Abstract
Many methods of synthesizing silver nanoparticles (Ag-NPs) by reducing Ag+ ions using aqueous/organic extracts of various plants have been reported in the past, but the methods are rather slow. In this investigation, silver nanoparticles were quickly synthesized from aqueous silver nitrate through a simple method using leaf extract of a plant—Cynodon dactylon which served as reducing agent, while sunlight acted as a catalyst. The formation of Ag-NPs was indicated by gradual change in colour and pH and confirmed by ultraviolet–visible spectroscopy. The Ag-NPs showed a surface plasmon resonance at 451 nm. Based on the decrease in pH, a possible mechanism of the synthesis of Ag-NPs involving hydroxyl (OH−) ions of polyphenols of the leaf extract is postulated. Ag-NPs having (111) and (200) crystal lattices were confirmed by X-ray diffraction. Scanning electron microscopy revealed the spherical nature of the Ag-NPs, while transmission electron microscopy showed that the nanoparticles were polydispersed with a size range of 8–10 nm. The synthesized Ag-NPs also demonstrated their antibacterial activity against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Salmonella typhimurium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanotechnology started with a boom during early twentyfirst century and synthesis of different metal nanoparticles using different physico-chemical methods such as inert gas condensation, chemical reduction and electrochemical methods has been well established [1–3]. However, these methods are not economical and convenient due to high cost, time requirements, toxicity of the raw materials used and byproducts generated [4]. To overcome these drawbacks, novel environment-friendly methods based on the approaches of ‘green chemistry’ for the synthesis of nanoparticles have been developed. Various biological agents such as microbial cell cultures and plant extracts containing secondary metabolites have been exploited for synthesizing metal nanoparticles [5–9]. The use of plant extracts containing secondary metabolites for the synthesis of nanoparticles is termed as ‘green synthesis’, as it is a simple, convenient, less energy-intensive and eco-friendly method. The use of plant extracts for the synthesis of nanoparticles is advantageous over microbial cultures as it eliminates the process of maintaining cell cultures. In this investigation, leaf extract of Cynodon dactylon which is a common occurring weed was used as a reducing agent for in vitro synthesis of silver nanoparticles (Ag-NPs) which were characterized appropriately. The antibacterial activity of the synthesized Ag-NPs against common pathogens is also presented and discussed in this paper.
Materials and methods
Chemicals
Silver nitrate (AgNO3) of analytical grade was purchased from E. Merck (India) Limited, Mumbai. Muller Hinton Agar was purchased from HiMedia Laboratories Pvt. Ltd., Mumbai.
Preparation of leaf extract
Healthy and fresh leaves of C. dactylon were collected from the campus of NEERI, Nagpur, India. The leaves were washed with distilled water and rinsed with 70 % ethyl alcohol for cleaning. The leaves were chopped to a size of approximately 2–3 mm and were used as starting biomass. 20 g of the biomass was boiled at 100 °C in 250 mL beaker for 2–3 min. The mixture was then cooled and filtered successively through Whatman filter paper no. 41 and 0.22 μm syringe filter to get leaf extract. The extract was used for the synthesis of silver nanoparticles and other experiments. The leaf extract was stored at 4 °C.
Biological synthesis of silver nanoparticles
AgNO3 (1 mM) and leaf extract were used as stock solution in triplicate for the synthesis of silver nanoparticles. 10 mL of leaf extract was mixed with 90 mL of 1 mM AgNO3 solution. The mixture was exposed to bright sunlight (35–40 °C) to initiate the reaction. A mixture containing 10 mL leaf extract and 90 mL distilled water was taken as control. Within few seconds, the light greenish colour of mixture turned to orange-brown indicating the formation of silver nanoparticles and no such phenomenon was observed in the control. The experiment was carried out for 1 h and samples were withdrawn for analysis using UV–visible spectrophotometer at various time intervals.
