Abstract
Although produced by meristems that are continuous along the stem length, marked differences in bark morphology and in microenvironment would suggest that main stem and twig bark might differ ecologically. Here, we examined: (1) how closely associated main stem and twig bark traits were, (2) how these associations varied across sites, and (3) used these associations to infer functional and ecological differences between twig and main stem bark. We measured density, water content, photosynthesis presence/absence, total, outer, inner, and relative thicknesses of main stem and twig bark from 85 species of angiosperms from six sites of contrasting precipitation, temperature, and fire regimes. Density and water content did not differ between main stems and twigs across species and sites. Species with thicker twig bark had disproportionately thicker main stem bark in most sites, but the slope and degree of association varied. Disproportionately thicker main stem bark for a given twig bark thickness in most fire-prone sites suggested stem protection near the ground. The savanna had the opposite trend, suggesting that selection also favors twig protection in these fire-prone habitats. A weak main stem-twig bark thickness association was observed in non fire-prone sites. The near-ubiquity of photosynthesis in twigs highlighted its likely ecological importance; variation in this activity was predicted by outer bark thickness in main stems. It seems that the ecology of twig bark can be generalized to main stem bark, but not for functions depending on the amount of bark, such as protection, storage, or photosynthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bark, the region including all tissues from the cambium to the outside of the stem (Romero 2013), performs multiple functions and represents an often massive C investment for plants. Although the meristems that produce bark are continuous from the stem tip to the base (Roth 1981), marked morphological differences can be observed between the bark of main stems and twigs on the same plant (Fig. 1). It is not clear to what extent divergent morphologies might reflect functional and ecological differences in twig and main stem bark. In general, all barks protect stems from herbivores, pathogens, fire, and desiccation (Dantas and Pausas 2013; Lawes et al. 2011; Romero et al. 2009), store water, starch, and other compounds (Scholz et al. 2007; Srivastava 1964), provide stem mechanical support (Niklas 1999), and can also photosynthesize (Pfanz et al. 2002). But given that twigs and main stems are subject to different microenvironments, e.g., temperature, exposure to light, fire regime, etc. (Patiño et al. 2009), differences in bark functional traits might be expected between twigs and main stems. Here, we compared bark traits in main stems and twigs across species from six ecologically contrasting sites to infer functional and ecological differences in the bark of these two maximally distant above ground levels of a plant.
Structure and diversity of bark. a Cross-section of bark showing its inner, mostly living, and outer, mostly dead, portions. Inner bark is composed of the secondary phloem, the photosynthate-translocating tissue, and the cortex, a primary and mostly parenchymatic tissue. In turn, outer bark can include a single periderm or a collection of periderms known collectively as “rhytidome.” A periderm is made up of three layers: the phelloderm, a usually thin layer of living tissue; the phellogen, also known as “cork cambium;” and the phellem, a layer of dead cells of varying thickness. Contrasting twig bark of b Exocarpus cupressiformis and c Eucalyptus tenuiramis. Main stem bark diversity in our sampling included d the thick, red, papery bark of Persoonia linearis, e the thick and fire-resistant corky bark of Alstonia actinophylla, f the smooth, photosynthetic and water-storing bark with papery phellem of Bursera instabilis, g the thick and hard bark of Exocarpus cupressiformis (compare with twig bark in b), and h the peeling bark with a basal “sock” of Eucalyptus tenuiramis (compare with twig bark in c). Scale b–c = 1 cm, d–h = 10 cm
To infer ecological differences in the bark of main stems and twigs, we examined how closely bark traits between these two levels were associated with one another. High degrees of association would imply functional similarity between main stem and twig bark, or at least that twig bark trait values can be used to extrapolate those of main stem bark. On the contrary, low associations or unrelated bark traits would suggest that main stem and twig bark traits diverge, likely because of different functional needs and thus different ecological contexts. In this case, twig bark trait values could not be used to extrapolate main stem bark ones. This rationale was applied to bark density, a trait linked with protection and mechanics (Niklas 1999; Romero et al. 2009; Rosell et al. 2014), and water content, a trait reflecting storage (Domec and Gartner 2002). Bark anatomy changes markedly with ontogeny [e.g., thickening of cell walls, accumulation of crushed phloem (Roth 1981)], so bark density would be expected to be associated in a proportional and positive way across species but higher in main stems than in twigs. If these ontogenetic changes affecting main stem bark structure are too extreme, main stem and twig bark density and water content could be uncoupled, reflecting strong ecological divergence. Comparisons were based on bark from 85 species of angiosperms from a broad phylogenetic range and from six contrasting sites, including tropical rain and dry forests, temperate woodlands, a xerophytic scrub, and a savanna (Table 1). Comparisons were performed on raw data and also taking into account phylogenetic relationships between species.
