Abstract
The recovery of predators has the potential to restore ecosystems and fundamentally alter the services they provide. One iconic example of this is keystone predation by sea otters in the Northeast Pacific. Here, we combine spatial time series of sea otter abundance, canopy kelp area, and benthic invertebrate abundance from Washington State, USA, to examine the shifting consequences of sea otter reintroduction for kelp and kelp forest communities. We leverage the spatial variation in sea otter recovery to understand connections between sea otters and the kelp forest community. Sea otter increases created a pronounced decline in sea otter prey—particularly kelp-grazing sea urchins—and led to an expansion of canopy kelps from the late 1980s until roughly 2000. However, while sea otter and kelp population growth rates were positively correlated prior to 2002, this association disappeared over the last two decades. This disconnect occurred despite surveys showing that sea otter prey have continued to decline. Kelp area trends are decoupled from both sea otter and benthic invertebrate abundance at current densities. Variability in kelp abundance has declined in the most recent 15 years, as it has the synchrony in kelp abundance among sites. Together, these findings suggest that initial nearshore community responses to sea otter population expansion follow predictably from trophic cascade theory, but now, other factors may be as or more important in influencing community dynamics. Thus, the utility of sea otter predation in ecosystem restoration must be considered within the context of complex and shifting environmental conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sustainable management and conservation of marine ecosystems hinges on understanding natural and anthropogenic pressures and structural forces that act on system stability (Knowlton 2004). While marine resources and ecosystem services in coastal zones are affected by climate and environmental variability as well as human activities like fishing, nutrient loading, and habitat alteration (e.g., Sherman and Duda 1999), species interactions also play an important and more nuanced role in marine ecosystem dynamics. For example, “keystone” species affect marine community structure and function to an extent that is disproportionate to their biomass (Paine 1969; Power et al. 1996). A classic example is the sea otter, Enhydra lutris, in coastal waters of the North Pacific Ocean from Alaska to California. Sea otter predation can severely reduce local densities of benthic grazing invertebrates such as sea urchins, thereby allowing kelp canopies to develop and expand (Estes and Palmisano 1974; Estes and Duggins 1995; Steneck et al. 2002; Watson and Estes 2011). The indirect effect of sea otters on kelp is important, given that kelp forests are among the most productive ecosystems on Earth (Mann 1973), support distinct fish, invertebrate, and understory algal communities (Duggins 1988; Ebeling and Laur 1988, Reisewitz et al. 2006; Markel and Shurin 2015), and perform ecosystem roles such as wave energy attenuation (Pinsky et al. 2013) and carbon storage (Wilmers et al. 2012). Similar community- and ecosystem-level consequences of sea otters have been noted in other coastal habitats as well (e.g., seagrass communities; Hughes et al. 2013).
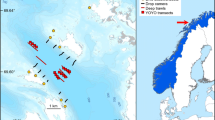
Sea otters are native to the coast of the Olympic Peninsula of Washington state, USA (Fig. 1), but were hunted to extirpation by the early 1900s (Lance et al. 2004). Reestablishment efforts began in 1969–1970, with 59 sea otters translocated to Washington from Amchitka Island, Alaska (Jameson et al. 1982). Despite high mortality in the early 1970s, the population eventually expanded (Fig. 1), surpassing 200 individuals by 1989 (Jameson 1993) and 600 by the late 1990s (Jameson and Jeffries 1999; Fig. 1). SCUBA surveys at multiple sites in 1987 indicated that otter densities were correlated with increased coverage of foliose and canopy-forming kelps, and reduced abundance and size of benthic invertebrates, including kelp-grazing sea urchins in the genera Mesocentrotus and Strongylocentrotus (Kvitek et al. 1989). Subsequent surveys in 1995 and 1999 showed further declines in invertebrate communities at these study sites (Kvitek et al. 1989, 1998, 2000). Kelp canopy area peaked at the scale of the Olympic Coast in the early 2000s (Fig. 1; WADNR 2017; Pfister et al. 2018).
Study area with survey site locations for invertebrate surveys (blue-labeled dots). Numbered areas along the coast indicate strata (kelp index map regions) within which WADNR calculates kelp area. Top right: time series of sea otter abundance on the Washington coast. Bottom right: time series of total kelp canopy area for kelp strata between 15.1 and 25.2
Since the subtidal community surveys in 1999, the Olympic Coast sea otter population has more than doubled (Fig. 1; Jeffries and Jameson 2014). We expect that increased top–down control by sea otters might have further suppressed benthic macroinvertebrates and increased kelp canopy cover; however, the total kelp canopy area has declined from a peak abundance in roughly 2005 (Fig. 1). The apparent decoupling of sea otter and kelp changes warrants renewed research to understand patterns of nearshore community change at the regional and landscape scales.
While sea otters are generally assumed to play a strong top–down role in shifting North Pacific coastal ecosystems from herbivore-dominated to algae-dominated (Estes and Duggins 1995), this generality has been both affirmed and challenged over the past 40 years (Watson and Estes 2011). There are numerous examples of Pacific coastal systems that are not herbivore-dominated in the absence of sea otters (Foster 1990; Lafferty 2004; Carter et al. 2007; Reed et al. 2011). For example, Reed et al. (2011) found that wave disturbance overwhelmed the effect of herbivory and nutrient availability in determining kelp forest dynamics in central and southern California. This example highlights the importance of other physical and biological interactions for structuring coastal habitats and encourages an explicit consideration of the spatiotemporal heterogeneity of coastal kelp systems. Such a landscape perspective on the drivers of heterogeneity and complexity has been used to improve understanding of kelp forest dynamics in California (Bell et al. 2015).
Here, we combine a 30-year time series of sea otter, kelp, and benthic invertebrate data along the Olympic Coast to better understand nearshore marine community dynamics at regional and landscape scales. We conduct spatiotemporal analyses on sea otter and kelp data from publicly available surveys and conduct new field sampling to extend the previous kelp forest invertebrate surveys conducted at focal sites by Kvitek et al. (1989, 1998, 2000). Our ability to understand the interactions between habitat, predators, and prey is essential for proper management and conservation of coastal species, habitats, ecosystems, and services, particularly in this area, where maintaining high native biodiversity and keystone species populations are explicit management objectives (Office of National Marine Sanctuaries 2008).
Materials and methods
Study locations
Our study sites fall within the Olympic Coast National Marine Sanctuary (OCNMS, designated in 1994). We conduct analyses at landscape- and local scales. For the landscape-scale analysis, we use long-term monitoring surveys of the abundance of sea otters and kelp canopy surface coverage for the entire OCNMS region. Below, we describe these data sources and detail how we connect these OCNMS-wide surveys to provide sea otter and kelp abundance at our sites.
