Abstract
A bench-scale integrated process based on submerged aerobic powdered activated carbon-membrane bioreactor (PAC-MBR) has been utilized and established for the treatment of landfill leachate. The results showed that the submerged PAC-MBR system effectively removed biodegradable trace organic compounds by the average removal rate about 71 % at optimum food to microorganism (F/M) ratio of 0.4 gCOD/g day under a HRT of 24 h. Adding nanofiltration (NF) process increased the treatment efficiency up to 99 %. Further, adding powdered activated carbon to activated sludge (AS) resulted in a higher adsorption capacity in comparison with AS. Adsorption isotherms were investigated and fitted by the Langmuir and Freundlich isotherm models in which the Langmuir model performed better. The specific oxygen uptake rate (SOUR) showed that adding PAC reduces the effects of COD on microorganism activities. NH3–N, TKN and Heavy metals removal efficiency amounted to 97 ± 2, 96 ± 2, and 99 ± 2 %, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In both industrialized and developing countries, the sanitary landfill method, owning to its economic advantages in terms of exploitation and capital costs, is a popular disposal way of solid waste materials. After landfilling, the generation of leachate is an unavoidable procedure in landfills. Leachates are defined as a complex aqueous effluent generated in consequence of decomposition of wastes, rainwater percolation, inherent moisture content of wastes, biochemical processes and physico–chemical reactions through waste cells [1]. With growing density of population and industrialization and excessive human consumption in the last decades, the quantity of the generated solid waste considerably increased. Landfill leachate (LFL) treatment has received more attention in recent years in order to minimize the adverse impacts of generated leachate on the environment. Further, the generated leachate would potentially contaminate the groundwater and surface waters [2]. Depending on the waste type and compaction, maturity, the rainfall patterns, landfill hydrology, also biochemical reactions occurring through the landfill and particularly landfill age, the leachate characteristics and composition vary significantly [3, 4]. LFLs contain significant amounts of organic pollutants, where humic-type substances, consist in an important group, also heavy metals, ammonia–nitrogen, and inorganic salts. Moreover, large amount of organic pollutants are refractory non-biodegradation fraction such as personal care products (PCPs), hormones, pharmaceuticals, halogenated hydrocarbons, pesticides, humic and folic acids. As time proceeds, the composition and properties of LFL vary widely with aging of the landfills [5]. The landfill leachate characteristics are represented by the key operational parameters such as the biological oxygen demand (BOD), chemical oxygen demand (COD), the BOD/COD ratio, suspended solids (SS), pH, total Kjeldahl nitrogen (TKN), ammonium nitrogen (NH3–N) and heavy metals. Among the mentioned properties, the BOD/COD ratio directly features the biodegradability of LFL. Therefore, with landfill aging, BOD/COD ratio decreases due to decomposition of most biodegradable portion of LFL and the non-biodegradable fraction of COD will greatly stay unchanged during this process. In particular, three stages of landfill leachates have been classified according to landfill age. In case of young LFL, high concentrations of organic matters mainly volatile fatty acids (VFAs) resulted in a high BOD/COD ratio. Also, the recalcitrant matter (e.g. folic and humic acids), and high concentrations of ammonium nitrogen stabilized leachate. It’s worth to note that there is no distinct cut off point between the intermediate and old LFL with about less than 0.2 BOD/COD ratios. Also, the same treatment methods may be used for both two later types by some researchers [6]. For the case of young leachates treatment, conventional biological methods have been extensively employed because of their easy operation and cost effectiveness. They are classified into anaerobic, anoxic and aerobic processes such as sequencing batch reactors (SBR), aerated lagoons, upflow anaerobic sludge blanket (UASB) reactors, conventional activated sludge (CAS) and etc. [7]. Treatment of stabilized leachate with low biodegradability due to containing recalcitrant and bio-refractory constituents presents a challenging problem which requires the integration of suitable additional techniques.
Currently, with the high stringent and restrictive level of discharge standards and of producing more stabilized leachates by the aging of landfills, many efforts have been made for evaluating appropriate treatment technologies to reach the satisfactory level of purification. It implies that new alternative and appropriate treatment methods must be proposed. Between the mostly used methods for this purpose, treatments based on membrane separation technology have broadly been emerged. Recently, membrane bioreactors (MBRs) have received considerable attentions due to their compact systems with smaller footprint, increase of biomass retention, processing stability, low sludge production and excellent effluent quality [8]. Membrane bioreactor technology has demonstrated a significant performance in treating old leachate. As the best treatment system, it proposes to combine MBR technology with nanofiltration (NF) process for the old landfill leachate treatment. The reason is that it is difficult to procure satisfactory effluent quality through using each method alone.