In addition to density and water content, we examined total bark thickness, by far the best studied trait in bark ecological studies (Paine et al. 2010). Thickness has a key role in fire protection, mechanical support, and water storage (Lawes et al. 2011; Midgley et al. 2010; Niklas 1999; Rosell et al. 2014; Scholz et al. 2007). Despite its functional significance, the patterns and causes of variation in bark thickness are unclear (Paine et al. 2010). Within individuals, main stems must have thicker bark than twigs because of a longer history of bark accumulation (Hoffmann and Solbrig 2003; Pinard et al. 1999; Poorter et al. 2014; Uhl and Kauffman 1990). However, it is unclear whether main stem bark thickness is proportional to that in twigs, disproportional but still associated, or uncoupled. Disproportionately thicker bark in main stems could reflect the need for higher protection, for example in fire-prone environments, where heat tends to be most intense near the ground (Pausas 2014). Protection from other agents may also be more important in main stems than in twigs given that trunk damage would cause greater growth setback than twig damage (Butler et al. 2012). We compared main stem and twig absolute bark thickness, and also relative thickness standardizing by stem size. The many functional roles of bark thickness make it a crucial trait in understanding whether bark differs functionally and ecologically along stems.
Most studies of the ecological significance of bark thickness have focused on total thickness (but see Graves et al. 2014; Rosell et al. 2014). However, bark has an inner living portion, and an outer portion composed of dead cells (Evert and Eichhorn 2006; Fig. 1a). Derived from different lateral meristems, these regions vary widely in their total and relative amounts across species (Roth 1981). Outer bark seems associated with protection against fire (Graves et al. 2014), and mechanical support (Romero 2013), whereas storage is likely dependent on the amount of inner, living bark (Rosell and Olson 2014). For this reason, variation in the relative amount of the two regions likely results in trade-offs between bark functions (Rosell et al. 2014). Observing that twigs and main stems differ in their relative amounts of inner and outer bark would suggest that certain functions are emphasized at the expense of others in different parts of the plant. These differences could provide information regarding ecological divergence that would remain hidden by focusing on total thickness only.
Finally, the presence of photosynthetic bark in main stems and twigs was compared. Recycling the CO2 from stem respiration (Teskey et al. 2008), bark photosynthesis has been found to contribute up to 11 % of the C in twig wood (Cernusak and Hutley 2011), and as much as 50 % of plant C gain during periods of water stress (Franco-Vizcaíno et al. 1990). Stem photosynthesis is limited by thickening of the outer bark, which acts as a light barrier (Pfanz et al. 2002; Saveyn et al. 2010). Therefore, photosynthesis would be expected to be more common in twigs, in which outer bark is thinner than in main stems. In addition to this main stem-twig comparison, we examined the association between photosynthetic bark and other functional traits. For example, because of its presumably greater metabolic activity, photosynthetic bark might require higher water content in its living tissues than non-photosynthetic bark. The ability of these traits to predict photosynthetic activity in main trunks was also tested. Exploring the traits associated with photosynthesis will contribute to understanding the ecology and physiology of this widespread bark role (Pfanz 2008).
Examining bark functional traits in a wide range of species and environments, we addressed the following questions:
-
1.
Are functional traits in twig and main stem bark associated across species?
-
2.
How do these main stem-twig bark trait associations change across sites?
-
3.
What do main stem-twig bark trait associations tell us about bark functional differences, and thus ecological divergence, between these extreme portions of stems?
We show that although bark density and water content are statistically indistinguishable between main stems and twigs across species, the main stem-twig bark thickness association varies notably with site, suggesting responses to varying environmental selective pressures.
Materials and methods
Localities and sampling
We collected 85 species from six localities in Australia and Mexico, providing a very wide range of precipitation, temperature, and fire regimes (Table 1). Sampling also covered an ample phylogenetic span, including 40 families and 20 orders of angiosperms (Electronic Supplementary Material, Table S1, Fig. S1), and a wide range of bark morphologies (Fig. 1b–h). We sampled bark from the bases of main trunks, and from sun-exposed twigs 1 m from the tip. We sampled five individuals per species, except in the rainforest, where three replicates were collected. We chose the largest individuals, which were assumed to represent the adult stage. Collections within species were quite homogeneous with 75 % of the sampled species having 15 cm or less variation in main stem diameter between individuals. Bark from twigs had mature characteristics (i.e., had outer bark) for virtually all species.