For local-scale analyses, we focus on ten kelp forest sites located within the range of OCNMS surveys for sea otters and kelp canopies (Fig. 1). Eight sites are on the outer coast, and two, Chibahdehl Rocks and Neah Bay, are inside the Strait of Juan de Fuca (Fig. 1). All sites feature subtidal rocky reef habitat with dense stands of canopy kelp (Nereocystis luetkeana and/or Macrocystis pyrifera) and understory red, brown, and green algae. Canopy-forming kelp forests generally occupy depths shallower than 10 m in OCNMS. SCUBA divers surveyed each site for benthic invertebrates in at least two of the 3 years (1987, 1995, and 1999) by Kvitek and colleagues (1989, 1998, and 2000) and 2015 (see below). Six sites (Teahwhit Head, Rock 305, Cape Johnson, Cape Alava, Anderson Point, and Neah Bay; Fig. 1) were surveyed in all 4 years (1989, 1995, 1999, and 2015). In addition, a winter sea urchin fishery occurred between the early 1980s and 1997 in the area including two of the sites, Chibadehl Rocks and Neah Bay (WDFW District 5), but available evidence does not suggest that harvest had an effect on sea urchin populations relative to the effect of sea otters (Kvitek 1989; Laidre and Jameson 2006). Commercial sea urchin fisheries never occurred at the other study sites.
For regional comparisons, we divided the ten sites into three geographic groups: northern (Neah Bay, Chibahdehl Rocks, and Tatoosh Island), central (Anderson Point, Point of the Arches, and Cape Alava), and southern (Cape Johnson, Rock 305, Teahwhit Head, and Destruction Island). These groupings are similar to the areas used to describe sea otter trends within OCNMS historically (Lance et al. 2004) and reflect distinct geographic patterns in kelp and sea otter trends (see “Results”). We also use these groupings to account for pseudo-replication in statistical analyses and to test for regional differences in biological relationships.
Finally, we calculated or compiled indices of several environmental features that may influence local nearshore community dynamics. For each site, we calculated an exposure metric, which was a composite of potential wind-driven wave energy, to use as an explanatory variable in statistical models (Supplement Fig. S1). We also summarized broad-scale oceanographic indices of summer (April–September) ocean temperature using the Pacific Decadal Oscillation (PDO; Mantua et al. 1997) and summer coastal upwelling (Coastal Upwelling Index (CUI); Bakun 1973) for each year and among-year averages for the longer time blocks described below. PDO and CUI estimates are large-scale oceanographic indicators relative to our study area, and do not vary among sites; we lack the detailed local data to construct additional site-level variables of temperature or upwelling for the entirety of the kelp and sea otter time series.
Sea otter abundance and distribution
We extracted sea otter location and abundance information from research reports (e.g., Lance et al. 2004; Jeffries and Jameson 2014) to examine shifts in otter abundance and distribution over the past several decades. Sea otter surveys along OCNMS have been conducted by a mix of aerial surveys and land-based observations since 1977. Surveys were approximately biennial through the 1980s and annual from 1989 to 2015 (but no surveys in 2009 or 2014) and are considered to be a complete census of the sea otter population (Lance et al. 2004; Jeffries and Jameson 2014). Sea otter surveys were conducted in summer and thus reflect summer distribution and abundance (Laidre et al. 2009). Available evidence does not suggest that summer and winter distributions of sea otters are substantially different in this region (Laidre et al. 2009). However, sea otters are highly mobile predators with substantial home ranges and seasonal patterns are uncertain.
To estimate trends in sea otter abundance at each focal site, we developed a kernel-smoothed distribution of otters along a “one-dimensional” coast (see Laidre et al. 2009; Shelton et al. 2017 for other examples) to incorporate uncertainty about how snapshot surveys translate to effective numbers of otters present at a given site. Using Esri ArcGIS, we created the “one-dimensional” coast by head-up digitizing a simplified or reduced resolution shoreline polyline that generally stayed within 2 km of shore and depths < 20 m (bathymetry based on Taylor et al. 2008, Grothe et al. 2010). We then divided that shoreline polyline into ~ 20,000 10-m-long segments and determined which 10 m segment was nearest to each of the sea otter survey points. This identified the approximate position of each observed sea otter along the entire length of the Olympic Peninsula coastline. We generated a smooth density of otters (units: number km−1) along the coastline using kernel density estimates, which approximates the observed otter data using a mixture of Gaussian (Normal) distributions. Specifically, we placed a Gaussian distribution centered at each observed sea otter location with a standard deviation h (the bandwidth) that corresponds to the estimated sea otter home-range size of 40 km for the Washington coast (h = 10.2; Laidre et al. 2009, their Fig. 3). After calculating the kernel probability density, we calculated the proportion of the total sea otter population that was present within a radius of 10 km of each focal site by integrating the probability density and multiplying by the total sea otter population. Due to uncertainty in the effective home-range size of sea otters, we used sensitivity analyses with a range of bandwidths (h = 5 and 15). The qualitative pattern of results did not change with alternate bandwidths.
We estimated the temporal trend in sea otter abundance at each site and OCNMS wide by regressing the natural logarithm of sea otter abundance against time. We used the whole time series beginning with the first year of kelp canopy survey (1989, see below), and separately for the two halves of the time series (1989–2001 and 2002–2015) to assess if trends shifted over time. As estimates of trends become progressively less precise with shorter time series, we do not further subdivide the time series. To facilitate comparison among sites that vary substantially in sea otter abundance, we standardized the number of sea otters by dividing by the average number of sea otters estimated at each site during the first 3 years of the kelp surveys (1989–91; \(N_{{j,{\text{base}}}}\)) and calculated a natural logarithm of this ratio: \({ \ln }\left( {\frac{{N_{j,t} }}{{N_{{j,{\text{base}}}} }}} \right)\). Using this index allows for sites across a large range of abundances to be visualized on the same axes and provides a graphical interpretation of sea otter trend (linear trends are exponential changes in abundance). We explored alternate years for dividing the time series as well, setting the break variously at 1999 to 2003; these breaks made little qualitative change to the results.
Kelp canopy area
To describe kelp abundance at each site, we used publicly available data from aerial overflight surveys of algae from the Washington Department of Natural Resources (Van Wagenen 2015; WADNR 2017). Surveys were conducted annually between 1989 and 2015 (but no survey in 1993) during peak kelp abundance for the region (late July or early August) and area calculations were obtained from analysis of aerial photographs (see Van Wagenen 2015; WADNR 2017 for detailed methods). Kelp canopies in this region consist of a mix of Macrocystis pyrifera and Nereocystis luetkeana. While overflight surveys differentiate between the two species, we are primarily interested in the total canopy habitat provided, and thus, we focus on the total surface coverage provided by the two species in the main text. We present parallel analyses for Macrocystis in the online supplement, but the two species’ abundances are strongly positively correlated in this region (Pearson’s r = 0.689; Pfister et al. 2018) and the overall pattern is shared for both species of kelp.