Owing to unique properties of NF, it has remarkable capability of removing complex and recalcitrant organic matters and heavy metals from landfill leachates [9, 10]. However, frequent membrane cleaning is indispensable and consequential operating costs increment as a result of required cleaning agents and occasionally membrane replacement also is expected. Many authors [11, 12] have demonstrated that membrane fouling remains one of the most adverse obstacles to the MBR implementation and the most challenging concern to encounter further MBR development. The main factors affecting membrane fouling include operative conditions, biomass characteristics, inorganic precipitates, extracellular polymers and colloids. According to some literatures [15, 16], the powdered activated carbon (PAC) as a supplementary treatment agent has been used in the LFL for three main objectives: (1) it can adsorb bio-refractory organic pollutants which cannot biologically degrade due to the substantial adsorption capacity, (2) the high surface area of nanoporous adsorbent increases growth sites for microorganisms and reactive sites between microorganisms and organic matters and (3) the final effect of PAC as a filtration aid can contribute to membrane fouling reduction. This effect was justified by depositing a permeable PAC layer with dynamic porosities over the membrane surface, making protection from the deposition of foulants on the membrane active layer [15, 16].
This study was aimed to assess and highlight the complementarities between an aerobic submerged MBR and NF membrane filtration capacity for an advanced treatment performance to remove the trace organic contaminants from landfill leachate. Also, the effect of combined system and PAC-MBR on biological treatment performance and the membrane fouling reduction were investigated.
Experimental
Landfill leachate and activated sludge (AS) characterizations
The raw leachate used in this study was obtained from the Kiasar landfill site in Sari, Mazandaran, Iran. Table 1 shows the main characteristics of raw leachate. AS culture was also collected from a conventional domestic wastewater plant located in sari, Iran. The characteristics of the AS were COD of 88 (mg/l), mix liquor suspended solid (MLSS) of 2500 (mg/l) and pH of 7.44. The sludge used in this study was aerated for 24 h. After this time, MLSS concentration reached to 3100 mg/l. In this study, the initial analyses with approximately BOD/COD of 0.3 reflected a non-biodegradable portion on the leachate.
Materials and analytical methods
All the chemicals were purchased as an analytical reagent grade. The thin film nano composite membrane was fabricated through a conventional interfacial polymerization technique which is described in our previous work [17]. Thin film nano composite membrane are composed of two layers. The general method involved the fabrication of polyamide layer on the prepared ultrafiltration support by the reaction of m-phenylenediamine and trimesoyl chloride. The ultrafiltration (UF) membrane was prepared using polysulphone (PSf) polymer via the phase inversion by immersion precipitation method and N,N-dimethylformamide (DMF) as solvent were provided by BASF Co. (Germany) [18]. The pure water flux of the synthesized UF was 51.21 l/m2 h under 0.8 bar vacuum pressure. As shown in Fig. 1, SEM images indicated a thin polyamide layer fabrication on the polysulfone (PSf) support membrane.
All the analytical methods were carried out based on the standard test methods [19]. Influent and effluent samples were taken regularly from the system. The COD analyses were executed by closed reflux method after filtration through Whatman filter papers (0.47 μ Millipore) Method 5220D [19]. UV254 was measured by a spectrophotometer Jenway 6305 with a cell length of 1 cm. The NH4–N (ammonia) concentrations were measured by using commercial Merck test kits. TKN value was obtained by a Kjeldahl autoanalyzer (KjelDigester K-446/K-449). pH of samples were measured by pH meter electrode (Model AD-1200 BENCH TOP). Conductivity was determined in line with Standard Method, 2510. MLSS concentration was determined by filtering the samples through a 0.47 μ Millipore paper filter and drying took place in a drying oven at 105 °C until a constant weight was reached [20]. Water used during all the experiments provided from laboratory distilled water. The employed PAC in the hybrid system was obtained from Sigma-Aldrich (No. 89440).
Sludge acclimatization
To adapt the AS for the leachate with turbulent and high strength medium, the AS was initially aerated for 24 h without adding wastewater. After that, glucose, as a substrate, was supplied to the reactor in order to promote easier adaptation of biomass to the complex leachate. Subsequently, low volumetric loading of diluted leachate was gradually introduced to the AS and aerating continuously for several days until the microorganisms could tolerate high COD concentration of leachate. During the adaptation procedure, the amount of COD, MLSS, mixed liquor volatile suspended solids (MLVSS) and pH parameters were regularly monitored. At the end of adaptation process, the MLSS concentration reached to 6200 mg/l.
Adsorption study
Determination of optimum dose of PAC
Batch experiments were conducted at the absence and presence of PAC as an adsorbent with different concentration ranging between 0.5 and 5 g/l. After the COD removal measurement in the reactor, the experiments were carried out under aeration at optimum HRT.
Isotherm model
From the various models that express the equilibrium between the adsorbates and adsorbents in the aqueous phase, the isotherm models are usually the best technique to represent this phenomenon. The Langmuir isotherm and the Freundlich isotherm as the most common models were employed to model the obtained data from the adsorption process. The linear forms of these equations are given as equation Langmuir and Freundlich through Eqs. (1) and (2), respectively
where q m (mg/g) is the maximum adsorption capacity, q e (mg/g) is the amount of COD adsorbed, C e (mg/l) is the equilibrium concentration of the adsorbate, K l is the Langmuir constant that related to energy of adsorption. K f and n are the Freundlich constants that related to adsorption capacity and adsorption intensity, respectively.
