Abstract
The effects of varying inoculum age and production scale upon the morphology and viability of Streptomyces clavuligerus were studied by analyzing visible and fluorescent light images acquired throughout pilot-plant and pre-industrial scale fermentations. Changes in production scale reveal that in 5 m3 fermentors, the maximum hyphal area obtained is double the value obtained in 0.5 m3 fermentors. It is probably due to the higher shear stresses acting upon hyphae in the 0.5 m3 fermentor caused by higher tip speeds observed in these. The morphological quantification based on elongation and branching rates allowed fermentations to be pattern classified into distinct physiological time zones namely elongation, branching, fragmentation, etc. The general pattern observed for fermentations inoculated with late exponential phase inocula was similar to the pattern of fermentations run with stationary phase inocula except that both the elongation and branching periods started earlier in the former case. Using the available staining technique and image acquisition system, the viability seemed to be generally high and constant throughout the time course of all the studied fermentations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The productivity of clavulanic acid from the filamentous bacteria, Streptomyces clavuligerus has undergone major improvements over the years due to the use of refined cultivation methods and selection of higher producing mutants. However, in order to continue improvement it is necessary to have a better understanding of the phenomena that affect the productivity.
Within this context, the morphology of the filamentous microorganism in submerged culture—which varies between “pellet” and “filamentous” forms depending on the genotype of the strain and prevailing culture conditions—plays a major role in process improvement efforts. It has been reported that in various fermentations morphology correlates with productivity [1, 2, 3, 4]. It is well known that morphology directly influences the rheology of the medium. In industrial submerged culture this can lead to highly viscous and pseudoplastic behavior which, in turn affects the necessary power input to the system to maintain the dissolved oxygen level above a critical value [3]. In severe cases, the oxygen limitation can be such that a lowered productivity is observed. The morphology of the organism can be modified by mechanical and chemical influences dependant on the culture conditions [1, 5]. Furthermore, the size and shape of biological structures in the fermentation broth influence the metabolic activity of the microorganisms [6]. Thus, morphology can be considered, in principle, to be just another manipulated process parameter such as pH and DO. However, process improvements based on morphological changes have proven to be rather difficult to implement. In fact, very little is known about the mechanisms and the overall kinetics that control the morphology that depend on the operating conditions.
The relationship that exists between the ability of a microorganism to grow, branch, fragment and produce primary and secondary metabolites to a greater or lesser extent could be analyzed in relation to the viable cell population, but no report of such studies, relating filamentous bacterial viability to changes in fermentation process parameters, has been published to date. The existing information has been mainly focused on flow cytometry determinations of bacterial activity without relating them to process conditions [7, 8]. To rapidly monitor activity and viability changes throughout a fermentation under complex media conditions, a different approach to plate colony counting using the serial dilution method would have to be used. Both improvement of microscopic instruments and the continuous development of new fluorescent stains have made fluorescent microscopy an attractive choice for the quantification of bacterial viability. Due to the focal importance of morphology and viability and their potential implications in process control, faster and more reliable measurements have to be undertaken. In an industrial context, non-biased, objective, and rapid quantification would be of particular value in prolonged fermentations, namely those run in fed-batch mode.
Various semi-automatic and automatic methods to quantify morphology have evolved in the last few years. Algorithms for quantifying dispersed morphology [9, 10, 11] and pelleted morphology [12, 13] have been developed. As the hyphae of actinomycetes are generally smaller in diameter than those of filamentous fungi, it is difficult to characterize their differentiation using conventional microscopy. However, Drouin et al. [14] and Mauss et al. [15] have succeeded in quantifying differentiation in Streptomyces hyphae using image analysis.
Over the years, many lab-scale fermentations involving submerged cultures of fungi and actinomycetes have been examined extensively with the intention of relating morphological parameters, obtained through digital image analysis and productivity to process conditions [6, 16, 17, 18]. However, despite clavulanic acid commercial importance, reports on the morphology aspects in real industrial conditions and scale are almost non-existent. The Scale-Up Deteriorated production Syndrome (SUDS) has been documented by Okada and Iwamatu [19] for S. hydroscopicus.