We examined kelp abundance at two scales. First, we used kelp area within discrete area strata along the coast to provide estimates of local kelp surface coverage, \(K_{j,t}\), for the strata containing each of our ten sites, j, in each year, t (Fig. 1). The strata (kelp index map regions) used by WADNR are substantially larger than the area surveyed during invertebrate surveys. Unfortunately, these strata are the smallest spatial unit for which it is appropriate to generate kelp area estimates (WADNR 2017). Second, we summed kelp surface coverage in all strata between Neah Bay and Destruction Island to provide an OCNMS-wide estimate of kelp area (Fig. 1).
We estimated the temporal trend in kelp canopy coverage at each site and OCNMS wide by regressing the natural logarithm of kelp area against time. We also calculated the standard deviation (SD) and coefficient of variation (CV = SD/mean) of observations around each trend. Thus, our measures of CV represent variability after accounting for the overall trend in area. We performed this analysis on the entire time series (1989–2015), and separately for the two halves of the time series (1989–2001 and 2002–2015) to determine if trends shifted over time. As with sea otter data, to facilitate comparison among sites that vary substantially in kelp area, we constructed a log index of kelp area; we standardized the area of kelp by dividing the average kelp area observed during the first 3 years of the survey (1989–91), \(K_{{j,{\text{base}}}}\), for site j and taking the natural logarithm of the ratio: \({ \ln }\left( {\frac{{K_{j,t} }}{{K_{{j,{\text{base}}}} }}} \right)\).
Finally, we calculated two metrics of population synchrony among sites to determine if kelp canopy area was correlated between two halves of the time series. We first calculated a measure of synchrony, \(\phi\), among all sites for each time period (1989–2001 and 2002–2015; Loreau and de Mazancourt 2008). The parameter \(\phi\) ranges between 0 (indicating uncorrelated fluctuations) and 1 (indicating perfect synchrony) using the community.sync function in R package synchrony (Gouhier and Guichard 2014). In addition, we calculated the pairwise correlations among sites for each time period and regressed the correlation against the shoreline distance between the two sites. To weight the information from each site equally, we calculated pairwise correlations on the standardized time series (raw canopy area minus the among-year mean and divided by the among-year standard deviation) rather than the raw kelp canopy area time series. We then used non-linear least squares to estimate an exponential correlation as a function of distance: \(C\left( d \right) = { \exp }\left( { - \frac{d}{V}} \right)\), where C is the pairwise correlation, d is distance in km, and V is a decay parameter controlling the rate at which correlation declines with distance. We calculated a separate decay parameter for each time period to assess how the spatial scale of correlation may have changed between periods. Because Neah Bay and Chibahdehl Rocks have functionally identical kelp canopy time series, we dropped Chibahdehl Rocks from this analysis to avoid pseudo-replication. Dropping Neah Bay instead of Chibahdehl Rocks only minimally affected the results.
Invertebrate SCUBA surveys
We conducted SCUBA surveys between 3 and 7 August 2015 and gathered historical survey information collected by Kvitek and colleagues in 1987, 1995, and 1999 (Kvitek et al. 1989, 1998, 2000). During 2015, SCUBA divers surveyed benthic communities in kelp beds at each site (Fig. 1) at depths between 5 and 10 m, along visual transects (30 m × 2 m, n = 4 transects per site). On each transect, divers counted large invertebrates (> 5 cm diameter: sea urchins, sea stars, sea cucumbers, crabs, bivalves, etc.).
For the 1987, 1995, and 1999 subtidal surveys, we extracted summary statistics on benthic invertebrate densities from Kvitek and colleagues (1989, 1998, 2000). We include surveys that occurred at the same sites and comparable depths (5–10 m). All surveys use standard quadrat and transect sampling methods, though the sample sizes vary among years (Tables S1, S2). We converted data from all subtidal surveys into units of counts m−2. Not all sites were sampled in each year, and some taxonomic groups of interest were not identified in available reports (e.g., sea stars were not reported for 1995). We used all available data for each site and year. When necessary, we combined quadrat and transect data using a weighted average with weights corresponding to the area surveyed by each type. We include only species that are large and readily identifiable to avoid concerns about the detection of cryptic species (e.g., chitons; class Polyplacophora). We present results for six species groups that are common members of the Olympic coast invertebrate community: sea urchins (genera Mesocentrotus and Strongylocentrotus), sea cucumbers (genera Cucumaria and Parastichopus), crab (primarily genera Pugettia and Cancer), bivalves (primarily rock scallops, Crassadoma gigantea), and sea stars (including genera Pisaster, Orthasterias, Dermasterias, Henricia, and Pycnopodia). Consistent with the previous research, we identified sea urchins as the dominant invertebrate grazer in this system and contrast the trends in sea urchin abundance with the other invertebrate groups. Based on published sea otter diet information, we classified these groups into broad categories of diet preference. Observed diets of sea otters vary with the available prey field and individual otter diet preference, so we identified sea urchins and crabs as preferred prey, sea stars and sea cucumbers as frequent prey, and rock scallops as rare prey (Estes et al. 2003; Laidre and Jameson 2006; Tinker et al. 2008; Walker et al. 2008). Other important prey for sea otters are not observed during our surveys due to tidal range (e.g., intertidal mussels Mytilus spp.) or habitat requirement (e.g., soft sediment species like clams).
Statistical analyses
To ask if local changes in sea otter abundance resulted in subsequent changes in kelp area among the ten focal sites, we regressed the estimated population growth rate of sea otter abundance against the growth rate of kelp area. We performed this analysis for the entire time series (1989–2015) and separately for each half of the study period (1989–2001 and 2002–2015), using region and otter growth rate as fixed effects. In the model with two time periods, we allowed for a period × otter growth rate interaction to ask if the relationship between sea otters and kelp shifted between periods. We also included our measure of wave exposure as a potential covariate in all models to explain variation among sites. Available indices of other potential environmental covariates such as the PDO or CUI are broad-scale ocean indicators and do not vary among sites in this study. Therefore, we did not include these environmental covariates in the models, as they will not explain among-site variation. However, we do ask if PDO or CUI shift between time periods that could explain distinct, coast-wide patterns in sea otter or kelp dynamics.
To assess the effect of otter abundance on the temporal variability of kelp cover, we constructed a linear regression using CV of kelp area in 2002–2015 as the response variable and region, wave exposure, difference in otter abundance between periods, and CV of kelp area in the 1989–2001 period as predictors. We explored only additive main effects due to a sample size of 10 and selected among models using AIC corrected for small sample sizes (AICc).