The relationship between adsorption capacity and food to microorganism (F/M) ratio
Five batch reactors containing 1 l of AS with PAC along with five equivalent batch reactors containing only AS were prepared and 1 l of fresh leachate with COD concentrations of 200, 400, 600, 800, 1000 mg/l, was added to them. Then, the mixed liquor was aerated. At the end of 24 h of aeration, COD removal rate was measured. Moreover, the MLSS in all ten batch reactors were measured. Adsorption capacities of AS and AS with PAC (q e, mg/g) were calculated using the Eq. (3) to find the relationship between adsorption capacity and F/M ratio (COD basis) [21]:
Laboratory leachate treatment setup
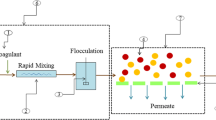
The setup of the leachate treatment was composed of a PAC-MBR hybrid system combined with the NF process. A cubic aeration tank with trapezoidal sides applied throughout the study was made of plexiglass at the dimension of 40 cm (length) × 25 cm (width) × 10 cm (depth) with a total volume of 10 l. The bottom of the tank was designed with a slight inclined angle. The studied PAC-MBR unit was constructed of a bioreactor for the biodegradation and a submerged UF membrane module. In order to assemble the PAC-MBR unit, the synthesized flat sheet UF membrane was folded as a leaf and glued together back-to-back with a permeate spacer between them with the active surface area of 0.04 m2. The open side of the folded sheet was connected to vacuum pomp to collect the permeate of a leaf. UF membrane modules were submerged vertically in the reactor. Two stone diffusers with a 20 cm length located underneath the membrane fed by an air compressor which provided a constant air flow rate of approximately 5.5 l/min in order to maintain DO concentration above 2–4 mg/l. Also, flow rate of air releases to the MBR module and providing shear stress to the membrane surface and refresh the membrane surface to prevent fouling. The treated leachate via AS process was pumped out through a UF membrane with a vacuum pump. The UF membrane initially enables to remove the biosolids, macromolecular refractive compounds and separation of the rated leachate from the AS. After that, the permeate was directed to the NF process in order to remove the most refractive non-biodegradable compounds and inorganic ones. The efficiency of NF membrane was based on the assessment of retention factor of low molecular resistant components and residual inorganic salts of the pretreated leachate after both biological treatment and ultrafiltration steps. Also, the changes of effluent conductivity were measured during the experiments. The subsequent NF filtration step was performed with a laboratory-scale cylindrical dead-end membrane filtration apparatus. This cell was made of stainless steel with a volume of 1100 ml. The filter diameter, height, and effective membrane area were 8, 22 and 50 cm2, respectively. The samples were filtered under the operational pressure of five bars, applied by nitrogen gas. Figure 2 illustrated the submerged PAC-MBR system along with NF system.
PAC-MBR start-up
In this research, the AS process was the first phase of leachate purification. This method provided partial oxidation of organic components and the removal of ammonium compounds. The biological treatment under aerobic conditions was performed in batch mode. Bioreactor was aerated vigorously with an air compressor at HRT of 24 h. The continuous agitation ensures homogeneous mixture of the liquor and prevents the biomass settling. Foaming was initially appeared with changing applied organic loading rates for a short time. This fact showed a common occurrence and was reduced to a minimum when steady-state condition was reached. At the same time, it was perceived that a biofilm layer of AS was formed on the bioreactor inner wall. When the thickness of adhesive biofilm layer attained to a maximum level, it was breaking from inner wall and mixed to circulation. At the first stage, the MLSS and MLVSS concentrations were about 6200 and 3940 mg/l. The bioreactor was operated in batch mode under different HRTs to evaluate the performance for COD removal exclusively. The hydraulic retention time (HRT) values were adjusted to 12, 24, 36 and 48 h. Also, the effect of F/M ratio was investigated through diluting the leachate with high organic loads with tap water ranging from 0.1 to 1 gCOD/g day. Experiments were run several times to assay the repeatability of the results. Among different heavy metals, nickel and chromium(III) exist commonly in high concentration in LFLs and these elements considered as an indicator of trace elements during our study. The temperature of the pilot medium was maintained by an aqua heater between 25 and 28 °C. The medium pH was measured daily to keep constant about 8.2 during the operation. At the end of each batch operation, the bioreactor content was sedimented for 1 h and 50 mL of the sample was carefully withdrawn from the clear supernatant with a pipette and passed through a Whatman filter paper with 0.47 µm pore size. Prior to filtration, filter papers were washed with distilled water to the elution of existence starch in the filters.
