cathode. The electrodes were connected to an external resistance of 100 Ω using concealed copper wires.
Operation of the MFCs
Screening experiments
Synthetic wastewater having acetate as carbon source with COD of 2000 ± 250 mg L−1 was used as anolyte for evaluating COD and sulphate removal in the MFCs. The acetate medium also contained (per gram of COD) NaHCO3, 1500 mg; NH4Cl, 318 mg; CaCl2·2H2O, 250 mg; MgSO4·7H2O, 64 mg; K2HPO4, 27 mg; and KH2PO4, 9 mg. Trace metals were added as FeSO4·6H2O, 10.00 mg L−1; MnSO4, 0.526 mg L−1; ZnSO4·7H2O, 0.106 mg L−1; H3BO3, 0.106 mg L−1; and CuSO4·5H2O, 4.5 μg L−1; CoCl2, 105.2 μg L−1; (NH4)6Mo7O24·4H2O, 105.2 μg L−1. Sulphate (200 mg L−1) was added to the anodic feed solution of MFC-2 and MFC-4 in the form of anhydrous Na2SO4. Aerated tap water was used as catholyte. Sludge from already working MFC treating synthetic wastewater of similar composition, of volume 5 mL, was used to inoculate the MFCs. The MFCs were operated in 72 h fed-batch mode as most of the sulphate and sulphide generated from sulphate reduction were removed within that time. After two batch cycles of operation, the system was considered to be under pseudo steady-state conditions, when the stable voltage output was reproducible in two or more cycles.
Kinetic studies and mass balance analysis
Later on, to perform kinetic studies and mass balance analysis related to sulphate and COD removal, the acrylic sheet MFCs were used with catalysed cathodes. The MFCs were operated in varying ranges of COD viz 200, 500, 1000, 2000, and 3000 mg L−1. For a COD of 3000 mg L−1, sulphate of 200, 250, 500, 1000, 2000, 3000, 4000, 5000, 6000, 7000, and 8000 mg L−1 was added to study the effect of sulphate/COD molar ratio and sulphate concentration on performance of MFC. These MFCs were also operated at a sulphate concentration of 200 and 3000 mg L−1 for the entire range of inlet CODs to understand the effect of COD/sulphate ratio on electricity generation. For the kinetic studies, sulphate of 50, 100, 150, 200, and 250 mg L−1 was added to wastewater with COD of 2000 mg L−1 in the MFCs. Overall, the MFCs were operated at different COD/sulphate of 0.067, 0.17, 0.33, 0.375, 0.43, 0.5, 0.6, 0.67, 0.75, 1, 1.5, 2.5, 3, 5, 6, 10, and 15.
Scaling up of the MFC treating real wastewater
Anodic chamber of the clayware MFC was fed with raw sewage of COD concentration around 500 ± 24 mg L−1, spiked with anhydrous Na2SO4 to have a sulphate concentration of 700 ± 5 mg L−1. Aerated tap water was used as catholyte. Sludge from an already working MFC treating synthetic wastewater, of volume 50 mL, was used to inoculate the MFC. The MFC was operated in a 72 h fed-batch mode.
Batch sulphide oxidation experiments
Batch tests were conducted to verify the feasibility of spontaneous sulphide oxidation. Single chambered electrolysis cells, constructed using 500 mL glass beakers, were used for this purpose. Total liquid volume of 450 mL was maintained during the operation. Graphite felt of projected surface area of 120 cm2 was used as anode. The cell was fed with a saline buffer containing NaCl (1 g L−1), NaH2PO4 (1.58 g L−1), and Na2HPO4 (6.38 g L−1), to maintain a near neutral pH, and Nitrogen gas (N2) was purged externally to maintain anaerobic condition in the cell. After that, sulphide in the form of Na2S was added in each cell to maintain a sulphide concentration of 100 ± 5 mg L−1. The cells were operated at a fed-batch time of 3 days and sulphur metabolites were analysed every 24 h. Each batch test was performed in triplicates. The batch tests aimed to investigate the influence of anode potential on electrochemical sulphide oxidation. This was achieved by controlling the potential at fixed levels between 0 to −500 mV (vs SHE) at intervals of 100 mV.