Analytical methods
Samples from the experiments were analyzed using a spectrophotometer (Shimadzu UV 1800) to monitor the rate of synthesis of nanoparticles in the solution. pH of the samples was measured using a pH meter (Eutech Instruments pH 510). The solution at the end of experiment was centrifuged (Thermo Scientific Heraeus Multifuge 1S-R) at 10,000×g and the pellet obtained was completely suspended in double distilled water by vigorous mixing. This suspension was transferred onto a petri plate and dried in oven at 60 °C overnight. The dried mixture was scrapped from the petri plate and its fine powder was analyzed by X-ray diffraction (XRD; Rigaku Miniflex II), to examine the crystalline nature of the nanoparticles in the mixture. Scanning electron microscopy (SEM; JEOL JXA-840A Electron probe micro-analyzer) and transmission electron microscopy (TEM; CM 200 Philips) were used to study the size and surface morphology of the particles. Sample for SEM analysis was prepared by drying the suspension on stub under IR lamp. The gold-coated mixture was observed under SEM at 5,000× magnifications. Sample for TEM analysis was prepared by drying the suspension on a carbon coated TEM grid under IR lamp. This dried mixture was observed under TEM at 120,000× magnification.
Antibacterial activity assessment
Antibacterial activity was assessed by agar well diffusion methods [10, 11], against the Gram-positive and Gram-negative microorganisms, viz. Escherichia coli (MTCC 4604), Staphylococcus aureus (NCIM 2127), Salmonella typhimurium (MTCC 733) and Pseudomonas aeruginosa (MTCC 4676) which are common pathogens. The microorganisms were grown on nutrient broth (Himedia) at 37 °C for 24 h. The concentrations of microbial inoculums were adjusted to 2.5 × 105 colony-forming units (CFU) per mL as measured with the McFarland turbidity standards using spectrophotometer at 600 nm. Muller Hinton Agar (MHA) plates were inoculated using a cotton swab over its entire surface with the bacterial suspensions. Three wells per plate were made and each was completely filled individually with (10 μg/mL) synthesized silver nanoparticles suspension, 1 mM AgNO3, and fresh leaf extract for simultaneous comparison of the antibacterial activity of each solution. The plates were incubated overnight at 37 °C and diameter of the bacterial inhibition zones in each of the plates was measured and compared after 24 h.
Results
Visual examination
The diluted leaf extract had a pale yellow colour and appeared turbid soon after adding AgNO3. After the solution was kept in sunlight, the intensity of the colour increased gradually from pale yellow to dark brown at the end of the experiment. The colour change in the solution is presented in Fig. 1a. Expectedly, under the same experimental conditions, control samples showed no change in colour.
a The figure shows change in colour of solution containing Cynodon dactylon plant leaf extract before addition (left) and after addition of 1 mM AgNO3 solution (right). b UV–visible spectra over a wavelength range of 200–700 nm recorded from aqueous C. dactylon leaf extract at different time intervals with 1 mM aqueous AgNO3
UV/visible spectrum analysis
Optical measurement is the prime technique for characterizing the biological synthesis of nanoparticles. Figure 1b shows the UV–visible spectra of the samples periodically withdrawn and subjected to UV–visible spectroscopy during the experiment. A broad peak located at 463 nm was observed after 5 min of exposure in sunlight. The height of the peak, i.e. the absorbance, increased with time till the end of experiment. However, gradual shift in the peak from 463 to 451 nm characteristic of surface plasmon resonance of silver nanoparticle formation is observed that remained constant till the end of experiment.
X-ray diffraction analysis
The dry powder of the nanoparticles was spread on a glass plate and subjected to XRD analysis. The diffracted intensities were recorded in the whole spectra of 2 theta (θ) angles ranging from 10° to 50°. As shown in Fig. 2, the XRD pattern showed two prominent peaks of different intensities and width at different 2θ values.
SEM analysis
The SEM micrograph provided further information about the morphological characteristics of the particles synthesized. It was confirmed that the particles were spherical in shape. The scanning electron micrograph of the nanoparticles at 5,000× magnifications is shown in the Fig. 3a.
TEM analysis
A TEM image of the nanoparticles is shown in the Fig. 3b at 120,000× magnifications. The silver nanoparticles were polydispersed and spherical in shape with a smooth surface morphology. The average diameter of the nanoparticles was found to be 8–10 nm. The TEM image also showed that the particles were more or less uniform in shape. A good number of Ag-NPs were scattered with only a few of them showing aggregates of different sizes.