The present study reports on the changes in morphology and viability parameters of industrial cultivations of Streptomyces clavuligerus, in response to two of the most influential process parameters, inoculum age and scale. Inoculum age has previously been shown as strongly affecting the production profile of clavulanic acid by Streptomyces clavuligerus [20]. On defined media Roubos et al. [21] have experienced high shear rate sensitivity in batch cultures of the same microorganism for tip speed between 0.91 and 4.1 m/s. Scale change is related to variations in shear rate conditions. Fragmentation of filamentous species is the result of the balance between the effect of hydrodynamic stresses, which favors hyphal breakage, and hyphal tensile strength, which resists it. Li et al. [22, 23] have noticed a decrease in the hyphal tensile strength of a recombinant strain of Aspergillus oryzae at different cultivation scales after the growth period. Such a decrease should induce a higher death rate and a lower viability. In the present work with complex media, viability is assessed using the BacLight double stain previously tested by Sebastine et al. [24] on Streptomyces grown in defined medium. More recently the same staining procedure has been applied by Stocks and Thomas [25] to monitor the viability of Saccharopolyspora erythraea, also in defined medium. The work focuses on the development of an image analysis system that can be used to quantitatively determine morphological aspects and the viability of Streptomyces clavuligerus under real, pilot and pre-industrial scale fermentation conditions using a complex cultivation medium. In doing so, considerations can be formulated, from a biochemical engineering perspective, about changes in morphology, biomass and viability, corresponding to changes in the age of the inoculum and changes in production scale. This understanding should hopefully lead to improved design and operation of industrial mycelial fermentations.
2 Materials and methods
2.1 Strain, culture and growth conditions
Approximately 1.5 ml of a spore suspension (1×107/ml) of an industrial isolate of Streptomyces clavuligerus supplied by CIPAN, S.A (Vala-do-Carregado, Portugal) was added to 400 ml of seed medium in 1500 ml high-lap baffled flasks. These were incubated at 25°C, 220 rpm for 48 h. The vegetative flask culture was used to inoculate the vegetative medium of the preculture tank. A 0.4% inoculum was used for an operating preculture volume of 100 dm3.
Streptomyces clavuligerus fed-batch cultivation was performed in a complex medium containing soy meal, glycerol, dextrin, phosphate and some microelements. A fully instrumented bioreactor with a total working volume of 0.5 m3 (T/D=0.33, D/H=0.33) was used in experiments run at pilot-plant scale conditions, whereas a bioreactor with a total working volume of 5 m3 (T/D=0.33, D/H=0.27) was used in experiments run at industrial scale conditions. T, D and H are respectively the reactor diameter, turbine diameter and reactor height. Both bioreactors had two stages of stirring with Rushton turbines of same size and configuration (6 vertical blades). The tip speeds for the impellers in the 5 m3 and 0.5 m3 bioreactors were 0.71 m/s and 0.95 m/s, respectively. This is lower than the values used by Roubos et al. [21]. During all runs the dissolved oxygen was maintained above 40% saturation by manipulation of the airflow rate. Samples were taken through adequate ports located in each bioreactor above the bottom impeller.
2.2 Experimental layout
A series of twelve fed-batch Streptomyces clavuligerus fermentations were undertaken. These fermentations represent changes of two of the most relevant levels of industrial process parameters, namely, in the age of the inoculum and production scale. Two industrial scale fermentations and four pilot-plant scale fermentations were inoculated with a 24 h, late exponential phase, 7% inoculum, whereas the remaining two industrial scale and four pilot-plant scale fermentations were carried out using an inoculum with approximately 48 h representing stationary phase mycelia at 7%.
2.3 Analytical methods
Samples were taken every 6 h. For determinations of the dry cell weight, broth samples were collected in 5 ml centrifuge tubes. The pipette was washed with 5 ml HCl 2 M. After centrifugation at 8000 rpm for 20 minutes, the supernatant was discarded and the pellet washed with 5 ml distilled water. The residue was dried to constant weight at 100°C, using a pre-dried gravimetric capsule.
Apparent viscosity measurement of the fermentation broth were undertaken using a Brookfield concentric cylinder viscosimeter (Model LVT, Wilmington, USA). Readings were taken using 500 ml samples at 25°C, 60 rpm and spindle number 3. In the present case viscosity is an indirect measurement of biomass [26].