Analysis and calculations
Chemical oxygen demand (COD) was measured according to closed reflux colorimetric method. Sulphate was measured by turbidimetric method. Sulphide was determined according to the iodometric titration method. The indication of “sulphide” described all sulphur species (H2S, HS−, and S2 −). Analysis of COD, sulphate, and sulphide was carried out as per the procedures described in Standard Methods [16]. Measurement of pH was done using a pH meter (Thermo, Kansas, USA). Samples were taken at 24 h intervals to measure the residual COD, sulphate, and sulphide over the course of the whole experiment.
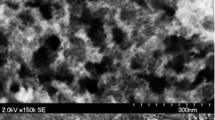
The MFCs were continuously monitored for voltage produced using a data acquisition system connected to a computer. Open circuit voltage (OCV) and operating voltage (OV) were measured daily. Polarization curves were obtained by measuring voltages at various external resistances from 10,000 Ω to 1 Ω to evaluate the relationship between voltage and current. For each point on the polarization curves, voltage readings were taken when the voltage stabilized. The current (I) in amperes (A) was calculated by Ohm’s law, I = V/R, where V is the potential drop in volts (V) across the external load resistance R in Ohms. The power output P in watts (W) and the power density (PD, W/m2) were calculated according to P = I × V and PD = I × V/S, respectively, where S (m2) is the projected surface area of the anode. Coulombic efficiency (CE) was deduced as, CE = (CP/CT × 100) %, where CP is the total number of coulombs calculated by integrating the current over time. CT is the theoretical number of coulombs that can be produced from the wastewater used as anolyte. CT was calculated from CT = FbS o v A /M o , where F is the Faraday’s constant (96,485 C/mol), b is the number of moles of electrons produced per mole of substrate (four for oxygen), S o is the reduction in amount of COD, v A is the liquid volume in the anodic chamber, and M o is the molecular weight of oxygen [17]. Pieces of anodes after use were analysed in energy dispersive X-ray spectroscopy (EDX) in Jeol, SEM (Japan) for elemental composition.
Results and discussion
Sulphate removal in the MFCs
MFC-1 and MFC-3 were operated without any sulphate in the inlet feed. Sulphate concentration in the MFCs decreased from 200 ± 12 to 10 ± 7 mg L−1 for MFC-2 and 5 ± 4 mg L−1 for MFC-4 on day 3, thereby giving a sulphate removal rate of 0.063 kg/m3.day for MFC-2 and 0.065 kg/m3.day for MFC-4 from an initial sulphate loading rate of 0.067 kg/m3.day (Fig. 1). Almost similar sulphate removal rate for MFC-2 without cathode catalyst and MFC-4 with cathode catalyst indicates that cathode catalyst did not have a significant impact on sulphate reduction or sulphide oxidation reactions. Zhou and Xing [18] operated an anaerobic filter for sulphate removal using different carbon sources as electron donors and observed a maximum sulphate removal rate of 8.6 kg/m3.day at an initial sulphate loading of 10 kg/m3.day using ethanol as carbon source and no sulphate removal was reported with acetate as carbon source. In addition, acetate was the final end product in the sulphate reduction reactions in all the cases using complex carbon sources. However, that is not a feasible solution while treating wastewater. In this study, synthetic wastewater with acetate as carbon source and later on raw sewage was successfully treated along with simultaneous sulphate removal. Majority of SRBs cannot directly use complex carbon sources as electron donors, which needs to be converted to lower molecular weight compounds by fermentative bacteria [18]. Hence, use of mixed culture of bacterial sludge proved to be beneficial for simultaneous removal of organic matter and sulphate while treating raw sewage.