Specific oxygen uptake rate (SOUR) determination
This section aims to explain the effects of using PAC on the activity of AS microorganisms. By assessing the oxygen consumption rate during a specific period of time, the SOUR can be calculated. A great deal of research is carried out and proposed different methods to measure the SOUR which is available in the literature [22]. In the method employed here, the aeration process was stopped for the intervals of 10 min and the amount of consumed oxygen was calculated every 2 min. Subsequently, the decrease of oxygen concentration in system was recorded. Finally, the aeration process was initiated at the end of the 10 min until the system reaches to the level of oxygen saturation. Then, SOUR was calculated by the Eq. (4):
Results and discussions
Biological treatment
Biological acclimatization
The adaptation process of AS to LFL lasted for 2 weeks. As can be seen in Fig. 3, at the early biological treatment of the acclimation process, the COD removal was quite low owing to the low degree of adaptation in the treatment process. As can be seen, the COD removal efficiency was increased by increasing the MLSS concentration. It seems that the reaction rate is directly proportionate to the MLSS concentration. Nonetheless, the sludge, suspensions physical properties and wastewater composition limit the increment of biomass concentration. Mainly, the decrement of oxygen transfer (mass transfer) and the increment of medium viscosity lead to low performance and high energy consumptions [23]. After several days of the run, the COD removal reached to the maximum reduction of 38 %. The adaptation process was stopped whenever the COD removal of system approached to the steady state conditions. The slow growth rate in microorganism population indicated that the total flora was strived to resist the shock imposed by the addition of complex leachate during this period of time. Further, there was not enough substrate for microorganisms to produce energy for cell growth until they could adapt with the leachate as a new substrate course. A proper volumetric index of sludge (SVI) level, normally below 100 ml/g, indicates better organic compounds removal. Well-settleable sludge is of great property in the conventional AS process. Quickly settling sludge resulted in sludge bulking problem and turbid effluent due to filamentous growth, small flocs and weakly structured [24]. The SVI value obtained during this experiment was 84.8 ml/g indicating a great sludge settleability.
Effect of HRT on the biological treatment
The effect of various HRTs on the AS performance in terms of COD removal rate is presented in Fig. 4. The HRTs of 12, 24, 36 and 48 h were set in the system gradually during the operation cycle. The organic removal rate increased from 45 to 69 % along with the increment of the HRT from 12 to 36 h. As the HRT increased from 36 h to 48 h, the COD removal efficiency decreased to less than 60 %. As the results revealed, the HRT of 24 h can be considered as the optimal HRT with more stable and reproducible results. HRT values greater than 24 h were not effectual against the efficiency. The MLSS concentration (bacteria population) and COD concentration (food) are plotted as a function of time, which is illustrated in Fig. 5. The growth of the bacteria in the AS is directly commensurate to the amount of available COD. The optimum concentration for biomass and COD was attained at the HRT of 24 h. Figure 5 shows the biomass growth in 60 h. At first, there was a large amount of food available compared to the amount of biomass. The initial concentrations of COD and MLSS were 2500 and 6200, respectively. The bacteria started consuming the COD and duplication, exponentially. The ascending trend of hollow circles is attributed to the growth of bacteria. On the contrary, the hollow circles have a descending trend which can be explained as a consequence of the decrease in the amount of available foods. The available foods began to decrease and bacterial proliferation began to slow. It is evident that the growth rate of bacteria was decreased as a consequence of the shortage in the available foods. Bacteria began to raceme and form the floc. Finally, the majority of the COD has been converted and there were not enough foods for the bacteria to consume. The final concentrations of COD and MLSS were 840 and 6300, respectively.
Effect of F/M on the biological treatment
As shown in Fig. 6, the correlation between F/M ratio and COD removal was investigated. The F/M ratio of 0.1 gCOD/g day was applied to system, which subsequently was increased stepwise to 1 gCOD/g day. The COD removal (%) increased primarily with an increase in F/M but it decreased gradually until it reached to the lowest value at F/M of 1 gCOD/g day. The average COD removal decreased from 62 to 50 % where the F/M ratio increased from 0.4 to 1.2 gCOD/g day. The performance profile represented that the maximum level of COD removal was obtained at F/M ratio of about 0.4 gCOD/g day. This experiment indicated good performance at low F/M ratio. The ratio of F/M is an important key operational parameter which extremely effects on the microbial composition, sludge properties and organic removal efficiency. All of them will impress on the membrane fouling and the process efficiency in PAC-MBRs. In several studies already conducted, no consensuses have been achieved on invariant optimal F/M ratio. High F/M ratios enable great driving force for microbial growth, metabolic activity and as a consequence, high overall rates of organic removal [25]. Nonetheless, too high values of F/M ratio may destroy hydrolyzing and methanation equilibrium, disturb the microbial ecology, and result in sludge deflocculating and eventually the reduction of the process efficiency. Low ratios of F/M enhance better organic removal efficiency and provide sludge flocculation and improvement of the biomass settleability in the reactor. However, too low F to M ratio limits cell growth and the deflocculation of sludge may also occur [26].