The HPLC procedure used for the clavulanic acid analysis was based on the modification of the method from the British Pharmacopoeia for potassium clavulanate salt and adapted to fermentation broths by CIPAN, SA. The titer is normalized with respect to the largest concentration obtained in the 12 runs.
The concentrations of oxygen and carbon dioxide in the inlet and outlet gas streams were measured using a gas analysis system composed of a paramagnetic oxygen analyzer (Model PMA-25, M&G, Ratingen-Lintorf, Germany) and an infrared carbon dioxide analyzer (Model SIFOR 200, Maihak, Hamburg, Germany). Data from the analyzers were transformed on-line to the metabolic rates of production and consumption, which were plotted against time for process interpretation and control.
2.4 Staining
As an average fermentation lasted for roughly 140 h, a sampling schedule had to be adopted. In general, samples for both the morphological and viability analysis were taken twice to three times a day, averaging 10–12 samples over the 140-hour period.
For the observation of morphology samples of the fermentation broth were diluted with distilled water and mixed in 50 ml glass flasks. The degree of dilution depended on the biomass and the morphology of the broth at that particular time interval. The dilution was necessary to obtain images with objects filling about 10% of the total area. It was verified that this dilution step did not modify the observed morphology (or viability). Approximately 30 μl of the diluted sample is evenly spread across the total area of a glass slide and then left to air-dry for a couple of hours. For every sample, four glass slides were prepared. After this fixation step, the mycelia were stained with crystal violet stain (Merck) and left to settle for 2 minutes. The unabsorbed stain was washed off with distilled water for a period of 2 minutes, and then the slide was left to dry. The dried slides were then identified and stored.
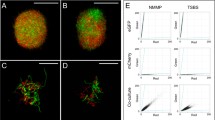
For the observation of the viability, the procedure developed by Sebastine et al. [24] was used. Freshly obtained samples of fermentation broth were diluted with distilled water in order to obtain images with objects filling 10% of the total area. To 1 ml of the diluted bacterial suspension, 3 μl of the mixed viability stain were added in a 1.5 ml micro-tube. The viability stain comprises a mixture of equal volumes of component A (3.34 mM SYTO 9 dye in anhydrous DMSO) and component B (20 mM propidium iodide in anhydrous DMSO) from L-7021 LIVE/DEAD Baclight Bacterial Viability Kit (Molecular Probes, Eugene, Oregon). The combined solution is thoroughly mixed and incubated in the dark for approximately 5 minutes. After incubation, 15 μl of the stained bacterial suspension are trapped between a glass slide and a 20 mm square cover slip. The slide can then be observed on the fluorescence microscope and the images acquired within 20 minutes of sample preparation because of the rapid loss of viability. When using the L-7021 LIVE/DEAD Baclight Bacterial Viability Kit, the hyphae are either stained green (if the membranes are intact) or they are stained red (if they contain damaged membranes). Total viability is given by the ratio of the areas of the hyphae that are stained green to the total area of the hyphae in that sample (red and green hyphae). This ratio indicates the fraction of viable biomass, i.e., biomass which has undamaged membrane.
2.5 Image analysis system
For the morphology analysis, the slides were viewed under phase contrast on an Olympus biological light microscope, Model CX40 (Olympus, Hamburg, Germany). It was found that the best observations were made at an objective magnification of ×20. At this magnification, small objects could be easily detected at the onset of a fermentation and a reasonable number of objects could easily be observed with a good resolution throughout most of the remaining samples. At this resolution, 50 images were captured for the morphological analysis using a COHU color camera (RGB system; USA) connected to a Matrox Meteor acquisition board (Matrox, Dorval, Canada) on an IBM compatible Intel-Pentium MMX personal computer (32 Mbytes of Ram and 166 MHz) with 9 Gb storage capacity. The images are captured via a PC-based image grabber—Matrox Inspector 2.1 (Matrox Electronic Systems, Ltd.) and stored in tiff image format (768×576 pixels) occupying 1.327 Mb of disk space per image. Each color primitive (R=red, G=green and B=Blue) is coded on 256 levels. The professional image analysis software VISILOG 5.1 (Noesis SA, Les Ulis, France) was used in the analysis of the morphology.