To examine changes in the abundance of invertebrate groups over time, we used permutation-based multivariate analysis of variance (PERMANOVA) to compare community structure across three time periods (1987, 1999, and 2015) or three regions (northern, central, and southern) using the adonis function in R (R Core Team 2017). We exclude data from 1995, because sea star data were absent. The taxa-specific average densities (individuals m−2) for each site-year region were used as the dependent variables, and converted to dissimilarity matrices using Manhattan log(x + 1) distances. Manhattan dissimilarity treats joint absences of species as informative; the more commonly used Bray–Curtis dissimilarity excluded information about joint absences (Legendre et al. 2005; Anderson et al. 2006). We performed randomizations within strata based on regions or time periods. We also tested whether community composition was had higher variability among regions or among time periods by examining multivariate dispersion in community composition using the betadisper function in R. To visualize differences among time periods or regions in invertebrate community structure, we used non-metric multidimensional scaling (nMDS) based on the nmds function and related variation individual taxa to community dissimilarity using the envfit function. All multivariate analyses and visualizations were conducted in the R package vegan. We also calculated proportional declines in mean abundance and used paired t tests to evaluate their significance.
Results
Spatiotemporal trends of sea otters and kelp
Sea otter density trends have followed three spatially distinct patterns along the Olympic Coast since the 1970s (Fig. 2a–c). Local trends in sea otters differ substantially from the region-wide trend. Near the most northerly study sites, sea otter densities increased sharply from the mid-1980s until the early 1990s before declining slightly and then remaining stable from the mid-1990s to present (Fig. 2a). Sea otter densities in the central region experienced exponential growth from the late 1970s until the mid-1990s, but have remained largely stable at approximately 1990 densities (Fig. 2b). This represents a longer period of increasing otter densities than the northernmost region. The increase in sea otter density has been strongest and most consistent in the southern region of the study area (Fig. 2c) with near exponential increases since the late 1970s; since roughly 2000, the rate of increase in the Destruction Island area has outpaced rates near Teahwhit Head and Cape Johnson/Rock 305. At present, the absolute abundance of sea otters is also greatest in the southern region; sea otter abundances in the northern region are the lowest by at least an order of magnitude [e.g., estimated 2015 sea otter abundances of 18, 207, and 439 for Tatoosh Island (northern region), Cape Alava (central), and Destruction Island (southern), respectively]. Cape Johnson and Rock 305 have essentially the same trend in Fig. 2c due to their proximity relative to the kernel bandwidth used for home-range estimation (Fig. 1, see “Methods”).
Time series for sea otters (left column, a–c) kelp area (right column, d–f) at sites divided into three regions (rows) in OCNMS. In all panels, points and dashed lines show log indices for sea otters and kelp area for individual sites. Both kelp area and sea otters are indexed, such that the average values for each site the 1989–1991 period are 0. Solid lines represent the summed OCNMS-wide values for otters and kelp area, respectively, index to the average value from 1989 to 1991
Further analysis of sea otter data shows that the distribution of the Olympic Coast population has shifted over time (see also Jeffries and Jameson 2014). The population has had a bimodal or multimodal distribution for much of the study period, with the most significant modes in the area between Cape Alava and Cape Johnson, and another further south near Destruction Island (Fig. 3). The center of gravity of the population was in the vicinity of Teahwhit Head in the late 1970s, but then shifted north to the area around Cape Alava for much of the 1980s and the 1990s. Starting in the late 1990s, the center of gravity shifted south to near Destruction Island, where it has remained. In recent years, sea otter observations have been rare inside the Strait of Juan de Fuca (Fig. 3, above dashed line) but common at most points to Point Grenville in the south (Fig. 3).
Kernel smoothed sea otter distributions for sea otters on the Washington coast. Grey densities show the probability distribution for each year, dots show the annual center of gravity (median) of the distribution, and bold dashed line shows smoothed trend in the center of gravity from a loess smoother. Prominent geographic points and invertebrate survey locations noted on the y-axis. Dashed horizontal line indicates the entrance to the Strait of Juan de Fuca at Tatoosh Island (see Fig. 1)
Canopy kelp area exhibited spatiotemporally distinct patterns in the three regions of the study area from 1989 to 2015 (Fig. 2d–f). Kelp area showed substantial interannual variation both at the individual sites and the OCNMS-wide scale (Fig. 1). While the area of kelp in absolute terms varied substantially among sites within a region (Pfister et al. 2018), regional differences in kelp trends within the Olympic Coast were most distinct. At the furthest north sites, kelp area indices showed no clear long-term trends, but displayed notably higher interannual variability at Tatoosh Island than Neah Bay and Chibahdehl Rocks inside the Strait of Juan de Fuca (Fig. 2d; note that Neah Bay and Chibahdehl Rocks are in the same kelp stratum and thus share a single time series; Fig. 1). The central region also showed within-region differences among sites (Fig. 2e). Canopy area at Cape Alava increased from 1989 to 2000 before stabilizing and even declining in recent years. Point of the Arches and Anderson Point decreased in the early 1990s before following a qualitative pattern similar to Cape Alava. Canopy area at Cape Alava was far less variable than the other two central sites. At the southern sites, canopy area generally increased until the early 2000s before stabilizing or declining slightly (Fig. 2f); as with the central region (Fig. 2e), there were differences in short-term trends across the four southern sites early in the time series.
Connections between sea otters, invertebrates, and kelp
We detected differences in the kelp population growth rate between periods with a region-wide average growth rate of 0.056 for 1989–2001 and of − 0.037 for the 2002–2015 period (difference between periods p = 0.012; Fig. 4). There was no corresponding evidence of persistent, regime-like differences between the two time periods in large-scale oceanographic indices of temperature [PDO: among-year summer mean(sd) = 0.44(1.00) and − 0.04 (0.93) for 1989–2001 and 2002–2015, respectively; t test p = 0.2] and coastal upwelling [CUI: among-year summer mean(sd) = 17.1 (7.4) and 16.6 (6.3) for 1989–2001 and 2002–2015, respectively; t test p =0.8].
While the temporal difference in kelp population growth rate is intriguing, more interesting is the interaction between sea otter population growth rate and time period. Sea otter growth had a positive relationship with kelp during the earlier period (point estimate of slope = 0.285) but a negative relationship during the later period (point estimate of slope = − 0.507; interaction term p = 0.045). There was no support for regional variation in kelp population growth after accounting for other factors (p = 0.128). The model considering only a single time period found no effect of sea otters on kelp (p = 0.40), but did find differences in kelp population growth rate among regions (p = 0.024). This result shows how the temporal context can substantively alter the interpretation of mechanisms driving kelp population growth. Importantly, our analyses should not be taken to suggest a discrete break in 2001—using alternate breakpoints in the time series between 1999 and 2003 yield qualitatively similar results. Rather, dividing the time series is a way to summarize changes in a continuous time series (Fig. 2).