When organic material is oxidized via sulphate reduction, eight electrons are accepted per molecule of sulphate. Because one molecule of oxygen can only accept four electrons, the electron accepting capacity of two moles of O2 equals 1 mol of SO 2−4 which is 0.67 g of O2 per g of SO 2−4 . This means that, for wastewaters with a COD/sulphate of 0.67, there is theoretically enough sulphate available to completely remove the organic matter (COD) via sulphate reduction. For COD/sulphate lower than 0.67, the amount of organic matter is insufficient for a complete reduction of the sulphate present and extra substrate should be added if removal of sulphate is the objective of the treatment. On the contrary, for wastewaters with a COD/sulphate exceeding 0.67, as in this present study with an average COD/sulphate of ten, a complete removal of the organic matter can only be achieved if in addition to sulphate reducing bacteria, other types of bacteria are also present [1]. In this study, in both the MFCs for an initial COD concentration of around 2000 mg L−1 and sulphate concentration of around 200 mg L−1, after sulphate reduction, COD available for electrogenesis is around 1870 mg L−1. However, after reduction of sulphate to sulphide, further oxidation of sulphide improves power generation, as sulphide is oxidized by donating electrons to the anode.
Oxidation of sulphide in the MFCs
Sulphide concentration was observed to increase from 0 to 48 ± 3 mg L−1 in anolyte of MFC-2 and 55 ± 6 mg L−1 for MFC-4 after 48 h, and then reduced to 5 ± 2 and 3 ± 1 mg L−1, respectively, in MFC-2 and MFC-4 within the next 24 h. Since, sulphate concentration did not increase even after 48 h when sulphide concentration reduced, it would be safe to assume that the sulphide was oxidized to elemental sulphur and not sulphate due to non-availability of oxygen. The graphite felt anode acted as the electron acceptor to support sulphide to sulphur oxidation. Zhao et al. [2] also concluded that elemental sulphur is the dominant oxidation product in the anodic chamber of a MFC and not sulphate. Sulphur entering MFC-2 in each batch, in form of sulphate, is 3.33 mg and that coming out from the effluent as sulphate and sulphide are, respectively, 0.17 and 0.25 mg, indicating an elemental sulphur deposition of 2.91 mg, considering negligible losses as gaseous H2S and metallic precipitates. Therefore, sulphur recovery in MFC-2, with a reaction time of 72 h is 87.4%. Similarly, for MFC-4, having cathode catalyst, sulphur recovery can be estimated from the sulphur mass balance within the reactor as 93.1%. After the experiments were over, the anode was removed from the anodic chamber and rinsed with deionized water to remove any sulphate species on the anode surface and was processed for the EDX analysis. While the EDX analysis of fresh graphite felt showed only the presence of Carbon, elemental sulphur deposition was observed on the graphite felt anodes of the MFCs with a weight percentage of 27.45% of the entire surface area. In other traditional aerobic biological methods for sulphide removal, sulphide is oxidized to sulphate; which could again be reduced to sulphide biologically if anaerobic conditions develop due to oxygen depletion in the clarifier, without actually solving the problem. Since, in MFCs, sulphide is converted to elemental sulphur only; hence, it is a better solution to the problem of sulphur removal.
Organic matter removal in the MFCs
Organic matter removal efficiencies in terms of COD in MFC-1, MFC-2, MFC-3, and MFC-4 were 78 ± 6, 93 ± 5, 87 ± 2 and 94 ± 5%, respectively. The absence of sulphate, in the feed of MFC-1 and MFC-3, stopped the metabolism of SRB, thus allowing only electrogenic bacteria to thrive, resulting in an excess of electron donors, as shown by higher effluent COD in MFC-1 and MFC-3 as compared to MFC-2 and MFC-4. Similar results were reported by Zhou and Xing [18]. The consumed sulphate and COD molar ratios increased with increasing sulphate concentration and the inlet sulphate/COD molar ratio up to a ratio of 1.14 (Fig. 2), which is slightly lower than the theoretical COD consumption for sulphate reduction. Further increase of the inlet molar ratio or sulphate concentration did not increase the molar ratio of sulphate and COD consumption. Molar ratio of consumed sulphate and COD curve indicates that rate of reduction of sulphate and oxidation of COD was not same (Table 1). Molar consumption of COD was higher up to an inlet sulphate/COD molar ratio of around 0.86. Molar consumption of sulphate became higher than COD oxidation rate with a further increase in inlet sulphate/COD molar ratio. Although it is a known fact that SRBs use organic matter as electron donors for reduction of sulphate, but the exact pathway of organic matter degradation and sulphate reduction is still unknown.