For fluorescent microscopy a reflected light fluorescence attachment model BX-FLA (Olympus, Hamburg, Germany) was fitted on to the existing biological light microscope. The required 50 images for the viability analysis were captured as previously described. Very often, a halo was formed around the green fluorescent hyphae due to over excitation, and an adjustment had to be made on the collector lens-focusing knob so as to diminish the amount of excitation light incident on the sample. In some cases, especially during the visualization of the first few samples, a second preparation had to be made because complete degradation of the sample on the slide had been attained. The same image analysis software was used in the analysis of viability images.
3 Image analysis
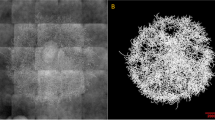
Two distinct image analysis programs, one for characterizing the dispersed morphology and the other for characterizing the viability of samples from submerged fermentations of S.clavuligerus were developed for each analysis. Only dispersed mycelia or loose entanglements (Fig. 1) were found in both types of image. No pellets could be observed in this complex cultivation medium.
4 Morphological analysis algorithm
Once the TIFF file has been opened and read, the RGB (red, green and blue) color system is changed to the HLS (hue, lightness and saturation) system and the L plane is selected. Object detection is then performed followed by binary image processing and noise filtering. This binary image is the starting point for the measurement of various object parameters including area, second-order moments and gyration radii. Non-filamentous objects (debris from cultivation medium) are excluded based on limits set for the size and the gyration radius [14, 15]. Objects have to be larger than 50 pixels and have a gyration radius larger than 1. After the final labeling of the binary image, the computation of the basic morphological and size descriptors is performed. The total area per object and the total number of end points per object were chosen as representative parameters for the morphological characterization, as they represent the degree of elongation and branching throughout a fermentation, respectively. The hyphal growth unit (HGU), given by the ratio between the total hyphal length and the total number of tips per object and which designates the predominance of branching and/or elongation was also monitored throughout.
4.1 Viability analysis algorithm
Once the TIFF file has been opened and read, the RGB color system is changed to the HLS system and the L plane is selected. After object detection and separation is achieved as described previously, a color segmentation procedure is applied which consists in thresholding the gray image in accordance to a set of predefined values designating red and green areas. The result of this procedure serves to characterize and separate objects that are entirely red and entirely green from those that are heterogeneously colored red and green. The latter is then separated into two images, one containing the red sections and the other containing the green sections. The measurements of the necessary parameters are then performed on the resulting images.
For the viability characterization, the fraction total viability was chosen as the representative parameter. This is given by the total area of green pixels divided by the total area of red and green pixels accounted for within an image.
5 Results
5.1 Data validation
Before interpreting the data, it is required that a statistical validation of the measurements be performed because the application of image analysis techniques leads to an enormous amount of data, and it would thus be inadequate to analyze them without a critical statistical validation. Thus, the choice of the number of images/objects to be analyzed (statistical sample size), the consistency of extracted features, but also the time spent to capture the images should be considered.
The usual validation process would have consisted in the analyses of several samples from different slide preparations, but this is made difficult with the use of vital stains. As a result, the test was carried out on one large set of 250 objects from various samples obtained during one fermentation for the morphological analysis and out of a set of 50 images for each of the samples taken from the same fermentation, for the viability analysis. These data were selected from a variety of samples taken during a fermentation so as to be representative of its time profile. The validation process consisted in analyzing the variation of the mean values for area as a function of the number of objects measured. Stabilization within 10% of the mean values calculated on 100 images was obtained for 90 objects. Therefore, in the case of the morphological analysis, the minimum number of statistically significant objects will be 90. The variation of the mean values for percent total viability as a function of the number of images analyzed was also undertaken and stabilization within 5% of the mean was obtained after roughly 30 images have been analyzed in the selected analyzed samples. In general, all the samples show this high stability in relation to total viability per image.
5.2 Comparison of runs
Using the morphology and viability programs previously outlined, the set of morphological and viability parameters described above were measured and calculated for the set of 12 fermentations. A vast heterogeneity of the mean values of the time profiles of total area (μm2), number of tips per object (tips/object), the hyphal growth unit (μm/tip) and fraction total viability was observed for the total group of twelve fermentations, although it could also be observed that the order of magnitude of certain parameters was the same. Each data point obtained is representative of the analysis of 50 images, constituting the measurement of at least 100 objects per data point. This heterogeneity is not surprising given the intense variability encountered in the process and culture variables, i.e., clavulanic acid HPLC titer (as % of maximum value of all 12 fermentations) and respiratory quotient (RQ). The primary metabolism is represented by the respiratory parameter RQ. The clavulanic acid titer is representative of the organism’s secondary metabolism.