After accounting for kelp population growth rates, the variability in kelp area declined at most sites between the two periods (Fig. S2). Specifically, bootstrapped estimates of CV showed that variability at all sites, but one (Tatoosh Island) declined, though the magnitude of decline varied substantially by region. The three northern sites had virtually no change in CV (changes of less than ± 0.05 for all sites), the central region showed declines in CV but with variability among sites (declines of 0.033, 0.343, and 0.351, for Cape Alava, Anderson Point, and Point of the Arches, respectively), while the southern sites showed substantial declines in CV (declines of 0.175 to 0.694). For most sites, these are large and biologically significant changes in kelp variability. Linear models showed that including kelp CV in 1989–2001 and change in the number of otters best predicted the CV from 2002 to 2015 (\(\Delta {\text{AICc}} = 0,\) adj. R2 = 0.63). A positive coefficient for kelp CV in 1989–2001 indicates that sites with high CV during the first period also tended to have higher CV during the second period. Interestingly, a negative coefficient for the change in otters indicates that increased sea otter abundance was associated with reduced kelp CV in 2002–2015 (point estimates correspond to an increase of approximately 13 otters leading to a decrease of 0.01 in CV). However, a model with kelp CV from 1989 to 2001 alone had nearly equivalent support as the best model (\(\Delta {\text{AICc}} = 0.2,\) adj. R2 = 0.41), suggesting that the effect of sea otter abundance is decidedly less important than site-to-site variation. As we only have ten sites for comparison, our statistical power and precision of these estimates is low. Estimates of wave exposure were not a significant explanatory variable for any aspect of kelp CV.
Finally, kelp forests showed a decline in synchrony among sites from 1989–2001 to 2002–2015. The measure of overall synchrony among sites, \(\phi\), declined dramatically from 0.72 (0.02) to 0.44 (0.34) between periods (jackknife standard deviation in parentheses; a \(\phi\) of 1 indicates perfect synchrony). This decline in synchrony is also evident in the spatial correlation among sites with the spatial correlation declining over notably shorter spatial scales in the 2002–2015 time period [Fig. 5; V = 111.93 (14.02) and 32.38 (5.10), for 1989–2001 and 2002–2015, respectively; mean (SE)]. Together, these results show that kelp coverage at our sites is less synchronous during the period of high sea otter abundance than low sea otter abundance.
The observed spatiotemporal patterns of benthic invertebrate abundance were starkly different from the patterns of sea otters and kelp. We found substantial declines in all five major taxonomic groups from 1987 to 2015 (Fig. 6). Sea urchins declined precipitously with the across-site mean density falling by more than 99% between 1987 and 2015 (from 3.7 to 0.01 m−2). While the other five species groups did not decline as dramatically as urchins, they all showed substantial declines from 1987 to 2015: bivalves (decline of 90%), sea cucumbers (86%), crabs (84%), and sea stars (70%). All of these declines were significant (paired t tests, p < 0.01 for all species groups). Only sea urchins showed a pattern in which the highest density occurred in the three sites that Kvitek et al. (1989) defined as outside of the range of sea otters (Neah Bay, Anderson Point, Point of the Arches; Fig. 7a). For the four other species groups, densities were not notably different between sites inside and outside of the otter range in 1987. Beyond declines in mean densities, all five species also show notable declines in the among-site variation in density; the among-site standard deviation fell by 75 to 99% for the species groups examined (Table 1).
Time series of densities of benthic invertebrates. Species are ordered roughly by otter prey diet frequency from common (top row) to rare (bottom row). Graphs show site means and SE. Points have been jittered slightly along x-axis to reduce overlap. Red filled points show sites designated by Kvitek et al. (1989) as outside the primary otter range in 1987. By 1995, all sites were within the otter range. Note that data on seastars are not available for 1995
Multivariate metrics of community composition showed significant variation in the benthic invertebrate community over years but not across regions (Fig. 7; Table 2). Not only was there a shift in mean community composition between the first survey (1987) and later survey years (1999, 2015), but community composition among sites became less variable after 1987 (Fig. 7). By all measures, the spatial variability in invertebrate densities has declined over the past 30 years.
Discussion
Sea otters are iconic keystone predators in coastal ecosystems of the northeastern Pacific, and their presence radically affects invertebrate and algal communities (Estes and Duggins 1995; Steneck et al. 2002; Watson and Estes 2011). Here, we revisit historical invertebrate surveys and complement these surveys with independent spatiotemporal data on kelp and sea otters in Washington’s OCNMS and new invertebrate surveys. Our analyses reveal a strong correlation between sea otter and kelp population growth at the local scale during the rapid expansion of sea otter populations. However, there is a temporal component to these associations: the relationship between kelp and sea otter growth rates shifted from positive during the 1990s to neutral or slightly negative post-2000 (Fig. 4). Together, our analyses demonstrate that regional trends in sea otter, kelp, and benthic invertebrate abundance are not necessarily evident at a finer spatial grain. They suggest that while a sea otter-derived trophic cascade initially drove changes in the nearshore community along the Washington coast, additional factors in more recent years may account for fundamental shifts in invertebrate community dynamics. In addition, contrary to predictions from trophic cascade theory, kelp and sea otter abundance are statistically decoupled when viewed at a regional scale and over the entirety of the 30-year period. The decoupling of otter and kelp abundance in more recent years, and the disconnect between regional and local-scale patterns, provides insight into theoretical expectations for short- vs long-term dynamics following the reintroduction of predators more generally (Sergio et al. 2014; Stier et al. 2016), and sparks intriguing hypotheses about the influence of top–down vs bottom–up forcing in temperate coastal habitats.
Trophic cascade theory predicts that increased sea otter abundance should reduce the abundance of their prey, including functionally important invertebrate grazers such as sea urchins. In turn, reduced grazer abundance should release kelp and other algae from top–down control and lead to increases in their abundance. Our results echo those of Kvitek et al. (1989, 1998, 2000) and demonstrate large, immediate, and persistent effects of sea otter expansion on the main invertebrate kelp grazer and a preferred prey, sea urchins. We also show that increases in otter abundance were correlated with declines for a broad suite of invertebrate species and multi-year increases in kelp area. These reductions in invertebrate abundances suggest that the consequences of sea otter populations for kelp forest community composition are not exclusively an immediate shift in state, but can manifest gradually over the span of decades (Watson and Estes 2011; Kenner et al. 2013). Furthermore, because invertebrate densities remain far below historical levels, the observed declines in kelp population growth rates (Fig. 4) and total area (Fig. 1) in OCNMS since 2000 are unlikely to be caused by increased invertebrate grazing pressure. Though the patterns we observed are consistent with the idea that invertebrate grazers can control kelp abundance when kelp is rare, but not when it is common (Ling et al. 2015), we do not have data to assess shifts over time in per capita interaction strengths between grazers and kelp. However, there is evidence from many other kelp forest systems that suggest urchin barrens and dense kelp forests form resilient, alternate stable states (Filbee-Dexter and Scheibling 2014; Ling et al. 2015). Concordantly, we suggest that forces acting at relatively small spatial scales and unrelated to otter or grazer abundance drive kelp abundance and community structure once the effects of sea otters have cascaded through the community.