Antibacterial test
The synthesized silver nanoparticles showed antibacterial activity against the tested microorganisms which was confirmed by the formation of inhibition zones of various diameters as shown in Fig. 4. The result of antibacterial test (diameters of zone of inhibition) is tabulated in Table 1. It was observed that the silver nanoparticles were most effective against P. aeruginosa followed by S. aureus, E. coli and S. typhimurium. The control sample containing only plant extract did not show antibacterial activity against any of the tested microorganisms. However, 1 mM AgNO3 solution also showed comparable antibacterial activity similar to silver nanoparticles.
Discussion
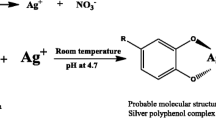
Synthesis of silver nanoparticles from AgNO3 solution requires the reduction of Ag+ ions to Ag. The components of leaf extract act as reducing agents in the synthesis of silver nanoparticles. A green synthetic route for the production of silver nanoparticles using aqueous C. dactylon leaf extract has been successfully demonstrated in the present investigation. Change in colour of the leaf extract solution from greenish yellow to brown during the experiment is the first indication of synthesis of silver nanoparticles. Experiments using other leaf extracts in the past have also shown identical colour changes [6–9], which is due to the excitation of surface plasmon resonance in the silver nanoparticles [12, 13]. The leaf extract was prepared by boiling the chopped C. dactylon leaves at 100 °C, and the enzymes such as nitrate reductase, which may serve as catalysts for the bioreduction reaction would be destroyed. Hence, the role of enzymes for catalyzing the bioreduction reaction can be nullified; this also demonstrates the presence of thermo-stable reducing agents in the extract. Several polyphenolic compounds are widely reported in plants. The leaf of C. dactylon contains polyphenols such as beta-setosterol, flavonoids, alkaloids, glycosides, tannic acids, triterpenoids, Vitamin-C, carotene, palmitic acid, etc. [14, 33]. They have been reported to serve as potential reducing agents for nanoparticles synthesis [15–17]. It has been reported that the OH− groups of such compounds play a vital role in the reaction [18]. Further, in the absence of active enzymes catalyzing the bioreduction reactions, sunlight is reported to act as a catalyst for the bioreduction reaction; and the irradiation effect of sunlight is responsible for this mechanism [19, 20]. So, exposure to bright sunlight was found to be enhancing the rate of the synthesis process. The mechanisms of the reduction reactions mediated by polyphenolic compounds and catalyzed by sunlight leading to the synthesis of silver nanoparticles are shown below.
A simultaneous reduction in pH is most probably because of the decrease in concentration of OH− in the solution, which also proves that polyphenolic compounds of C. dactylon with OH− functional group serve as reducing agents. The intensity of colour (i.e. the absorbance at λmax) constantly increased during the experiment. This shows that concentration of polyphenols in the leaf extract is sufficient to carry out the reduction of 1 mM AgNO3 for 1 h. The synthesis of Ag-NPs started within 5 min of experiment. In addiion, it was observed from the shift in the absorbance peak through UV/Vis spectra from 463 to 451 nm. It has been earlier reported that Ag-NPs of different sizes and shapes depict surface plasmon resonance effect at different wavelengths [13, 21]. This shows a change in surface plasmon resonance of Ag-NPs synthesized during the first 30 min of experiment which may be due to the change in size of Ag-NPs. Increasing intensity of the surface plasmon resonance at constant wavelength (451 nm) after 30 min till the end of experiment shows a steady increase in concentration of Ag-NPs of constant size and shape as a function of time without any shift in the peak wavelength.