Due to the variety of conditions investigated, the approach used for result presentation and discussion is to divide the set of runs into four groups, each consisting of a set of fermentations undertaken at the same process conditions (Table 1).
Figure 2 illustrates the average time profiles of total hyphal area, HGU, viability, product formation and RQ, for the four fermentations constituting Group A (cf. Table 1). The corresponding profiles for a particular fermentation (run 5) are also shown. The morphological pattern encountered in this group is essentially fragmentation. From the 25th hour of fermentation to the final 20 hours of senescence, a continuous fragmentation pattern is observed, which involves the reduction in the total hyphal area from a maximum value of 450 μm2 (with an average of 8 tips/obj) to 240 μm2 (2.5 tips/obj). Broth viscosity increases until the 70th hour and remains constant or decreases, to a minimal value of 60% of its maximum. Clavulanic acid production starts after the initial lag phase has been completed (after 5 h). It then boosts up at 25 h of fermentation, when the carbon dioxide production rate starts to decrease. The maximal antibiotic production rate is obtained shortly before the viscosity maximum. The boost in the production of the secondary metabolite seems to be associated with a decrease in the area of the hyphae. During this production phase there is a steady decrease in the length and branching of the hyphae, but this decrease is much more accentuated in terms of area than in terms of the number of tips, indicating the formation of smaller but still highly branched hyphae. The latter can also be the result of continuous fragmentation and is characteristic of fed-batch fermentations where size heterogeneity is the result of a heterogeneous population in terms of age. Total viability is very high, ranging between 80% and 90%. The constancy of the viability can be observed in all Group A fermentations.
Figure 3 presents for Group B runs (same scale as Group A, but with 48 h inocula), the same average profiles of morphological, viability and process parameters as before, and also highlights a particular fermentation (run 7) belonging to this group. The morphological pattern encountered in this group is considerably different from profiles of Group A. A longer lag phase is observed with the 48 h inocula (0–20 h), i.e., the time taken before rapid elongation sets in, for maximal hyphal area to be attained, is longer than in Group A fermentations. For both groups, the boost in clavulanic acid production is observed after the maximum hyphal areas have been reached. Nevertheless, the maximum dimensions for both groups are similar (approximately 450–500 μm2 in both cases). The fact that clavulanic acid production is non-zero at the onset of most fermentations in this group and that it is zero for Group A, indicates that the preculture development is a predetermining factor for a systematically run fermentation. This has implications in terms of the duration of the lag phase, which is 5 h for Group A and roughly 20 h for Group B. Within Group B, varying lag phase durations can be detected, caused perhaps by the delay or anticipation of inoculation time, with inocula typically of 48 h of age. The area and the number of tips decrease during this phase, but the decrease in area is more accentuated than the decrease in the number of tips until roughly 80 h of fermentation indicating that on average, during the rapid production phase, the hyphae are smaller but still highly branched. From 90 h to the end of the fermentation the small and unbranched hyphae do not fragment further, probably since highly fragmented hyphae cannot be further reduced in size. These hyphae are still producing clavulanic acid, but the observed productivity is decreased. In spite of the higher inoculum age, the viability is in the same range as for Group A, around 90%.
In Fig. 4 the average profiles of morphological, viability and process parameters for Group C runs are presented (higher scale than Groups A and B, and 24 h inocula). Off-gas analysis for the fermentation run 15 is not available. The clavulanic acid titer for fermentation 14 is much higher than that for fermentation 15; in fact the maximum clavulanic acid titer obtained in the twelve runs is that observed in fermentation 14 (100% HPLC titer). The average viability profile is constant and high, ranging between 85 and 93%. It is observed that the morphological pattern encountered has some similarities with the profile of Group A fermentations. A short lag phase (0–5 h) is also observed with the 24 h inocula at industrial conditions, i.e., the adaptation time taken before elongation occurs to attain maximal hyphal area is similar to Group A fermentations. The initial secondary metabolite production is also nil in Group C, indicating that the inocula were still actively growing. This is consistent with the fact that during the initial 20 h of fermentation a significant increase in the size of the hyphae is observed indicating a rapid growth phase. It is interesting to note that the clavulanic acid production curve observed in Group C is linear during the entire fermentation run, in contrast with Group A and B where the curves exhibited different production gradients. An important morphological difference observed in Group C, relative to Groups A and B, is the maximum total hyphal area. The maximum total hyphal area in this group is double the value obtained in relation to the fermentations run at pilot-plant scale, where the tip speed is about 25% higher. However this maximum area of 800 μm2 is very quickly brought down to roughly half its value within a few hours. At the end of the runs, the hyphal area has reached values similar to those observed in Groups A and B. Broth viscosity reaches its maximal value, which is about 20% higher than for groups A and B, between 70 and 100 h and remains constant.