A promising hypothesis for the decoupling of kelp and otter growth rates after 2001 is an increase in the influence of abiotic factors. Oceanographic dynamics in the late 1990s and the early 2000s in the Northeast Pacific have been the subject of intense study, because they were characterized by major El Niño and La Niña events (PDO; Mantua et al. 1997; Mantua and Hare 2002). However, we find no evidence for systematic differences in either the PDO or CUI over the course of the two time periods explored here; both study periods include periods of high and low values for both PDO and CUI, and on average, the two periods do not differ. Furthermore, in recent years, overall synchrony in kelp canopy area declined as did spatial correlation among sites in kelp canopy area. As an increased importance of shared oceanographic forces would be expected to lead to increased synchronization among sites, not a decline, we find no strong evidence for increased importance of oceanographic forces on kelp dynamics in the absence of grazing invertebrates. It remains possible that kelp dynamics have become more strongly influenced by sea-surface temperature, upwelling, nutrient availability, and other bottom–up forces over time (Pfister et al. 2018), but such drivers must be acting primarily at a local rather than a regional scale. Validating that hypothesis would require new, local-scale models of abiotic forcing and variability.
At the local scale, we expected that the variability in kelp area would be strongly related to wave exposure at a given site (Reed et al. 2011; Bell et al. 2015). However, while kelp CV varied substantially among sites, it was unrelated to calculated wave exposure values (Fig. S1). Surprisingly, Neah Bay, a site largely protected from wave exposure, has had nearly equivalent kelp CVs to five sites on the outer coast since 2002, including Cape Alava and Destruction Island (Fig. S2; detrended CV of approximately 0.2). We speculate that this may be driven predominantly by the fact that we only have information about kelp area during the summer when seasonal wave energy on the coast is relatively low. An alternative prediction is that kelp CV in Neah Bay would decline if sea otters became established in Neah Bay, as increases in otters are associated with declines in kelp CV. We cannot exclude the possibility of an effect of the sea urchin fishery on kelp variability at Neah Bay, but the fishery has been closed since 1997 and cannot be considered a strong driver of the ecosystem in recent years. While we cannot definitively identify what caused the decoupling of otter and kelp dynamics in OCNMS, shifts in factors controlling alternate states within ecological communities are not without precedent in other systems (Bellwood et al. 2006; Petraitis et al. 2009). Our study adds richness and complexity to the classic trophic cascade explanation for the dynamics of kelp forest communities.
We know of no other study that has examined the interaction between otter abundance and kelp variability, but suggest that this is a particularly interesting avenue of future research due to the connection between canopy kelps, benthic community structure (Arkema et al. 2009), and various ecosystem services (Wilmers et al. 2012; Pinsky and Fogarty 2012). Indeed, incorporating the effects of keystone species and ecosystem engineers in marine planning seems especially wise when ecological endpoints include conservation and restoration of biogenic habitats such as kelp forests. For benthic invertebrates, both our multivariate and univariate analyses show that invertebrate communities cluster by year rather than spatial region, suggesting that the primary driver of communities is a temporal rather than a spatial process (Figs. 6, 7). Thus, both kelp and invertebrates show evidence of homogenization in concert with the expansion of sea otters, which aligns with the previous suggestions that sea urchin-dominated habitats may show more variability than sea otter controlled habitats (Watson and Estes 2011). We acknowledge many potential consequences for other groups including understory algae (Watson and Estes 2011) and fish (Markel and Shurin 2015) that we cannot explore here.
Our data do not allow us to examine year-to-year changes in the linkages between sea otters, benthic invertebrates, and kelp, opening the possibility that invertebrate communities substantially shifted during the years between surveys in a way that can explain kelp variation. While there is ample evidence that other factors have affected the abundance of some invertebrate groups (e.g., sea star wasting disease outbreak in 2013–4; Eisenlord et al. 2016), personal observations of one authors (AOS) between 2004 and 2008 at two sites—Tatoosh Island and Point of the Arches—do not support radical changes in invertebrate abundances during the 1999–2015 gap in our invertebrate time series. We cannot exclude the possibility of variability in invertebrate communities driving the patterns, but we suggest that it is an unlikely cause.
Looking to the future, sea otter numbers appear to have stabilized in much of the northern and central regions of the OCNMS (Fig. 2) and may be at or near carrying capacity in these regions. Invertebrate densities in these regions are very low, which begs the question of how sea otter populations are maintained with very low prey abundances. Rocky subtidal and kelp forest habitats support higher densities of sea otters in Washington than either sandy bottom or estuarine habitats (Laidre et al. 2001, 2002), suggesting that sea otters are not likely to simply be foraging in nearby sandy habitats, but they may be capturing invertebrates in deeper or shallower rocky habitats that were not included in our surveys or historical surveys. Furthermore, we note that the abundance of sea otters in 2015 (n > 1400) is substantially above published estimates of carrying capacity for equivalent region of the Washington outer coast (922–1189; Laidre et al. 2002), suggesting that either the carrying capacity of otters needs to be revisited or, if carrying capacity estimates are correct, the population is predicted to decline in the coming years.
In conclusion, place-based management of resources and ecosystem services is of great importance in coastal regions, and informed, holistic management requires accounting for the dynamics of keystone species and major biogenic habitats. Along the Olympic Coast, place-based management is a priority at both the state level—for example, the recently drafted Washington state marine spatial plan (http://www.msp.wa.gov/wp-content/uploads/2017/draft_MSP_and_appendices.pdf)—and the federal level, as practiced by OCNMS (Office of National Marine Sanctuaries 2008) and the adjacent Olympic National Park (National Park Service 2008). The reestablishment of a healthy sea otter population in this region has already yielded considerable ecosystem change, through trophic cascade dynamics that have enabled kelp canopy habitats to expand. However, our research shows that kelp canopy dynamics are now being influenced predominantly by drivers other than otter abundance and such variation now occurs at smaller spatial scale. This apparent decoupling poses a challenge to spatial management of marine resources in the area, because the new prevalent mechanisms must be identified to anticipate further change and understand how management actions interact with natural variation. A second challenge may be in revising management objectives for sea otters, which have been prioritized as keystone species that have major impacts on ecosystem structure and functioning, biodiversity, and other attributes (Watson and Estes 2011; Wilmers et al. 2012). Our work suggests that their keystone effect on kelp forests either diminished over time or is obscured by environmental factors. The current ecological dynamics of sea otters at sites along the Olympic Coast need further study to determine how their tremendous predatory demands are impacting other habitats and potentially introducing new management tradeoffs in habitats beyond the shallow kelp forest studied here.