Bark density and water content
We measured density (g cm−3) as dry weight/fresh volume for the total bark and also the inner living bark alone. We also calculated water content (%) for total and inner bark as (fresh weight–dry weight)/dry weight. For total bark measurements of main stems, we cut blocks approximately 1 cm high and wide, with variable depth according to bark thickness. For inner bark measurements, we cut a replicate block and removed the outer bark. For total bark measurements of twigs, blocks of total bark were 1 cm high, 0.5 cm wide, with depth equal to bark thickness. Additional blocks were sampled and the outer bark removed for inner bark measurements. We used the water displacement method with an analytical balance to measure fresh volume and dried samples at 100 °C for 4 days to measure dry weight (Williamson and Wiemann 2010).
Thickness of total, outer, and inner bark
We measured the thicknesses of total, inner, and outer bark on main stems and twigs. We defined outer bark as the portion made up of phellem or rhytidome, and inner bark as the living portion made up of secondary phloem, cortex, and phelloderm (Fig. 1a). For main stems, we measured these thicknesses using digital calipers at the point of maximum total thickness. We used tissue aspect, color, and moisture in cross-section to divide inner from outer bark, with the aid of a hand lens when needed. For twigs, we measured total bark thickness directly on samples, and outer and inner bark thickness on thin sections using light microscopy. One twig sample per species was processed anatomically for this purpose. We fixed samples upon collection in 70 % aqueous ethanol, and processed them for light microscopy following Carlquist (1982), staining sections with safranin and alcian blue (Ruzin 1999). We photographed sections and measured outer and inner bark thickness at three different points per sample using ImageJ (Schneider et al. 2012). Sections were not available for six species (see Table S1). We calculated relative main stem bark thickness (%) as bark thickness/main stem radius × 100. The same formula was applied for relative bark thickness in twigs.
Photosynthetic activity in bark
We assessed the presence of photosynthetic bark in main stems and twigs by scraping off the rhytidome or phellem to uncover a green phelloderm (Fig. 1a). We tabulated the presence of photosynthetic bark in twigs and main stems to examine to what degree photosynthetic twigs predicted photosynthetic main stems. We tested for differences in traits between photosynthetic and non-photosynthetic bark using t or Wilcox tests. We used the traits that differed significantly (i.e., total bark water content and total and outer bark thickness; Table S2) to predict the presence of bark photosynthesis in main stems fitting a logistic regression (Rosell et al. 2007). In preliminary logistic models, we tested the significance of these predictors through Wald tests, and evaluated their contribution to the model comparing models with and without each predictor through likelihood ratio tests (Kleinbaum and Klein 2010). The final logistic model for main stems included outer bark thickness as the only significant predictor (see “Results”). We examined the global fit of this final model through the Akaike information criterion (AIC) and the Hosmer and Lemeshow test as implemented in the R package MKmisc (Kohl 2012), and plotted the model with the R package popbio (Stubben and Milligan 2007). We used the regression coefficient associated with outer bark thickness to calculate the decrease in the log odds of observing photosynthetic bark per unit of bark thickness increase. Log-transforming outer thickness resulted in a better model fit. Base 2 was used for this log transformation, so that the odds ratio of observing photosynthetic main stem bark (calculated by exponentiation of the estimated coefficient of outer thickness) could be associated with a twofold increase in outer bark thickness. We could not fit a logistic model for twigs given the low number of non-photosynthetic twigs in our sample (see “Results”).
Main stem-twig bark associations across species
We calculated functional trait means per species to assess main stem-twig associations. When traits were associated (as indicated by R 2), we examined whether the scaling relationship was constant (isometry, scaling slope = 1 on log10-transformed traits; Electronic Supplementary Material, Fig S2a, b). When isometry was observed, we further tested whether the intercept was zero, suggesting that main stem and twig traits were indistinguishable from a statistical point of view (Fig. S2a), or differed from zero, suggesting trait proportionality (Fig. S2b). Isometry with a zero intercept would imply that main stem and twig bark are functionally and ecologically similar regarding a particular trait, and that main stem trait values can be straightforwardly extrapolated from twig sampling and vice versa. While still suggesting association, proportionality in main stem-twig trait values (isometry with non-zero intercept) would imply that accumulated ontogenetic changes in main stem bark might cause ecological and functional differences from twig bark.