Adsorption study
Effect of PAC on the biological treatment
Variations of COD removal were assessed for each PAC concentration. The percentage of COD removal increased steeply from 38 to 56 % by adding PAC concentration from 0.5 to 2 g/l. The increment of PAC concentrations up to 2 g/l, did not increased the COD removal efficiency significantly and remained almost unchanged. Adding adsorbent had an effect on microorganism activities and subsequently on COD removal. The use of PAC-MBR hybrid system improved the properties of AS and MBR filtration efficiency. PAC as an aiding filter adsorbs the colloidal substances and dissolved organic matter, the back diffusion of existent organic particles increases at the interfacial region of membrane–liquid and substantially decreases the hydrodynamic layer thickness on membrane surface limiting the mass transfer [27, 28] (Fig. 7).
Adsorption isotherms
Figure 8 shows the adsorption of COD for two Langmuir and Fruendlich isotherms. Although both isotherm models are capable of handling the obtained experimental data. The Langmuir constants (K l and q m) and Freundlich constants (K f and n) were calculated and the values are tabulated in Table 2. Also, the regression correlation coefficients (R 2) of the linear plots are presented in Table 2. Adsorption on COD can be fitted by both Langmuir and Freundlich isotherms with R 2 > 0.99, which demonstrates that the both adsorbents can be described very well by the mentioned models. The maximum capacity of adsorption (q m) and K L values in the PAC-AS was higher than AS. This means that the PAC played an important role in improving the performance of the system which enhanced the COD removal in comparison with the non-PAC reactor [29, 30]. The n value of PAC-AS was higher than AS, suggesting that the adsorption of COD is favorable for PAC-AS.
Effect of F/M ratio on adsorption capacity
The adsorption capacity of COD onto an adsorbent is proportional to the ratio of F/M. The experimental data of different F/M ratios and adsorption capacities are illustrated in Fig. 9, which shows that the adsorption capacity changes with different F/M ratios. The adsorption capacity (q) of the system as a function of the F/M ration (x) is given through the following equation [31]:
From the obtained data, it can be found that in both PAC and non-PAC reactors, the adsorption capacity is directly related to the F/M ratio and a coefficient. The values of a coefficient in the PAC reactor is higher than AS reactor which means that the adsorption of COD can be more easily performed when PAC is added to the reactor (Table 3).
The relationship between SOUR and AS microorganisms
Figure 10 shows that the decrease in oxygen concentration versus time was a linear plot and the slope of this plot determines the oxygen uptake rate. It can also be found that the amount of oxygen consumption by microorganisms was low when there was not any powdered activated carbon in the AS system. The oxygen concentration reached to 2.37 mg/l as the aeration process was stopped and, it was increased to 4.1 mg/l during the aeration process. The addition of PAC to the AS increased oxygen consumption rate, since PAC can increase reactive sites among microorganisms and organic matters. Consequently, microorganisms need higher amount of oxygen to biodegrade available organic matters [32].
Figure 11 indicates that the addition of PAC increased the SOUR as a consequence of increasing the activities of microorganism. In fact, the absorbent provided active area for the growth of microorganisms and Formation of floc on absorbent is more comfortable and subsequently the oxygen consumption rate was increased. As a result, the microorganism generated a significant amount of energy and produces new biomass, which causes a substantial speed-up in the biological degradation of microorganism in the AS and consequently, increases the SOUR to 46 %.
PAC-MBR performance
At the present work, the AS process was the first stage of landfill leachate treatment. Additional PAC enhanced the adsorption of pollutant and improved microorganism activities and increased the COD removal by the creation of active sites for the production of biomass. At the subsequent step of our experiment, the biological process was detached by ultrafiltration to separate the poorly sediment AS and to remain refractive macromolecular substances after the biological treatment from the rated leachate. Fast biodegradable organic molecules fraction was removed during HRT of 24 h. The fraction of slow biodegradable organic was approximately removed totally. This fact probably was thanks to the application of UF membrane that prevented the microorganisms washing off. Figure 12 represents the reproducible results of COD reduction during the batch experiment in PAC-MBR with optimum F/M value of 0.4 gCOD/g l, HRT value of 24 h. The COD reduced from about 2500 to below 550 mg/l. The mixed liquor suspended solids concentration was measured per day for 42 days. After the LFL enters the aeration tank, bacteria become entangled and adsorbed substrate to the floc. Bacteria growth on adsorbent and cells become larger during periods of rapid growth and during the experiment MLSS concentration was reached to 9300 mg/l. Both of the changes in MLSS concentration versus time and the relationship between MLSS concentration and COD removal rate were illustrated in Fig. 12. During the experiment, the trend of COD removal was incremental along with increasing MLSS concentration but not for a short time. Also, the maximum organic removal rate of 78 % was obtained after 38 days. After reaching to maximum growth, a constant MLSS value was attained and the biomass growth was stabilized for several days. There was a slight decrease in the MLSS concentration between 26th and 30th days. It’s speculated that the MLSS reduction was mainly due to the sharp drop of liquor pH from 8.4 to about 5.5. Since pH value of near the neutrality is more favorable for proliferation of bacterial with different biodiversity levels [24]. TKN and ammonia (NH4–N) removal efficiencies for raw and treated leachate were analyzed. Due to the great value of ammonia concentration (1951 mg/l), the LFL could be classified as an intermediate leachate. The nitrification rate of ammonia (NH4–N to NO3–N) in the PAC-MBR system equaled to 88 %. Despite the aerobic conditions in a submerged PAC-MBR which causes lower nitrogen removal rate by continuous aeration [33], a significant removal efficiency was obtained due to the long sludge retention time (SRT) (SRT >30 days) in the PAC-MBR. Also, the significant color removal more than 67 % was observed through the PAC-MBR process at optimum conditions.