References
Anderson MJ, Ellingsen KE, McArdle BH (2006) Multivariate dispersion as a measure of beta diversity. Ecol Lett 9:683–693
Arkema KK, Reed DC, Schroeter SC (2009) Direct and indirect effects of giant kelp determine benthic community structure and dynamics. Ecology 90:3126–3137
Bakun, A. 1973. Coastal upwelling indices, west coast of North America, 1946–71. U.S. Department of Commerce, NOAA Technical Report NMFS–SSRF–671. Accessed at https://www.pfeg.noaa.gov/products/PFEL/modeled/indices/upwelling/NA/data_download.html on July 5, 2018
Bell TW, Cavanaugh KC, Reed DC, Siegel DA (2015) Geographical variability in the controls of giant kelp biomass dynamics. J Biogeogr 42:2010–2021
Bellwood DR, Hughes TP, Hoey AS (2006) Sleeping functional group drives coral-reef recovery. Curr Biol 16:2434–2439
Carter SK, VanBlaricom GR, Allen BL (2007) Testing the generality of the trophic cascade paradigm for sea otters: a case study with kelp forests in northern Washington, USA. Hydrobiologia 579:233–249
Duggins DO (1988) The effects of kelp forests on nearshore environments biomass, detritus and altered flow. In: Van Blaricom G, Estes J (eds) The community ecology of sea otters. Springer, Berlin
Ebeling A, Laur D (1988) Fish populations in kelp forests without sea otters: effects of severe storm damage and destructive urchin grazing. In: VanBlaricom G, Estes J (eds) The community ecology of sea otters. Springer, Berlin, pp 169–191
Eisenlord ME, Groner ML, Yoshioka RM, Elliott J, Maynard J, Fradkin S, Turner M, Pyne K, Rivlin N, van Hooidonk R, Harvell CD (2016) Ochre star mortality during the 2014 wasting disease epizootic: role of population size structure and temperature. Philos Trans R Soc B 371:20150212
Estes JA, Duggins DO (1995) Sea otters and kelp forests in Alaska: generality and variation in a community ecological paradigm. Ecol Monogr 65:75–100
Estes JA, Palmisano JF (1974) Sea otters: their role in structuring nearshore communities. Science 185:1058–1060
Estes JA, Riedman ML, Staedler MM, Tinker MT, Lyon BE (2003) Individual variation in prey selection by sea otters: patterns, causes and implications. J Anim Ecol 72(1):144–155. https://doi.org/10.1046/j.1365-2656.2003.00690.x
Filbee-Dexter K, Scheibling RE (2014) Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Mar Ecol Prog Ser 495:1–25
Foster MS (1990) Organization of macroalgal assemblages in the Northeast Pacific—the assumption of homogeneity and the illusion of generality. Hydrobiologia 192:21–33
Gouhier TC, Guichard F (2014) Synchrony: quantifying variability in space and time. Methods Ecol Evol 5:524–533
Grothe PR, Taylor LA, Eakins BW, Carignan KS, Warnken RR, Lim E, Caldwell RJ (2010) Digital elevation model of Taholah, Washington: procedures, data sources and analysis. NOAA Tech, Boulder
Hughes BB, Eby R, Van Dyke E, Tinker MT, Marks CI, Johnson KS, Wasson K (2013) Recovery of a top predator mediates negative eutrophic effects on seagrass. Proc Natl Acad Sci USA 110:15313–15318
Jameson RJ (1993) Survey of a translocated sea otter population. IUCN Otter Special Group Bull 8:2–4
Jameson RJ, Jeffries S (1999) Results of the 1999 survey of the reintroduced sea otter population in Washington State. IUCN Otter Special Group Bull 16:79–85
Jameson RJ, Kenyon KW, Johnson AM, Wight HW (1982) History and status of translocated sea otter populations in North America. Wildlife Soc B 10:100–107
Jeffries S, Jameson R (2014) Results of the 2013 survey of the reintroduced sea otter population in Washington State. Washington Department of Fish and Wildlife
Kenner MC, Estes JA, Tinker MT, Bodkin JL, Cowen RK, Harrold C, Hatfield BB, Novak M, Rassweiler A, Reed DC (2013) A multi-decade time series of kelp forest community structure at San Nicolas Island, California (USA). Ecol Lett 94:2654–2655
Knowlton N (2004) Multiple “stable” states and the conservation of marine ecosystems. Prog Oceanogr 60:387–396
Kvitek RG, Shull D, Canestro D, Bowlby EC, Troutman BL (1989) Sea otters and benthic prey communities in Washington State. Mar Mammal Sci 5:266–280
Kvitek RG, Iampietro PJ, Thomas K (1998) Sea otters and benthic prey communities: a direct test of the sea otter as keystone predator in Washington state. Mar Mammal Sci 14:895–902
Kvitek RG, Iampietro PJ, Thomas K (2000) Quantitative assessment of sea otter benthic prey communities within the Olympic Coast National Marine Sanctuary: 1999 re-survey of 1995 and 1985 monitoring stations. Final report to the Olympic Coast National Marine Sanctuary
Lafferty KD (2004) Fishing for lobsters indirectly increases epidemics in sea urchins. Ecol Appl 14:1566–1573
Laidre KL, Jameson RJ (2006) Foraging patterns and prey selection in an increasing and expanding sea otter population. J Mammal 87:799–807
Laidre KL, Jameson RJ, Demaster DP (2001) An estimation of carrying capacity for sea otters along the California coast. Mar Mammal Sci 17:294–309
Laidre KL, Jameson RJ, Jeffries SJ, Hobbs RC, Bowlby CE, VanBlaricom GR (2002) Estimates of carrying capacity for sea otters in Washington state. Wildlife Soc B 30:1172–1181
Laidre KL, Jameson RJ, Gurarie E, Jeffries SJ, Allen H (2009) Spatial habitat use patterns of sea otters in coastal Washington. J Mammal 90:906–917
Lance MM, Richardson SA, Allen HL (2004) Washington state recovery plan for the sea otter. Washington Department of Fish and Wildlife, Olympia
Legendre P, Borcard D, Peres-Neto PR (2005) analyzing beta diversity: partitioning the spatial variation of community composition data. Ecol Monogr 75:435–450
Ling SD, Scheibling E, Rassweiler A, Johnson CR, Shears N, Connell SD, Salomon AK, Norderhaug KM, Perez-Matus A, Hernandez JC, Clemente S, Blamey LK, Hereu B, Ballesteros E, Sala E, Garrabou J, Cebrian E, Zabala M, Fujita D, Johnson LE (2015) Global regime shift dynamics of catastrophic sea urchin overgrazing. Phil Trans R Soc B 370:20130269. https://doi.org/10.1098/rstb.2013.0269
Loreau M, de Mazancourt C (2008) Species synchrony and its drivers: neutral and nonneutral community dynamics in fluctuating environments. Am Nat 172:E48–E66
Mann KH (1973) Seaweeds: their productivity and strategy for growth: The role of large marine algae in coastal productivity is far more important than has been suspected. Science 182:975–981
Mantua NJ, Hare SR (2002) The Pacific decadal oscillation. J Oceanogr 58:35–44
Mantua N, Hare S, Zhang Y, Wallace J, Francis R (1997) A Pacific interdecadal climate oscillation with impacts on salmon production. Bull Am Meteorol Soc 78:1069–1079
Markel RW, Shurin JB (2015) Indirect effects of sea otters on rockfish (Sebastes spp.) in giant kelp forests. Ecology 96:2877–2890
National Park Service (2008) Final general management plan and environmental impact statement. Olympic National Park, Washington, D.C.