A third scaling possibility is allometry, a scenario in which change in one variable produces disproportionate change in the other (scaling slope ≠ 1; Fig. S2c). Allometry can occur with slopes >1, suggesting that main stem bark traits increase disproportionately in value as twig bark trait values increase, or with slopes <1, with the disproportionate increase occurring in twigs (Fig. S2c). A fourth scenario implies a lack of trait association, and thus decoupling in function and ecology between main stems and twigs (Fig. S2d). To examine trait association, we fit standardized major axis regressions (SMA) (Warton et al. 2006) using the R package smatr (Warton et al. 2012). Main stem and twig traits were respectively designated y and x variables. Data were log10 transformed to linearize relationships and meet statistical assumptions.
To take into account phylogenetic relationships in our inferences, we refitted SMA regressions based on phylogenetically independent contrasts (PICs) (Felsenstein 1985). We built a phylogeny using the Angiosperm Phylogeny Group backbone and literature for particular groups (Fig. S1). We used the bladj command in Phylocom version 4.2 (Webb et al. 2008) to assign branch lengths with the divergence times of Wikstrom et al. (2001). We calculated PICs using the R package ape (Paradis et al. 2004) and refitted SMA regressions forcing a zero origin. All analyses were performed in R version 3.0.2 (R Development Core Team 2013).
Main stem-twig bark associations across sites
After assessing the general main stem-twig bark scaling relationships across species, we examined whether these relationships changed across sites. We fitted SMA regressions with site as a categorical independent variable and tested for the significance of a twig bark × site term, which would suggest different main stem-twig scaling (slopes) across sites. When the interaction term was not significant, we tested whether intercepts differed between sites.
Results
Stems varied from small shrubs (<1 cm in diameter) to large trunks (>70 cm in diameter). Twigs varied from 0.7 to 3 cm in diameter. Main stems were surrounded by bark 0.4 mm thick in shrubby species to more than 5 cm in Eucalyptus crebra, which represented 1–41 % of the main stem radius. Twig bark thickness varied from 0.3 to 5.3 mm, which represented 8–42 % of the twig radius. Outer bark in main stems varied widely in thickness, from a few cell layers to more than 4 cm thick. At the level of twigs, accumulation of outer bark was more limited. However, variation in this outer layer was still considerable, ranging from a few cells to more than 3 mm thick. Inner bark thickness ranged from 0.3 mm to 3 cm in main stems, and from 0.2 mm to more than 4 mm in twigs (Table 2).
Main stem-twig bark associations across species
Bark traits reflecting density and water content in main stems tended to have the same values as in twigs. Density and water content of main stem and twig bark were very closely associated (R 2 ≥ 0.57, P < 0.001; Table 3), scaling with slopes indistinguishable from unity (isometry; Electronic Supplemental Material, Fig. S2a), and intercepts not differing from zero (Table 3; Fig. 2), hence the interpretation of equivalent values. Both density and water content varied widely across species, with very similar ranges between twigs and main stems. Regarding water content, main stem inner bark tended to have higher and more variable values than whole bark across species (Table 2). Traits of inner bark had very similar correlations and slopes to whole bark traits. Results were very similar based on PICs (Table 3).
In contrast with density and water content, the various thickness traits scaled allometrically between main stems and twigs. Total, outer, and inner bark thicknesses of main stems were closely associated with those of twigs (0.37 ≤ R 2 ≤ 0.43; Table 3), indicating that species with thicker bark in main stems also had thicker bark in twigs. However, this relationship was not proportional (isometric). The main stem-twig scaling slope for bark thickness was >1 (1.45–1.53 based on raw data, and 1.11–1.61 based on PICs; Table 3), meaning that moving from thinner to thicker twig bark across species, main stem bark increased disproportionately in thickness (Fig. S2c). The same was observed for outer and inner bark (Table 3). In contrast with raw thicknesses, relative bark thickness was less strongly correlated between twigs and main stems across species (R 2 = 0.17). Very similar results were recovered for SMA regressions based on PICs (Table 3).
Regarding photosynthetic activity, about half of the species (45 %) had photosynthetic bark on main stems and almost all did on twigs (94 %). No species photosynthesized on main stems without also doing so on twigs, implying that photosynthesis in young stems can be lost but is not acquired later in ontogeny. Photosynthetic barks in twigs did not differ in any trait from the five non-photosynthetic ones (Electronic Supplementary Material, Table S2). This lack of differences and the small number of non-photosynthetic twig barks precluded us from fitting a logistic regression to model photosynthetic activity in twigs.