Figure 5 presents for Group D runs the average profiles of morphological, viability and process parameters observed for the 5 m3 scale bioreactor and with stationary phase inocula. Off-gas analysis for Group D fermentations is not available. The viability, as in other groups remains relatively constant and high (Fig. 4). The morphological pattern encountered in this group is typical of other fermentation runs with 48 h inocula (Group B). A longer lag phase (0–20 h) is also observed with the 48 h inocula at industrial conditions, i.e., the adaptation time necessary for the hypha to commence rapid elongation in order for maximal hyphal area to be attained is similar to Group B fermentations. An important morphological difference observed in Group D (and Group C), relative to Groups A and B, is the maximum total hyphal area (800 μm2). The maximum total hyphal area in this group is double the value obtained in relation to the fermentations run at pilot-plant scale. This is probably due to the lower shear stress acting on hyphal elements in the industrial bioreactor caused by the lower tip speeds encountered. Viscosity is in the same range as for Group C.
In terms of total hyphal area, there are significant differences between groups during the exponential growth phase only: maximal hyphal area is higher for the higher scale, probably due to reduced fragmentation at the lower tip speeds used in the higher scale. The hyphal area decreases significantly after the exponential growth phase. HGU was not found discriminating (i.e., the general pattern followed by this variable was not significantly different across the four groups). Viability was for all groups and through all process phases similar and very high (i.e., >90%), indicating that lysed mycelia were readily emptied of intracellular material and further fragmented. The antibiotic titer is significantly higher for the higher scale runs from the transition growth phase onwards. Namely, for the 5 m3 fermentation runs with more active inocula (group C) the titer at the end of the fermentation were the highest. This result is linked to the reduction in mycelia fragmentation obtained in the higher scale (more active biomass) and higher specific activity of biomass from 24 h-inocula (higher specific production rates).
To conclude, the staining technique employed in the viability study gives the percentage of hyphal area containing intact membranes relative to the total hyphal area (indicating cells with intact and perforated membranes). Therefore, it does not distinguish between viable, metabolically active (producing and reproducing) cells and non-producing and non-reproducing viable cells. The measured viability was generally always very high (in the order of 80–90%) and relatively constant during the time course of all the studied fermentations. These results are in accordance with those reported by Andersson et al. [27] who showed that dead cells formed an insignificant part of the bacterial population during growth of Escherichia coli in high cell density fed-batch cultures. Evidence of this was found while using a dye (p-iodonitrotetrazodium violet), which distinguishes viable and reproducing cells, as well as viable metabolic active cells, having a reduced ability to divide. The question arises whether the complete mycelium is stained. Sebastine et al. [24] who investigated the change in viability of S.clavuligerus in defined media, observed generally high percentage viabilities throughout the time course of all studied fermentations. He calculated viability as the ratio of green filaments to total mycelia area, as measured by brightfield microscopy. He indicates also that there is no unstained mycelial regions. In the present study two devices were used for image capture, which makes a direct comparison difficult. More investigation is probably necessary to elucidate that question. It should be noted that the viability analysis with the BacLight double stain is delicate and may be prone to inaccuracies if the quality of the image is poor, especially concerning the visualization of the red regions.
6 Conclusion
A set of 12 fermentations, divided into four groups, were run in industrial conditions, in order to examine the effect of inoculum age and scale. Even though each group consisted of a set of fermentations carried out at identical process conditions, batch to batch reproducibility could not be exactly achieved, as is the case with most bioprocesses. Inoculation of production bioreactors is based on standard age procedures and not on constant physiological time, contributing to variable biomass concentration and metabolic activity of the preculture at the instant of inoculation.