Office of National Marine Sanctuaries (2008) Olympic Coast National Marine Sanctuary condition report 2008. US Department of Commerce, National Oceanic and Atmospheric Administration, Office of National Marine Sanctuaries, Silver Spring
Paine RT (1969) A note on trophic complexity and community stability. Am Nat 103:91–93
Petraitis PS, Methratta ET, Rhile EC, Vidargas NA, Dudgeon SR (2009) Experimental confirmation of multiple community states in a marine ecosystem. Oecologia 161:139–148
Pfister CA, Berry HD, Mumford T (2018) The dynamics of kelp forests in the Northeast Pacific Ocean and the relationship with environmental drivers. J Ecol. https://doi.org/10.1111/1365-2745.12908
Pinsky ML, Fogarty M (2012) Lagged social-ecological responses to climate and range shifts in fisheries. Clim Change 115:883–891
Pinsky ML, Guannel G, Arkema KK (2013) Quantifying wave attenuation to inform coastal habitat conservation. Ecosphere 4(8):95. https://doi.org/10.1890/ES13-00080.1
Power ME, Tilman D, Estes JA, Menge BA, Bond WJ, Mills LS, Daily G, Castilla JC, Lubchenco J, Paine RT (1996) Challenges in the quest for keystones. Bioscience 46:609–620
R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reed DC, Rassweiler A, Carr MH, Cavanaugh KC, Malone DP, Siegel DA (2011) Wave disturbance overwhelms top-down and bottom-up control of primary production in California kelp forests. Ecology 92:2108–2116
Reisewitz SE, Estes JA, Simenstad CA (2006) Indirect food web interactions: sea otters and kelp forest fishes in the Aleutian archipelago. Oecologia 146:623–631
Sergio F, Schmitz OJ, Krebs CJ, Holt RD, Heithaus MR, Wirsing AJ, Ripple WJ, Ritchie E, Ainley D, Oro D, Jhala Y, Hiraldo F, Korpimäki E (2014) Towards a cohesive, holistic view of top predation: a definition, synthesis and perspective. Oikos 123:1234–1243
Shelton AO, Francis T, Feist BE, Williams GE, Lindquist A, Levin P (2017) Forty years of seagrass population stability and resilience in an urbanizing estuary. J Ecol 105:458–470
Sherman K, Duda AM (1999) An ecosystem approach to global assessment and management of coastal waters. Mar Ecol Prog Ser 190:271–287
Steneck R, Graham M, Bourque B, Corbett D, Erlandson J, Estes J, Tegner M (2002) Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ Conserv 29:436–459
Stier AC, Samhouri JF, Novak M, Marshall KN, Ward EJ, Holt RD, Levin PS (2016) Ecosystem context and historical contingency in apex predator recoveries. Sci Adv 2:e1501769
Taylor LA, Eakins BW, Carignan KS, Warnken RR, Sazonova T, Schoolcraft DC (2008) Digital Elevation Model of La Push, Washington: procedures, data sources and analysis. NOAA Tech, Boulder
Tinker MT, Bentall G, Estes JA (2008) Food limitation leads to behavioral diversification and dietary specialization in sea otters. Proc Nat Acad Sci 105(2):560–565. https://doi.org/10.1073/pnas.0709263105
Van Wagenen RF (2015) Washington Coastal kelp resources—port townsend to the Columbia River, summer 2014. Washington Department of Natural Resources, Olympia
WADNR (2017) “Kelp monitoring—Olympic Peninsula” Washington State Department of Natural Resources, Olympia, WA. http://data-wadnr.opendata.arcgis.com/datasets/kelp-monitoring-olympic-peninsula Accessed: 1 Sept 2017
Walker KA, Davis JW, Duffield DA (2008) Activity budgets and prey consumption of Sea Otters (Enhydra lutris kenyoni) in Washington. Aquatic Mammals 34:393–401
Watson J, Estes JA (2011) Stability, resilience, and phase shifts in rocky subtidal communities along the west coast of Vancouver Island, Canada. Ecol Monogr 81:215–239
Wilmers CC, Estes JA, Edwards M, Laidre KL, Konar B (2012) Do trophic cascades affect the storage and flux of atmospheric carbon? An analysis of sea otters and kelp forests. Front Ecol Environ 10:409–415
Acknowledgements
We thank H Jackson and G Galasso for piloting the research vessels during field work, and the United States Coast Guard station at Neah Bay for providing docking space. We thank Washington Department of Fish and Wildlife for their excellent sea otter surveys. C. Pfister provided thoughtful discussions, field assistance, and comments on the manuscript. J. Hale, K. Laidre, and two anonymous reviewers provided comments that improved the manuscript. Order of authorship was determined in part by efficiency of SCUBA-based navigation of Tatoosh Island while dodging menacing Steller sea lions. This study was supported by funding from the National Marine Fisheries Service, the Office of National Marine Sanctuaries, and the NOAA Integrated Ecosystem Assessment program.
Author information
Authors and Affiliations
Contributions
AOS, CJH, JFS, KSA, BEF, KEF, NT, and GDW designed the surveys and performed field work. AOS, CJH, and JFS analyzed the data. AOS, CJH, and JFS wrote the manuscript; other authors provided editorial advice. BEF created Fig. 1.
Corresponding author
Additional information
Communicated by Jonathan Shurin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shelton, A.O., Harvey, C.J., Samhouri, J.F. et al. From the predictable to the unexpected: kelp forest and benthic invertebrate community dynamics following decades of sea otter expansion. Oecologia 188, 1105–1119 (2018). https://doi.org/10.1007/s00442-018-4263-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-018-4263-7