In contrast with twigs, main stem photosynthesis was associated with other functional traits and could be predicted well by a logistic model. Photosynthetic barks in main stems tended to have thinner total (P < 0.01) and outer bark (P < 0.001), and higher water content (P < 0.01; Electronic Supplemental Material, Table S2). However, outer bark thickness was the only significant trait in the final logistic regression predicting photosynthetic bark. For example, when included along with outer bark thickness as a predictor in a preliminary model, water content was not significant (P = 0.11), and the likelihood ratio test pointed to the model without water content as better (\(\chi^{ 2}\) (1) = 3.29, P = 0.07). The fit was improved when outer bark thickness was log2 transformed (AIC = 87.54 and 95.43 for the models with transformed and untransformed predictor, respectively), and the model fitted the data well (Hosmer and Lemeshow test statistic = 6.90, 8 df, P = 0.55). Outer bark thickness had a coefficient of −0.704 (P < 0.001), so the odds ratio of observing photosynthetic bark decreased 50 % when the thickness of outer bark was doubled (Fig. 3).
Probability of observing photosynthetic bark on main stems based on outer bark thickness (mm; plotted in log2 scale). Solid line represents the fitted logistic model. Histograms for the occurrence of photosynthetic (upper) and non-photosynthetic bark (lower) also shown for different thickness of outer bark
Main stem-twig bark associations across sites
Main stem-twig bark associations for bark density and water content did not differ across sites. No significant differences in slopes or intercepts were detected when the term “site” was added to the SMA regressions (Electronic Supplementary Material, Table S3).
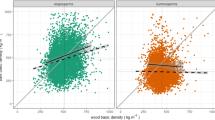
In contrast, all thickness traits (total, outer, inner, and relative bark) scaled differently across sites. The association between twig and main stem total bark thickness increased slightly when site was included in models (R 2 = 0.48 with site vs. 0.43 without site; Table 4). Although relatively modest, this site effect modified slopes (significant twig bark thickness × site term), which ranged from 0.81 to 3.35 (Table 4, first column; Fig. 4a). All sites had slopes >1 (Electronic Supplemental Material, Fig. S2c), except for the savanna. The shallow slope of the savanna indicated disproportionately higher allocation to twig bark for increasingly thicker main stem bark. Despite a good global fit, the fire-free dry and rainforests, and the fire-prone xerophytic shrubland had wide intra-community scatter and non-significant correlations (Table 4). In these sites, main stem bark thickness was uncoupled from twig bark thickness. The models predicting outer and inner bark thickness in main stems based on twig values and site yielded very similar results to that for total bark thickness (Table 4, second and third column).
Covariation of a total (mm) and b relative bark thickness (%) between main stem and twigs. Different lines were fitted per site using standardized major axis regression (Table 4). Dotted line represents 1:1 relationship
Relative bark thickness scaled in a similar way across sites. Species with thicker main stem bark for a given diameter also had thicker twig bark for a given diameter (R 2 = 0.50). Sites had a common scaling slope of 1.83, indicating disproportionate increases in relative thickness in main stems when compared with twigs (Table 4, fourth column). Despite the good global fit (R 2 = 0.50), there was high dispersion within sites, with only the cool temperate woodland and the xerophytic shrubland having significant correlations (Table 4). Sites differed in intercept indicating that the increase in relative thickness in main stems when compared with twigs was higher in some sites (Electronic Supplemental Material, Fig. S2b). The temperate and the cool woodlands, two sites subject to fire, had the highest intercepts, and thus proportionately higher allocation to main stems. In contrast, the fire-free tropical dry and rainforests, and the frequently burned savanna, had the lowest intercepts, but also high dispersion and non-significant correlations within site (Table 4; Fig. 4b).
Discussion
Main stem-twig bark associations across species
Main stem and twig bark were expected to vary in density and water content, because of the marked changes that bark undergoes throughout ontogeny (Junikka 1994). These changes include the maturation of thin-walled parenchyma cells into thick-walled sclereids, accumulation of crushed phloem, and often the loss of cortex as consecutive phellogens produce outer bark (see Fig. 1a; Roth 1981; Srivastava 1964). Any of these processes might be expected to raise the density of main stem bark via the accumulation of dense wall material and the elimination of empty space. Though density and water content varied markedly across species, within each species twig and main stem values were statistically indistinguishable, i.e., across species these traits scaled isometrically with non-zero intercepts (Figs. 2; S2a). Like wood density (Chave et al. 2009), bark density is likely a key trait that is involved in many bark functions such as mechanics, storage, and defense (Romero et al. 2009; Rosell et al. 2014). That this summarizing trait was found to be equivalent between twigs and main stems may suggest that other traits, such as certain bark tissue mechanical properties or C storage per unit of dry biomass, could be very similar if not equivalent along stems.