As illustrated in Fig. 13, the membrane flux rate in PAC-MBR decreased with increasing F/M ratio. The result may be ascribed to the agglomeration of the biomass on the membrane surface area and the formation of the cake layer. Otherwise, it is expressed that the suspensions were not directly responsible for flux reduction. In fact, the exponentially increasing dissolved substances and viscosity resulted in concentration polarization. In other words, the increment of biomass concentration causes viscosity increment, which had an effect on the boundary layer thickness and the concentration polarization [34].
UV254 removal
Organic matters in raw leachate could be estimated in terms of UV254. The UV254 measurement procedure was conducted every 4 days. As it is shown in the Fig. 14, the PAC-MBR was capable of eliminating 96 % organic matters during the experimental run. During the operation, PAC-MBR expressed a good compatibility with the existed organic matters of the leachate which resulted in a decrease in UV254. Increasing the removal of UV254 showed that aromatic compounds could be easily removed by PAC-MBR. It is because of the fact that PAC has an unsaturated bond or negatively charged fraction which gains the ability to eliminate aromatic compounds through the adsorption process. However, particulate organic matters could be more easily rejected by the membrane [35].
Supplementary treatment
Although the hybrid treatment method of landfill leachate yielded rather high degrees of purification, and then the introduction of PAC again brought improvement of its quality, the effluent discharge standards still have not been achieved. The reported results reconfirmed the limitation of PAC-MBRs in the removal of some biologically recalcitrant trace organic compounds and inorganic impurities. The permeate of PAC-MBR systems was subjected to further purification step with the NF process and measurement procedure was conducted every 4 days. The various concentrations of effluent COD from PAC-MBR systems were between 550 and 850 mg/l. As shown in Fig. 15, the COD values decreased to below 50 mg/l in the permeate stream and substantial COD removals were attained. NF process complemented the PAC-MBR treatment more effectively, above 98 % removal. However, the increment of the inlet organic loading was led to a fast decrease in flux rate in the NF membrane. According to Nghiem et al. [37], hydrophobic particles can absorb to NF membrane and diffuse through the condensed polymeric matrix. Subsequently, the considerable transmission of these hydrophobic compounds occurs through the ultrathin active layer. Conversely, major amount of these hydrophobic components can be removed significantly by PAC-MBR as represented in the previous section. Coupling PAC-MBR treatment with NF membrane filtration resulted in complementary removal of hydrophilic and hydrophobic trace organic components. Nitrogen removal rate of pretreatment leachate during the NF process in terms of TKN and ammonia were also analyzed and listed in Table 4. TKN and NH4–N were removed by PAC-MBR from 88 to 91 %, and by NF process from 96 to 97 %, respectively. The TKN removal performance in PAC-MBR was greater than that of the NF process. It is evident that a significant fraction of TKN is NH 4–N and the NF membranes are not typically capable of rejecting monovalent ions. During the NF process the removals of chromium (III) and nickel were increased to 100 and 99 %, respectively [38, 39]. Conductivity rejection was measured during the NF process for every 4 days. Nonetheless, the conductivity rejection did not recognize as an authentic indicator to determine the removal efficiency of organic particles by the tight NF membrane. With reducing effluent concentration of COD from PAC-MBR (Fig. 15), a slightly decrement was observed in the conductivity rejection. The low rejection of monovalent ions in NF membranes was expected since NF membranes had a good rejection of divalent ions, as observed by the conductivity removal of 87 %.
Conclusion
Compared with the classical biological systems such as CAS, PAC-MBRs as a versatile technology exhibited great potentials in treating highly polluted landfill leachates with high persistent compounds regardless of their age. The obtained results indicated that PAC-MBR treatment and NF membrane filtration can be integrated to enhance removal rate of trace organic contaminants in a wide range. Whereas, an important fraction of organic matter has been removed by the primary PAC-MBR treatment. COD removal rate was achieved up to 75 % by the PAC-MBR unit and improved up to 94 % by the NF process. Based on the obtained results, considerable amount of TKN which majorly consists of (NH4–N), was efficiently removed by the PAC-MBR. The mean removal value of TKN and NH4–N by the PAC-MBR were around 91 and 88 %, respectively. The phosphorus content of feed leachate reached to below discharge limit value by 99 % reduction during the hybrid treatment system Adsorption isotherms showed that the adsorption of COD fitted well with the Langmuir and Freundlich, showing correlation coefficients (R 2) of 0.998 and 0.997, respectively. The adsorption capacities were increased after the addition of powdered activated carbon. SOUR measurement indicated the toxic effects of landfill leachate on activated sludge microorganisms were substantially decreased after the addition of PAC. The addition of powdered activated carbon into the Activated Sludge increased the activated sludge microorganisms SOUR by reducing the toxic effects of COD on microorganism activities and by acting as reaction site for substrates and microorganisms.