Electricity generation from the MFCs
The four MFCs made from acrylic sheet were fed with synthetic wastewater for 2 months in this study. Already acclimatized inoculum collected from a different MFC treating synthetic wastewater of similar concentration was used; hence, start-up time required for acclimatization of bacteria could be avoided. However, it took 2–3 days for biofilm formation on anode; hence, there was a delay in voltage response (Fig. 3). By the second cycle, voltage generation stabilized and reached an average operating voltage (OV) of 29 ± 6, 24 ± 4, 62 ± 4, and 57 ± 5 mV in MFC-1, MFC-2, MFC-3, and MFC-4, respectively. The presence of sulphate in the anolyte of MFC-2 and MFC-4 was expected to reduce the voltage response than the corresponding MFCs with the same configuration and without sulphate in anolyte, because sulphate reducing bacteria (SRB) used substantial amount of organic matter for the reduction of sulphate [18]. Gibb’s free energy for oxidation of organic matter for sulphate reduction is more negative than electrogenic oxidation of the same substrate, thereby making sulphate reduction a more favourable reaction [18]. However, the presence of sulphide, produced from the reduction of sulphate, added to the electricity generation in MFC-2 and MFC-4, thus compensating for the electron loss due to sulphate reduction. Sulphide produced from reduction of sulphate in MFC-2 and MFC-4 acted as electron donors [19]. The presence of sulphide in anolyte could lower the anode potential to facilitate electricity generation according to the Nernst equation, thus allowing the MFCs to maintain a stable operating voltage [4]. A maximum power density of 6.85, 8.03, 29.26, and 23.63 mW/m2 was obtained in MFC-1, MFC-2, MFC-3, and MFC-4, respectively. Power outputs increased with use of cathode catalyst. MFC-3 offered the highest CE of 15.2% (Table 1). In MFC-2, contribution of sulphide to the charge harvested was 20.43%; while that for MFC-4, contribution of sulphide to charge harvested was only 10.68%, though CE was observed to increase from 5.95% in MFC-2 to 13.72% in MFC-4 due to the presence of cathode catalyst. This shows the contribution of catalyst in increasing operating voltage, power production, and CE. However, cathode catalyst had very little contribution to improve sulphide oxidation or COD removal efficiency. In general, lower CE in the studies can be due to precipitation of metallic sulphides in anodic sludge and also to some extent because of diffusion of sulphide to the cathodic compartment. In addition, at the pH levels in the anodic chamber (6.8–7.2), equilibrium exists between HS− and H2S. The latter can diffuse through the membrane separating the anode and cathode either as a gas or dissolved in liquid [11]. This results in lower current capture than theoretically predicted. Other than that, oxygen diffusion into the anodic chamber and mass transfer limitations can also cause lower Coulombic efficiency. Low Coulombic efficiency and low power density of the MFC systems indicate the need for improvement of the systems.