The strong main stem-twig bark density association provides evidence for functional coordination along stems. Wood density is strongly associated between main stems and branches (Swenson and Enquist 2008). In turn, bark and wood density are strongly correlated (Poorter et al. 2014; Rosell et al. 2014). These sets of correlations in combination with our results would suggest an overall coordination between main stem and branch traits and between wood and bark traits. This coordination would also imply the inclusion of bark in the spectra of variation that have been described for wood (Chave et al. 2009; Reich 2014).
As for density, differences were expected for main stem and twig bark thickness because of bark accumulation and difference in functional needs. However, we observed that twig and main stem bark were associated, with species having disproportionately thicker main stem bark than twig bark (Figs. 4a, S2c). Outer and inner bark thickness had similar main stem-twig scaling as total bark (Table 3). High variation in inner and outer bark amounts is observed across species. However, within an individual, the two lateral meristems producing outer and inner bark (the phellogen and the vascular cambium; Fig. 1a), seem to produce associated and predictable amounts of bark between main stem and twigs.
Bark photosynthesis also changed markedly between main stems and twigs, and as expected, was limited by outer bark thickness. Although photosynthetic barks had higher water content (Table S3), the logistic model predicted main stem photosynthesis quite well based solely on outer bark thickness. Outer bark thickness as a limitation for photosynthesis has been discussed (Gibson 1983; Pfanz et al. 2002; Wittmann and Pfanz 2008), but the thickness thresholds impeding this activity were unknown. We observed that the probability of photosynthetic activity decreased rapidly with the increase in outer bark thickness. For outer bark of 1 mm, this probability was just 50 %. Practically no bark was photosynthetic with more than 4 mm of outer bark (Fig. 3). We treated photosynthesis as a presence/absence trait, but quantifying bark chlorophyll could indicate whether photosynthetic ability changes continuously with outer bark thickness and thus photon flux. However it is analyzed, though, our data showing that as outer bark becomes thicker photosynthesis is quickly lost suggests that the advantages associated with even a thin outer bark are able to offset the C gain by photosynthetic activity. This C gain-protection trade-off was also manifest across communities. The savanna, the site with the most frequent fires, had the lowest percentage of species with main stem bark photosynthesis (18 %), which was congruent with thick protective outer bark (Graves et al. 2014). In contrast, the non fire-prone tropical dry forest and the fire-prone cool temperate woodland had the highest percentages (~70 %). That the fire-prone woodland had such a large percentage of photosynthetic species could be the result of a bias toward a reseeding or basal resprouting strategy (Clarke et al. 2013) of the mostly shrubby or small-statured tree species in this community. Because these species are released from selective pressures favoring stem persistence, they would be free to bear thin outer bark, permitting photosynthesis.
Main stem-twig bark associations across sites
As expected, the relationship between twig and main stem bark thickness (scaling slopes) varied across localities, likely reflecting differing ecological contexts (Fig. 4). Although site affected scaling, the bulk of main stem thickness variation was explained by twig thickness, and the inclusion of site increased R 2 only slightly (from 0.43 to 0.48). The relatively minor role of site is congruent with the high variation in bark thickness observed within sites (Paine et al. 2010; Poorter et al. 2014) and the coexistence of different ecological strategies usually observed within plant communities regarding bark and many other traits (Dantas and Pausas 2013; Wright et al. 2004).
In practically all communities, species with thicker twig bark had disproportionately thicker total bark in main stems (slopes >1; Table 4). This disproportionately higher allocation to main stem bark was expected for fire-prone sites, but not for non-fire prone systems. Slopes >1 were actually observed in dry and rain forests, which are not subjected to fire (Table 1). However, the non-significant correlations within these two sites complicated slope interpretation, but highlighted that non fire-prone sites could tend to have uncoupled main stem-twig bark thickness scaling, and perhaps lower functional coordination between these two levels. The savanna was the only site with a slope <1, i.e., disproportionately higher allocation to twig bark. This observation could be interpreted as the result of bark loss on main stems given the very frequent ground fires fueled by the tall grassy understory of our monsoonal savanna (Cernusak et al. 2006; Pausas 2014). These fires could erode the trunk outer bark, thus leading to disproportionately thicker total twig bark. However, this scenario is rejected by our data, which showed higher net allocation also to the inner bark of savanna twigs (Table 4), a stem portion that does not usually burn. However, fire still seems to have a potential role in reducing outer bark thickness. The main stem-twig thickness slope was lower for the outer (0.57) than for the inner bark (0.73) in the savanna trees (Table 4), a difference that was consistent with greater erosion of outer bark in trunks.