Abbreviations
- PAC:
-
Powdered activated carbon
- MBR:
-
Membrane bioreactor
- F/M :
-
Food to microorganism
- NF:
-
Nanofiltration
- AS:
-
Activated sludge
- SOUR:
-
Specific oxygen uptake rate
- LFL:
-
Landfill leachate
- PCPs:
-
Personal care products
- BOD:
-
Biological oxygen demand
- COD:
-
Chemical oxygen demand
- SS:
-
Suspended solids
- TKN:
-
Total Kjeldahl nitrogen
- NH3–N:
-
Ammonium nitrogen
- VFAs:
-
Volatile fatty acids
- SBR:
-
Sequencing batch reactors
- UASB:
-
Upflow anaerobic sludge blanket
- CAS:
-
Conventional activated sludge
- UF:
-
Ultrafiltration
- PSf:
-
Polysulphone
- DMF:
-
N,N-Dimethylformamide
- MLVSS:
-
Mixed liquor volatile suspended solids
- MLSS:
-
Mix liquor suspended solid
- HRT:
-
Hydraulic retention time
- SVI:
-
Volumetric index of sludge
- R 2 :
-
Regression correlation coefficients
- SRT:
-
Sludge retention time
- q m :
-
Maximum adsorption capacity
- q e :
-
Amount of COD adsorbed
- C e :
-
Equilibrium concentration of the adsorbate
- K l :
-
Energy of adsorption
- K f :
-
Adsorption capacity
- n :
-
Adsorption intensity
- a :
-
Coefficient
- q :
-
Adsorption capacity
- x :
-
Function of the F/M ration
References
Ahmed FN, Lan CQ (2012) Treatment of landfill leachate using membr ane bioreactors: a review. Desalination 287:41–54
Wiszniowski J, Robert D, Surmacz-Gorska J, Miksch K, Weber JV (2006) Landfill leachate treatment methods: A review. J Environ Chem Lett 4:51–61
Renou S, Givaudan JG, Poulain S, Dirassouyan F, Moulin P (2008) Landfill leachate treatment: review and opportunity. J Hazard Mater 150:468–493
Baig S, Coulomb I, Courant P, Liechti P (1999) Treatment of landfill leachates: lapeyrouse and Satrod case studies. Ozone Sci Eng 21:1–22
Kjeldsen P, Barlaz MA, Rooker AP, Baun A, Ledin A, Christensen TH (2002) Present and long-term composition of MSW landfill leachate: a review. Crit Rev Environ Sci Technol 32:297–336
Bohdziewicz J, Kwarciak A (2008) The application of hybrid system UASB reactor-RO in landfill leachate treatment. Desalination 222:128–134
Kurniawan TA, Lo W, Chan GYS (2006) Physico–chemical treatments for removal of recalcitrant contaminants from landfill leachate. J Hazard Mater 129:80–100
Yu J, He C et al (2014) Removal of perfluorinated compounds by membrane bioreactor with powdered activated carbon (PAC): adsorption onto sludge and PAC. Desalination 334(1):23–28
Lu X, Bian X, Shi L (2002) Preparation and characterization of NF composite membrane. J Membr Sci 210:3–11
Wanga Guanghui, Fana Zheng, Wua Dexin, Qina Lei, Zhanga Guoliang, Gaoa Congjie, Mengb Qin (2014) Anoxic/aerobic granular active carbon assisted MBR integrated with nanofiltration and reverse osmosis for advanced treatment of municipal landfill leachate. Desalination 349:136–144
Ince M, Senturk E, Onkal G, Keskinler EB (2010) Further treatment of landfill leachate by nanofiltration and microfiltration—PAC. Desalination 255:52–60
Remy M, Potier V, Temmink H, Rulkensb W (2010) Why low powdered activated carbon addition reduces membrane fouling in MBRs. Water Res J 44:861–867
Satyawali Y, Balakrishnan M (2009) Effect of PAC addition on sludge properties in an MBR treating high strength wastewater. Water Res J 43:1577–1588
Tammaro Marco, Salluzzo Antonio, Perfetto Raffaele, Lancia Amedeo (2014) A comparative evaluation of biological activated carbon and activated sludge processes for the treatment of tannery wastewater. J Environ Chem Eng 2(3):1445–1455
Torretta Vincenzo, Urbini Giordano, Raboni Massimo, Copelli Sabrina, Viotti Paolo, Luciano Antonella, Mancini Giuseppe (2013) Effect of powdered activated carbon to reduce fouling in membrane bioreactors: a sustainable solution. Case study. J Sustain 5(4):1501–1509
Yinga Z, Ping G (2006) Effect of powdered activated carbon dosage on retarding membrane fouling in MBR. J Sep Purif Technol 52:154–160
Rahimpour A, Jahanshahi M, Peyravi M (2014) Development of pilot scale nanofiltration system for yeast industry wastewater treatment. J Environ Health Sci Eng 6:12–55
Peyravi M, Rahimpour A, Jahanshahi M (2012) Thin film composite membranes with modified polysulfone supports for organic solvent nanofiltration. J Membr Sci 423–424:225–237