Effect of different inlet COD/sulphate ratios
As shown in Fig. 4a, there was directly an ascending trend in the maximum power as the initial COD concentration was increased from 200 mg L−1 up to 3000 mg L−1 with a constant initial sulphate concentration of 200 mg L−1 that is with gradually increasing COD/sulphate ratio. As discussed earlier, COD/sulphate ratio should be greater than 0.67 for complete sulphate removal. It was also observed that contribution of sulphide to the actual charge harvested increased with decreasing COD-to-sulphate ratio. Power outputs increased with the up-going initial substrate concentrations as Coulombic efficiency decreased, in a polynomial equation described by the following equation:
where y is the Coulombic efficiency (%) and x is the ratio of initial COD to sulphate. This equation is valid only when sulphate is available in limited conditions that are at COD/sulphate higher than 1 and the maximum power that can be obtained obviously depends on the electrode material. Power densities obtained in co-substrate MFC system do not fit the Monod-like response so well as those reported in other MFCs with single substrate, such as acetate, because sulphide served as an alternative electron donor for electrochemically active microbes in the anode compartment. Similar observations were reported by Zhang et al. [19]. When COD is present in limited condition that is with COD/sulphate being less than 1, the Coulombic efficiency can be represented by Eq. 2 and Fig. 4b:
The main limitations of these two equations are in its boundary conditions, majorly the surface area and material of the electrode. The equations do not consider the surface area of electrode and electrode materials, which are important factors affecting power generation. Coming up with an universal equation that will give power output or Coulombic efficiency is difficult. One has to incorporate the surface area of electrode actually available for oxygen reduction at the cathode and the actual surface area where bacterial attachment can take place and electrochemistry of electrodes, as well. However, these equations give a fairly good idea on how the Coulombic efficiency or power production might vary just with change in the ratio of COD and sulphate, when other conditions are constant.
Coulombic efficiency was also observed to decrease with increasing sulphate loading rates at a constant COD loading. This indicates that majority of contribution to voltage generation was from COD and not from sulphide oxidation, which is also evident from the mass balance calculations in Table 1. A similar observation was also reported by Rabaey, Van De Sompel [11] who observed a decrease in Coulombic efficiency from 29.5 to 14.6% when the sulphide concentration was increased from 100 to 300 mg L−1. Due to higher sulphate concentration in anolyte, sulphide concentration inside the reactor was higher than the maximum tolerable limit of 150 mg L−1 for methanogens or SRBs. However, due to instantaneous oxidation of sulphide at the anode, it did not cause any toxicity to the biofilm, which is evident from the smooth operation of the MFCs. This is another advantage of operating MFCs, which can tolerate sulphur concentration as high as 1200 mg L−1 as observed in the present experiments with high sulphur concentrations.
Substrate mass balance in the MFCs
Two moles of oxygen are required per mole of sulphide for complete oxidation into sulphate making the COD equivalent of one mole of sulphide as two [1]. Table 1 shows the mass balance of COD, sulphate, and sulphide in an average cycle in the acrylic sheet MFCs. Theoretical coulombs that can be generated for substrate degradation in the MFCs are also calculated and compared with the actual coulombs generated to get an idea of the efficiency of the MFCs. Though, COD equivalent of 1 mol of sulphide is considered to be two; however, in a MFC, sulphide is not completely oxidized to sulphate, it is only oxidized to elemental sulphur. Oxidation of 1 molecule of sulphide to sulphur donates only two electrons, thereby reducing the theoretical charge transfer to 1/4th of that obtained from oxidation of sulphide to sulphate. Hence, MFC-2 and MFC-4 showed slightly lower voltage output than MFC-1 and MFC-3.
Elemental sulphur was the primary final product of sulphate reduction and subsequent sulphide oxidation. The presence of elemental sulphur on the anode surface was confirmed by EDX analysis which showed the presence of 27.45% of sulphur in the used carbon felt anode. However, exact quantification of sulphur deposits was not possible due to heterogeneity of the electrode, which is only possible by mass balance calculations which were 87.4 and 93.1% in MFC-2 and MFC-4, respectively. Actual sulphur recovery will be lesser than the estimated percentage if sulphur released in the form of H2S or precipitated as metallic sulphides are taken into consideration.