The morphological quantification allowed the classification of fermentations into distinct physiological zones. In general, fermentations run with stationary phase (48 h) inocula (independently of scale) followed a pattern that consisted in an initial lag phase of roughly 20 h, followed by elongation, branching and a final fragmentation phase which followed on up to the end of the fermentation. The general pattern observed for fermentations inoculated with late exponential phase inocula (24 h) was similar, except that both the elongation and branching periods started earlier. Moreover, clavulanic acid production was higher in fermentations with 24 h inocula.
The study of the effect of changes in production scale reveal that in the 5 m3 fermentor, the maximum average hyphal area obtained (800 μm2) is almost double the value of the hyphal area obtained in the 0.5 m3 fermentor (400 μm2). This is probably due to the fact that the tip speed in the larger fermentor was less than the tip speed in the smaller fermentor, and therefore the shear stress acting on the hyphal elements in the smaller bioreactor is larger, thus causing these hyphae to fragment. These results indicate that this industrial isolate of Streptomyces clavuligerus is shear sensitive in the agitation speed range tested, like many other filamentous microorganisms. Nevertheless, this increase in hyphal area did not seem to affect the clavulanic acid production profile. Although the maximum hyphal area observed in 5 m3 is considerably larger than the area observed in pilot-plant scale fermentors, it is quickly reduced to roughly 600 μm2 after a few hours. However, the morphological behavior observed during the studied industrial cultivation of S.clavuligerus is similar to that observed by Pons et al. [28] in Streptomyces ambofaciens with a complex medium: a fragmentation and a decrease of HGU were observed at the onset of the production of spiramycin. Similar findings have been described by Li et al. for A. oryzae [29]. These phenomena might be general and should be investigated in more detail. By the use of image analysis, this work gives a quantitative description of how changes on two of the most influential industrial process parameters namely, inoculum age and scale change, can affect the morphology and apparent viability of S.clavuligerus and consequently the productivity of fed-batch fermentations. It can be used to investigate the effect of other operation conditions likely to affect the productivity at industrial scale such as mixing time and pulse-feeding [29].
References
Metz B, Kossen NWF (1977) The growth of moulds in the form of pellets—a literature review. Biotech Bioeng 19:781–799
Krabben P, Nielsen J (1998) Modelling the mycelial morphology of Penicillium species in submerged cultures. Adv Biochem Eng/Biotechnol 60:125–152
Metz B, Kossen NWF, van Suijdam LC (1979) The rheology of mould suspensions. Adv Biochem Eng 11:104–156
Whitaker A, Long PA (1973) Fungal pelleting. Process Biochem 11:27–31
Vecht-Lifshitz SE, Magdassi S, Braun S (1990) Pellet formation and cellular aggregation in S. tendae. Biotechnol Bioeng 35:890–896
Schügerl K, Wittler R, Lorenz T (1983) The use of molds in pellet form. Trends Biotechnol 1:10–123
Davey HM, Kell DB (1996) Flow cytometry and cell sorting of heterogeneous microbiological populations: The importance of single cell analysis. Microbiol Rev 12:641–696
Virta M, Lineri S, Kankaanpää P, Karp M, Peltonen K, Nuutila J, Lilius E (1998) Determination of compliment-mediated killing of bacteria by viability staining and bioluminescence. App Environ Microbiol 2:515–519
Adams HL, Thomas CR (1988) The use of image analysis for morphological measurements on filamentous organisms. Biotechnol Bioeng 32:707–712
Packer HL, Thomas CR (1990) Morphological measurements on filamentous microorganisms by fully automatic image analysis. Biotechnol Bioeng 35:870–881
Tucker KG, Kelly T, Delgrazia P, Thomas CR (1992) Fully automatic measurements of mycelial morphology by image analysis. Biotechnol Prog 8:353–359
Cox PW, Thomas CR (1992) Classification and measurement of fungal pellets by automated image analysis. Biotechnol Bioeng 39:945–952