The XRD pattern (Fig. 2) of the Ag-NPs gives an insight about the crystalline structure of the Ag-NPs and reveals that the diffraction pattern corresponds to the lattices of crystalline spherical Ag-NPs. The width of peaks obtained at two different 2θ values (38.02° and 46.06°) also signifies the size of nanoparticles. It is well known that the lesser the width of peak, the larger is the size of nanoparticles. From the diffraction pattern, it is revealed that the nanoparticles synthesized are polydispersed and have different sizes. The size and shape of the Ag-NPs were confirmed by SEM and TEM analysis. The synthesized nanoparticles were found to be spherical in shape having a diameter of around 8–10 nm. Similar approaches for nanoparticles synthesis using plants like Azadirachta indica, Glycine max, Cinnamon zeylanicum, Camellia sinensis, Medicago sativa, Brassica juncea, Carica papaya, Cinnamon camphora, etc. have also been reported to synthesize nanoparticles having wide range of size of 8–70 nm and all of them also produced polydispersed nanoparticles [6–9, 22–25]. Our premise was based on exploitation of a common plant occurring in almost all terrestrial ecosystems, so that efficacy of the plant can be tried by many other researchers for development of a techno-economically viable process in the near and far future.
The antibacterial activity of the synthesized Ag-NPs was confirmed against four bacteria viz. S. aureus, E. coli, P. aeruginosa and S. typhimurium and the radii of zone of inhibition are tabulated in Table 1 and shown in Fig. 4. However, the nanoparticles showed moderate antibacterial effect against Gram-negative E. coli and S. typhimurium but a stronger effect against Gram-positive S. aureus and Gram-negative P. aeruginosa. It has been reported that Ag-NPs interact with bacterial membrane protein and DNA at preferential sites as it has high affinity towards sulfur and phosphorous compounds. This interaction leads to the disruption of cell membrane, increasing the cell permeability and DNA damage which leads to cell death and inhibition of bacterial growth. Thus, the antibacterial activity of Ag-NPs is largely dependent on the cell wall and cell membrane composition of the bacteria due to which the Gram-negative bacteria should be more susceptible to the nanoparticles than Gram-positive bacteria.
Higher generation time of P. aeruginosa (39 min) as compared to other Gram-negative bacteria E. coli (20 min) and S. typhimurium (22 min) might be the reason for higher susceptibility of P. aeruginosa for Ag-NPs. Similarly, a higher generation time of Gram-positive S. aureus (30 min) might be the reason for higher antibacterial affect of Ag-NPs as compared to Gram-Negative E. coli and S. typhimurium. MubarakAli et al. [26] have shown strong antibacterial activity of Ag-NPs against both S. aureus and E. coli. However, 1 mM AgNO3 solution showed highest antibacterial activity, while the leaf extract prepared by boiling did not show antibacterial effect against any of the test pathogens. The antibacterial effect of the Ag-NPs was comparable to that of AgNO3 as observed from the zones of inhibition mentioned in Table 1. However, it is more convenient to use silver as nanoparticles than AgNO3, since the latter shows acute toxicity to human and animals and is also irritating to the skin, eyes and mucous membrane. Moreover, Ag-NPs and other nanoparticles can be used to prepare and develop nanomedicine, new generation of antimicrobials, drug delivery systems, biosensors and various other applications such as silver-based dressings, silver-coated medicinal devices, nanogels and nanolotions [27–32].
Conclusion
Silver nanoparticles were successfully prepared using C. dactylon leaf extract as the starting raw material. The method involves the bioreduction of silver ions by the plant extract and the secondary metabolites consisting of polyphenols most probably play the role of potential reducing agent. UV/Vis spectroscopy reveals the surface plasmon property and the increase in silver nanoparticles concentration in the reaction mixture during the course of experiment. XRD analysis and TEM images confirm the surface morphology, shape and size of the silver nanoparticles. Average size estimated from our studies is 8–10 nm. Synthesized silver nanoparticles act as an effective antibacterial agent against common pathogens. Silver nanoparticles can be aptly termed as “Silver Bullets” owing to their potential antibacterial activity. This is a green, economical and rapid method for the fabrication of silver nanoparticles. However, further investigation is required for identifying the active biomolecule present in C. dactylon leaf extract which mediates nanoparticles production.