Kinetics of substrate degradation
Steady removals of COD and sulphate were achieved during the bioelectricity generation process in the MFCs. With sulphate of 200 mg L−1, COD of 2000 mg L−1, and fed-batch duration of 72 h, the average sulphate removal efficiencies reached 93 ± 2 and 96 ± 1%, respectively, for MFC-2 (without cathode catalyst) and MFC-4 (with cathode catalyst). COD removals in the MFCs were, respectively, 78 ± 6, 93 ± 5, and 87 ± 2 and 94 ± 5% for MFC-1, MFC-2, MFC-3, and MFC-4. Sulphate is anaerobically reduced to sulphide by SRBs [3]. Sulphide is oxidized by sulphide oxidizing bacteria with the energy from decomposition of organic matters or it can be oxidized abiotically [4]. Therefore, the presence of sulphate as co-substrate in MFC-2 and MFC-4, along with acetate improved COD removal efficiency of the system.
Order of removal of COD, sulphate, and sulphide was determined by average rate technique, where rate of reaction of COD removal and sulphate reduction, r (m mol/L.day), is given by the following equation:
where k (day−1) is the overall rate constant and A is the initial COD or sulphate concentration in m mol/L. Order of reaction is given by a. The initial rate of the reaction of COD removal was calculated at different COD concentrations like 200, 500, 1000, 2000, and 3000 mg L−1 (Table S1). Similarly, the initial rate of the reaction of sulphate removal was calculated at various sulphate concentrations like 50, 100, 150, 200, and 250 mg L−1.
The order of reaction a is 1.006 ± 0.010 as calculated from Eq. 3 using values of rate and the initial concentration of substrate from trial 1 to 5 (Table S1, Supplementary material) which depicts the condition in MFC-1, and the reaction rate constant k is 0.26 ± 0.01 day−1. Therefore, the reaction in MFC-1 can be well depicted by the following first-order reaction:
where A t is the COD concentration (mg L−1) at any time t (days), and the reaction rate constant for acetate as carbon source is 0.26 ± 0.01 day−1. Similarly, from trial 6 to 10, the order of the reaction for the MFC with a cathode catalyst was calculated as 1.004 ± 0.006, and the reaction rate constant is 0.29 ± 0.01 day−1. Hence, the reaction in MFC-3 can also be depicted by Eq. 4. The presence of sulphate in MFC-2 improved the reaction rate as the rate constant increased to 0.31 ± 0.01 day−1. However, as can be seen in Table S1, rate of COD removal and sulphate reduction is not the same with higher COD removal rate than that of sulphate removal, indicating contribution of fermentative, electrogenic, and other methanogenic bacteria inside the anodic chamber to COD removal. A similar observation was reported by Liu, Zhang [4]. The presence of catalyst in MFC-4 had no role in improving the reaction rate constant further from that of MFC-2 and it was 0.31 ± 0.01 day−1. The order of sulphate removal in MFC-2 and MFC-4 was 0.99 ± 0.03 and 1.02 ± 0.01 with the respective reaction rate constants being 0.320 ± 0.003 and 0.320 ± 0.002 day−1.
Performance of MFC treating raw sewage spiked with sulphate
To obtain an operating voltage of 300 mV, from blackwater of COD 500 mg L−1 and with a sulphate content of 200 mg L−1, coulombs to be generated by the MFC should be 777,600 C. Assuming a conservative Coulombic efficiency of 13%, theoretical coulombs generated would be 3,987,692. For a COD and sulphate removal efficiency of around 90 and 95%, respectively, outlet COD would be 50 mg L−1 and outlet sulphate would be 10 mg L−1. Therefore, COD consumed for sulphate reduction would be 127 mg L−1. Assuming an 85% conversion of sulphate into sulphide and 80% conversion of sulphide to sulphur, sulphide produced in the MFC would be 53 mg L−1 and that in the effluent would be 10 mg L−1. Hence, about 43 mg L−1 of sulphide would be oxidized to sulphur. Hence, total COD available for electrogenesis would be 340 mg L−1. To obtain the previously mentioned operating voltage, therefore, anodic chamber volume of the MFC should be 1000 mL. For an anode of projected surface area 300 cm2, sustainable power density that can be obtained is 30 mW/m2.