Reichl U, King R, Gilles ED (1992) Characterisation of pellet morphology during submerged growth of Streptomyces tendae by image analysis. Biotechnol Bioeng 39:164–170
Drouin JF, Louvel L, Vanhoutte B, Vivier H, Pons MN, Germain P (1997) Quantitative characterisation of cellular differentiation of Streptomyces ambofaciens in submerged culture by image analysis. Biotechnol Tech 11:819–824
Mauss P, Drouin JF, Pons MN, Vivier H, Germain P, Louvel L, Vanhoutte B (1997) Location of respiration activity in filamentous bacteria by image analysis. Biotechnol Tech 11:813–817
Belmar-Beiny MT, Thomas CR (1991) Morphology and clavulanic acid production of Streptomyces clavuligerus: Effect of stirrer speed in batch fermentations. Biotechnol Bioeng 37:456–462
Braun S, Vecht-Lifshitz SE (1991) Mycelial morphology and metabolite production. Trends Biotechnol 9:63–68
Paul GC, Priede MA, Thomas CR (1999) Relationship between morphology and citric acid production in submerged Aspergillus niger fermentations. Biochem Eng J 3:121–129
Okada S, Iwamatu S (1997) Scaleup production of milbemycin by Streptomyces hydroscopicus subspecies Aureolacrimosus with control of internal pressure, temperature, aeration and agitation. J Chem Technol Biotechnol 70:179–187
Neves AA, Vieira LM, Menezes JC (2001) Effects of preculture variability on clavulanic acid fermentation. Biotechnol Bioeng 72:628–633
Roubos JA, Krabben P, Luiten RGM, Verbruggen HB, Heijnen JJ (2001) A quantitative approach to characterizing cell lysis by mechanical agitation of Streptomyces clavuligerus. Biotechnol Prog 17:336–347
Li ZJ, Shukla V, Wenger K, Fordyce A, Pedersen AG, Marten M (2002) Estimation of hyphal tensile strength in production-scale Aspergillus oryzae fungal fermentations. Biotechnol Bioteng 77:601–613
Li ZJ, Bhargawa S, Marten MR (2002) Measurements of the fragmentation rate constant imply that the tensile strength of fungal hyphae can change significantly during growth. Biotechnol Lett 24:1–7
Sebastine IM, Stocks SM, Cox PW, Thomas CR (1999) Characterisation of percent viability of Streptomyces clavuligerus using image analysis. Biotechnol Tech 13:419–423
Stocks SM, Thomas CR (2001) Viability, strength and fragmentation of Saccharopolyspora erythrea in submerged fermentation. Biotechnol Bioeng 75:702–709
Neves AA, Pereira DI, Vieira LM, Menezes JC (2000) Real time monitoring biomass concentration in Streptomyces clavuligerus cultivations with industrial media using a capacitance probe. J Biotechnol 84:45–52
Andersson LC, Strandberg L, Enfors S (1996) Cell segregation and lysis have profound effects on growth of Escherichia coli in high cell density fed batch cultures. Biotechnol Prog 12:190–195
Pons MN, Drouin JF, Louvel L, Vanhoutte B, Vivier H, Germain P (1998) Physiological investigations by image analysis. J Biotechnol 65:3–14
Bhargava S, Wenger K, Marten MR (2003) Pulse addition of limiting-carbon during Aspergillus oryzae fermentation leads to improved productivity of a recombinant enzyme. Biotechnol Bioeng 82:111–117
Acknowledgements
This work was partially financed by the PRAXIS XXI program. LSP acknowledges Fundação para a Ciência e a Tecnologia for their research grant under PRAXIS XXI program (FCT-BM/98/15210), The French Embassy and The British and The Portuguese (ICCTI) Research Councils for financing stays in LSGC-CNRS, France and in Prof. C. Thomas’ (Birmingham University, UK) lab. The authors wish to express their gratitude to CIPAN S.A. for partially financing the project and for permission to publish the above results. The authors thank Prof. Muge and co-workers at the Materials Department at I.S.T for contributing one of the image analysis algorithms.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00449-004-0362-4
Rights and permissions
About this article
Cite this article
Pinto, L.S., Vieira, L.M., Pons, M.N. et al. Morphology and viability analysis of Streptomyces clavuligerus in industrial cultivation systems. Bioprocess Biosyst Eng 26, 177–184 (2004). https://doi.org/10.1007/s00449-003-0349-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-003-0349-6