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington, DC
Standard Methods for the Examination of Water and Wastewater. American Public Health Association, Washington, DC
Kong L, Xiong Y et al (2013) Preparation and characterization of a hierarchical porous char from sewage sludge with superior adsorption capacity for toluene by a new two-step pore-fabricating process. Bioresour Technol 146:457–462
Kristensen HG, Jørgensen PE, Henze M (1992) Characterization of functional microorganism groups and substrate in activated sludge and wastewater by AUR, NUR and OUR. J Water Sci Technol 25(6):43–57
Chan YJ, Chong MF, Law CL, Hassell DG (2009) A review on anaerobic–aerobic treatment of industrial and municipal wastewater. Chem Eng J 155:1–18
Qing-Yuan H, L M, Wang C, Ji M (2015) Influence of powdered activated carbon addition on water quality, sludge properties, and microbial characteristics in the biological treatment of commingled industrial wastewater. J Hazard Mater 295:1–8
Lobos J, Wisniewski C, Heran M, Grasmick A (2008) Sequencing versus continuous membrane bioreactors: effect of substrate to biomass ratio (F/M) on process performance. J Membr Sci 317:71–77
Lobos J, Wisniewski C, Heran M, Grasmick A (2005) Effects of starvation conditions on biomass behavior for minimization of sludge production in membrane bioreactors. J Water Sci Technol 51:35
Winzeler HB, Belfort G (1993) Enhanced performance for pressure driven membrane processes: the argument for fluid instabilities. J Membr Sci 80:35–47
Wang D, Hu Q-y et al. (2015) Evaluating the removal of organic fraction of commingled chemical industrial wastewater by activated sludge process augmented with powdered activated carbon. Arab. J. Chem
Onga S-A, Toorisaka E, Hirata M, Hano T (2010) Adsorption and toxicity of heavy metals on activated sludge. Scie Asia 1513–1874:36.204
Ong SA, Lim PE, Seng CE (2003) Effects of adsorbents and copper (II) on activated sludge microorganisms and sequencing batch reactor treatment process. J Hazard Mater B103:263–277
Zhang X, Li X, Zhang Q, Peng Q, Zhang W, Gao F (2014) New insight into the biological treatment by activated sludge: the role of adsorption process. Bioresour Technol 153:160–164
HagMan M, la Cour Jansen J (2007) Oxygen uptake rate measurements for application at wastewater treatment plants. Vatten 63:131–138
Sun S, Nàcher CPI, Merkey B, Zhou Q, Xia S, Yang D et al (2010) Effective biological nitrogen removal treatment processes for domestic wastewaters with low C/N ratios: a review. J Environ Eng Sci 27:111–126
Li AJ, Li XY, Yu HQ (2011) Effect of the food-to-microorganism (F/M) ratio on the formation and size of aerobic sludge granules. Process Biochem J 46:2269–2276
Lia J, Zhaoa L, Qina L, Tiana X, Wanga A, Zhoua Y, Mengb L, Chenc Y (2016) Removal of refractory organics in nanofiltration concentrates of municipal solid waste leachate treatment plants by combined Fenton oxidative-coagulation with photo—Fenton processes. Chemosphere 146:442–449
Abdulhakeem AA, Tadkaewa N, McDonaldb JA, Khanb SJ, Pricec WE, Nghiema LD (2010) Combining MBR and NF/RO membrane filtration for the removal of trace organics in indirect potable water reuse applications. J Membr Sci 365:206–215
Kappela C, Kempermanb AJB, Temminka H, Zwijnenburga A, Rijnaartsc HHM, Nijmeijerb K (2014) Impacts of NF concentrate recirculation on membrane performance in an integrated MBR and NF membrane process for wastewater treatment. J Membr Sci 453:359–368
Barakat MA (2011) New trends in removing heavy metals from industrial wastewater. Arab J Chem 4:361–377
Al-Rashdi BAM, Johnson DJ, Hilal N (2013) Removal of heavy metal ions by nanofiltration. Desalination 315:2–17
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peyravi, M., Jahanshahi, M., Alimoradi, M. et al. Old landfill leachate treatment through multistage process: membrane adsorption bioreactor and nanofitration. Bioprocess Biosyst Eng 39, 1803–1816 (2016). https://doi.org/10.1007/s00449-016-1655-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-016-1655-0