Based on this concept, a clayware MFC of effective volume 900 mL was operated on raw sewage of COD concentration of 500 mg L−1 spiked with sulphate of concentration 200 mg L−1. Figure 5 shows the changes in the production of operating voltage during the performance of the MFC. The MFC obtained a power density of 54.4 mW/m2 and a Coulombic efficiency of 13.95% with COD and sulphate removal efficiencies of 90 and 95%, respectively. Equation 1, predicting CE from COD/sulphate ratio in feed, is validated in this experiment. The high Coulombic efficiency is not only due to the catalyst used but also due to higher ratio of COD and sulphate. As evident from Fig. 5, the voltage was not stable due to difference in availability of electrons at the anode. Hence, polarization was performed just after giving feed in the 10th cycle, to get an idea of the maximum power output, which was higher than the sustainable power that could be obtained from the MFC.
Abiotic sulphide oxidation
Maximum sulphide oxidation rate could be observed at an anode potential of -200 mV (vs. Ag/AgCl) (Fig. 6). Majority of sulphide oxidation occurred within the first 24 h. Oxidation of sulphide could not be obtained at potentials lower than -400 mV (vs Ag/AgCl) and whatever sulphide was oxidized again got reduced back to sulphide. These observations indicate that elemental sulphur produced by electrochemical oxidation remains active, i.e., it could be further oxidized or reduced [20]. To avoid reduction of already produced sulphur products at any potential, fresh feed and fresh electrodes were used in each feed cycle. As oxidation progressed, the pH of the solution decreased due to the release of protons generated as the sulphide is removed from the solution. In the abiotic cells, the pH dropped from 7.01 to 6.65. However, this drop of pH was not observed in the MFCs, due to the presence of the proton exchange membrane.
Researchers have reported losses in current output and reduced wastewater treatment efficiency of MFCs treating sulphide or sulphate rich wastewaters with time, due to deposition of elemental sulphur on the electrode surface [21]. The effluent from the MFC can be transferred to an electrolytic cell to minimize losses on deposition of elemental sulphur on the anode of MFC, which meets the expectation of elemental sulphur recovery [6]. The power required for the electrolytic cell can be generated from the MFC. This way operation will also help in the sense that for maximizing power generation from sulphate reduction and organic matter oxidation, lower anode potentials are required, and for maximizing sulphide oxidation, higher anode potentials are beneficial [11].
Conclusions
Successful sulphate and COD removals from synthetic wastewater with wide range of COD and sulphate concentrations as well as sulphate rich sewage along with simultaneous energy harvesting were explored in a MFC with recovery of elemental sulphur. Results and mass balance of COD and sulphate indicate that this type of MFC is a promising scalable system for actual applications, which can achieve almost 72% of elemental sulphur recovery from sulphate laden wastewater. Raw sewage spiked with sulphate was also treated in a low-cost ceramic MFC to obtain 97% sulphate removal efficiency. COD and sulphate removal had different reaction rates, but both followed first-order reactions.