References
Raffi M, Hussain F, Bhatti TM, Akhtar JI, Hameed A, Hasan MM (2008) J Mater Sci Technol 24:192–196
Guzmán MG, Dille J, Godet S (2009) Int Jr Chem Biomol Eng 2:104–111
Yin B, Ma H, Wang S, Chen S (2003) J Phys Chem B 107:8898–8904
Buzea C, Blandino IIP, Robbie K (2007) Biointerphases 2:MR17–MR172
Gericke M, Pinches A (2006) Hydrometallurgy 83:132–140
Shahverdi AR, Minaeian S, Shahverdi HR, Jamalifar H, Nohi AA (2007) Process Biochem 42:919–923
Tripathi A, Chandrasekaran N, Raichur AM, Mukherjee A (2009) J Biomed Nanotechnol 5:93–98
Vivekanandhan S, Misra M, Mohanty AK (2009) J Nanosci Nanotechnol 9:6828–6833
Begum NA, Mondal S, Basu S, Laskar RA, Manda D (2009) Colloids Surf B Biointerfaces 71:113–118
Pal S, Tak YK, Song JM (2007) Appl Environ Microbiol 73:1712–1720
Deans SG, Ritchie G (1987) Int J Food Microbiol 5:165–180
Mulvaney P (1996) Langmuir 12:788–800
Kelly KL, Coronado E, Zhao LL, Schatz GC (2003) J Phys Chem B 107:668–677
Nagori BP, Solanki R (2011) Res J Med Plant 5:508–514
Kasthuri J, Veerapandian S, Rajendiran N (2009) Colloids Surf B Biointerfaces 68:55–60
Kakazu E, Murakami T, Akamatsu K, Sugawara T, Kikuchi R, Nakao S (2010) J Memb Sci 354:1–5
Chen HM, Hsin CF, Liu RS, Lee JF, Jang LY (2007) J Phys Chem C 111:5909–5914
Durán N, Marcato PD, Durán M, Yadav A, Gade A, Rai M (2011) Appl Microbiol Biotechnol 90:1609–1624
Zarchi AAK, Mokhtari N, Arfan M, Rehman T, Ali M, Amini M, Majidi RF, Shahverdi AR (2011) Appl Phys A 103:349–353
Rastogi L, Arunachalam (2011) J Mat Chem Phys 129:558–563
González AL, Noguez C (2007) J Comp Theor Nanosci 4:231–238
Torresdey JLG, Gomez E, Videa JRP, Parsons JG, Troiani H, Yacaman MJ (2003) Langmuir 19:1357–1361
Haverkamp RG, Marshall AT, Agterveld DV (2007) J Nanopart Res 9:697–700
Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X, Wang H, Wang Y, Shao W, He N, Hong J, Chen C (2007) Nanotechnology 18:105104–105115
Jain D, Daima HK, Kachhwaha S, Kothari SL (2009) Dig J Nanomat Biostruct 4:557–563
MubarakAli D, Thajuddin N, Jeganathan K, Gunasekaran M (2011) Colloids Surf, B 85:360–365
Senarath-Yapa MD, Phimphivong S, Coym JW, Wirth MJ, Aspinwall CA, Saavedra SS (2007) Langmuir 23:12624–12633
Haes AJ, Duyne RPV (2002) J Am Chem Soc 124:10596–10604
Jain J, Arora S, Rajwade JM, Omray P, Khandelwal S, Paknikar KM (2009) Mol Pharmaceut 6:1388–1401
Lv Y, Liu H, Wang Z, Liu S, Hao L, Sang Y, Liu D, Wang J, Boughton RI (2009) J Memb Sci 331:50–56
Singh M, Singh S, Prasad S, Gambhir IS (2008) Dig J Nanomater Bios 3:115–122
Rai M, Yadav A, Gade A (2009) Biotechnol Adv 27:76–83
Jananie RK, Priya V, Vijayalakshmi K (2011) N Y Sci J 4:16–20
Acknowledgments
Authors are thankful to the Director, CSIR-NEERI, for his invaluable help in extending all facilities at the institute. Authors are also thankful to Department of Science and Technology, and Planning Commission, Government of India for providing funds to CSIR-NEERI to carry out the research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sahu, N., Soni, D., Chandrashekhar, B. et al. Synthesis and characterization of silver nanoparticles using Cynodon dactylon leaves and assessment of their antibacterial activity. Bioprocess Biosyst Eng 36, 999–1004 (2013). https://doi.org/10.1007/s00449-012-0841-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-012-0841-y