References
Lens PNL, Visser A, Janssen AJH, Hulshoff Pol LW, Lettinga G (1998) Biotechnological treatment of sulfate-rich wastewaters. Crit Rev Env Sci Technol 28(1):41–88
Zhao F, Rahunen N, Varcoe JR, Chandra A, Avignone-Rossa C, Thumser AE et al (2008) Activated carbon cloth as anode for sulfate removal in a microbial fuel cell. Environ Sci Technol 42(13):4971–4976
Lee DJ, Liu, X W eng HL (2014) Sulfate and organic carbon removal by microbial fuel cell with sulfate-reducing bacteria and sulfide-oxidising bacteria anodic biofilm. Bioresour Technol 156:14–19
Liu H, Zhang B, Liu Y, Wang Z, Hao L: (2015) Continuous bioelectricity generation with simultaneous sulfide and organics removals in an anaerobic baffled stacking microbial fuel cell. Int J Hydrogen Energy 40(25):8128–8136
Raschitor A, Soreanu G, Fernandez-Marchante CM, Lobato J, Cañizares P, Cretescu I et al (2015) Bioelectro–Claus processes using MFC technology: influence of co-substrate. Bioresour Technol 189:94–98
Liu J, Feng Y, He W, Gong Y, Qu Y, Ren N (2014) A novel boost circuit design and in situ electricity application for elemental sulfur recovery. J Power Sources 248:317–322
Cai J, Zheng P, Qaisar M, Sun P (2014) Effect of electrode types on simultaneous anaerobic sulfide and nitrate removal in microbial fuel cell. Sep Purif Technol 134:20–25
Kelly PT, He Z (2014) Nutrients removal and recovery in bioelectrochemical systems: a review. Bioresour Technol 153:351–360
Dutta PK, Keller J, Yuan Z, Rozendal RA, Rabaey K (2009) Role of sulfur during acetate oxidation in biological anodes. Environ Sci Technol 43(10):3839–3845
Ghangrekar MM, Murthy SSR, Behera M, Duteanu N (2010) Effect of sulfate concentration in the wastewater on microbial fuel cell performance. Environ Eng Manage J 9(9):1227–1234
Rabaey K, Van De Sompel K, Maignien L, Boon N, Aelterman P, Clauwaert P et al (2006) Microbial fuel cells for sulfide removal. Environ Sci Technol 40(17):5218–5224
Ahn Y, Ivanov I, Nagaiah TC, Bordoloi A, Logan BE (2014) Mesoporous nitrogen-rich carbon materials as cathode catalysts in microbial fuel cells. J Power Sources 269:212–215
Qiu D, Bu G, Zhao B, Lin Z, Pu L, Pan L et al (2014) In situ growth of mesoporous Co3O4 nanoparticles on graphene as a high-performance anode material for lithium-ion batteries. Mater Lett 119:12–15
Zhu T, Chen JS, Lou XW (2010) Shape-controlled synthesis of porous Co3O4 nanostructures for application in supercapacitors. J Mater Chem 20(33):7015–7020
Xiao X, Liu X, Zhao H, Chen D, Liu F, Xiang J et al: (2012) Facile shape control of Co3O4 and the effect of the crystal plane on electrochemical performance. Adv Mater 24(42):5762–5766
APHA WEF (1998) Standard methods for the examination of water and wastewater 20th Edition-4500-NO3-D nitrate electrode method. American Public Health Association, Washington
Liu H, Logan BE (2004) Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ Sci Technol 38(14):4040–4046
Zhou J, Xing J (2015) Effect of electron donors on the performance of haloalkaliphilic sulfate-reducing bioreactors for flue gas treatment and microbial degradation patterns related to sulfate reduction of different electron donors. Biochem Eng J 96:14–22
Zhang J, Zhang B, Tian C, Ye Z, Liu Y, Lei Z et al (2013) Simultaneous sulfide removal and electricity generation with corn stover biomass as co-substrate in microbial fuel cells. Bioresour Technol 138:198–203
Dutta PK, Rabaey K, Yuan Z, Keller J (2008) Spontaneous electrochemical removal of aqueous sulfide. Water Res 42(20):4965–4975
Dutta PK, Rozendal RA, Yuan Z, Rabaey K, Keller J: (2009) Electrochemical regeneration of sulfur loaded electrodes. Electrochem Commun 11(7):1437–1440
Acknowledgements
Grant received from the Department of Science and Technology, Govt. of India (File No. DST/INT/UK/P-101/2014) to undertake this work is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chatterjee, P., Ghangrekar, M.M., Rao, S. et al. Biotic conversion of sulphate to sulphide and abiotic conversion of sulphide to sulphur in a microbial fuel cell using cobalt oxide octahedrons as cathode catalyst. Bioprocess Biosyst Eng 40, 759–768 (2017). https://doi.org/10.1007/s00449-017-1741-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-017-1741-y