The explanation for the contrasting scaling of the savanna remains unclear, but fire could still be involved. Protection against fire has been the main explanation for thick bark at the level of both main stems and twigs (Pausas 2014). It could be argued that the twigs of the shrubs and small trees that represent half of our sampling (Electronic Supplementary Material, Table S1) could have thick bark because of ground fire exposure, and that these short-statured species could be lowering the main stem-twig bark thickness slope for the whole savanna. However, the trend for thick-barked twigs is also observed in large-statured trees (Fig. 4a), suggesting that even the twigs of larger plants could be exposed to extreme temperatures caused by ground fires. Quantification of the twig-trunk bark relationship in additional savannas will define whether disproportionately thicker twig bark is a characteristic of these habitats.
Relative bark thickness showed somewhat different scaling trends than raw thickness. In contrast with raw thickness, the R 2 of the main stem-twig bark relative thickness association increased considerably when taking site into account (from 0.17 to 0.50). This large contribution of site was likely observed because, despite high intra-site dispersion, sites occupied different regions in the main stem-twig relative thickness plot. The rainforest had a combination of low relative bark thickness values for both main stems and twigs, whereas the savanna had the highest values for both variables (Fig. 4b). Another fire-prone site, the temperate woodland, had high values for main stem relative thickness, but not for twigs. Within sites, relative thickness was in general non-significantly or weakly associated between main stems and twigs (Table 4). This observation suggests that relative allocation to bark in comparison with wood tended to be uncoupled between main stems and twigs. However, this uncoupling in relative allocation does not necessarily mean functional or ecological differences for bark of these two stem levels.
Main stem-twig bark associations and the inference of bark functional and ecological divergence
Despite conspicuous differences in bark external morphology and factors such as fire affecting canopies and main stems differently, the bark traits analyzed here were statistically indistinguishable or changed more or less predictably in most sites between twigs and main stems. These results have positive implications for bark studies. For example, ecologists can sample twig bark and extrapolate its density and water content to main stem bark across species. Likewise, twig bark thickness can be used to estimate main stem bark thickness, at least in fire-prone habitats. Despite the possibility of deriving bark trait values from one level to the other, the ecology of bark is likely to differ along stems. For example, bark thickness scaled disproportionately in main stems when compared to twigs. This scaling would strongly affect functions that depend on total bark amount, such as bark mechanics (Paine et al. 2010; Rosell and Olson 2014), on inner bark, such as water and starch storage, or on outer bark, such as fire protection, and as shown here, the presence of photosynthetic inner bark. Our results indicate that main stem bark is, from an ecological point of view, much more than simply a thicker version of twig bark.
Author contribution statement
J. A. R. and M. W. conceived and designed the study. J. A. R., M. C. and C. L. collected the data. J. A. R., M. C., C. L., and M. W. analyzed the data and wrote the manuscript.
References
Butler DW, Gleason SM, Westoby M (2012) Setbacks to shoot growth are common in woody plants, so how are shoots of some species safer than others? Ecology 93:1275–1282. doi:10.1890/11-1017.1
Carlquist S (1982) The use of ethylendiamine in softening hard plant structures for paraffin sectioning. Stain Technol 57:311–317
Cernusak LA, Hutley LB (2011) Stable isotopes reveal the contribution of corticular photosynthesis to growth in branches of Eucalyptus miniata. Plant Physiol 155:515–523. doi:10.1104/pp.110.163337
Cernusak LA, Hutley LB, Beringer J, Tapper NJ (2006) Stem and leaf gas exchange and their responses to fire in a north Australian tropical savanna. Plant Cell Environ 29:632–646. doi:10.1111/j.1365-3040.2005.01442.x
Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE (2009) Towards a worldwide wood economics spectrum. Ecol Lett 12:351–366. doi:10.1111/j.1461-0248.2009.01285.x
Clarke PJ, et al. (2013) Resprouting as a key functional trait: how buds, protection and resources drive persistence after fire. New Phytol 197:19–35. doi:10.1111/nph.12001
Dantas VDL, Pausas JG (2013) The lanky and the corky: fire-escape strategies in savanna woody species. J Ecol 101:2454–2463
Domec JC, Gartner BL (2002) How do water transport and water storage differ in coniferous earlywood and latewood? J Exp Bot 53:2369–2379. doi:10.1093/Jxb/Erf100
Evert RF, Eichhorn SE (2006) Esau’s plant anatomy: meristems, cells, and tissues of the plant body: their structure, function, and development. Wiley, Hoboken
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15
Franco-Vizcaíno E, Goldstein G, Ting IP (1990) Comparative gas exchange of leaves and bark in three stem succulents of Baja California. Am J Bot 77:1272–1278
Gibson AC (1983) Anatomy of photosynthetic old stems of non succulent dicotyledons from North American deserts. Bot Gaz 144:347–362
Graves SJ, Rifai SW, Putz FE (2014) Outer bark thickness decreases more with height on stems of fire-resistant than fire-sensitive Floridian oaks (Quercus spp.; Fagaceae). Am J Bot 101:2183–2188. doi:10.3732/ajb.1400412
Hoffmann WA, Solbrig OT (2003) The role of top kill in the differential response of savanna woody species to fire. For Ecol Manage 180:273–286. doi:10.1016/s0378-1127(02)00566-2
Junikka L (1994) Survey of English macroscopic bark terminology. IAWA J 15:3–45
Kleinbaum DG, Klein M (2010) Logistic regression. A self-learning text. Springer, New York
Kohl M (2012) MKmisc: miscellaneous functions from M Kohl. R package version 0.91
Lawes MJ, Richards A, Dathe J, Midgley JJ (2011) Bark thickness determines fire resistance of selected tree species from fire-prone tropical savanna in north Australia. Plant Ecol 212:2057–2069. doi:10.1007/s11258-011-9954-7
Midgley JJ, Lawes MJ, Chamaille-Jammes S (2010) Savanna woody plant dynamics: the role of fire and herbivory, separately and synergistically. Aust J Bot 58:1–11. doi:10.1071/bt09034
Murphy BP, et al. (2013) Fire regimes of Australia: a pyrogeographic model system. J Biogeogr 40:1048–1058. doi:10.1111/jbi.12065
Niklas KJ (1999) The mechanical role of bark. Am J Bot 86:465–469. doi:10.2307/2656806
Paine CET, Stahl C, Courtois EA, Patiño S, Sarmiento C, Baraloto C (2010) Functional explanations for variation in bark thickness in tropical rain forest trees. Funct Ecol 24:1202–1210. doi:10.1111/j.1365-2435.2010.01736.x
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290. doi:10.1093/Bioinformatics/Btg412
Patiño S, et al. (2009) Branch xylem density variations across the Amazon Basin. Biogeosciences 6:545–568. doi:10.5194/bg-6-545-2009
Pausas JG (2014) Bark thickness and fire regime. Funct Ecol. doi:10.1111/1365-2435.12372
Pfanz H (2008) Bark photosynthesis. Trees Struct Funct 22:137–138. doi:10.1007/s00468-007-0196-1
Pfanz H, Aschan G, Langenfeld-Heyser R, Wittmann C, Loose M (2002) Ecology and ecophysiology of tree stems: corticular and wood photosynthesis. Naturwissenschaften 89:147–162. doi:10.1007/s00114-002-0309-z
Pinard MA, Lutz FE, Licona JC (1999) Tree mortality and vine proliferation following a wildfire in a subhumid tropical forest in eastern Bolivia. For Ecol Manage 116:247–252. doi:10.1016/s0378-1127(98)00447-2
Poorter L, McNeil A, Hurtado VH, Prins H, Putz J (2014) Bark traits and life history strategies of tropical dry and moist forest trees. Funct Ecol 28:232–242. doi:10.1111/1365-2435.12158
R Development Core Team (2013) R: A language and environment for statistical computing, v. 3.0.2 edn. R Foundation for Statistical Computing, Vienna
Reich PB (2014) The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. J Ecol 102:275–301. doi:10.1111/1365-2745.12211
Romero C (2013) Bark structure and functional ecology. In: Cunningham AB, Campbell BM, Luckert MK (eds) Bark: use, management, and commerce in Africa, vol 17. The New York Botanical Garden Press, New York, pp 5–25
Romero C, Bolker BM, Edwards CE (2009) Stem responses to damage: the evolutionary ecology of Quercus species in contrasting fire regimes. New Phytol 182:261–271. doi:10.1111/j.1469-8137.2008.02733.x
Rosell JA, Olson ME (2014) The evolution of bark mechanics and storage across habitats in a clade of tropical trees. Am J Bot 101:764–777
Rosell JA, Olson ME, Aguirre-Hernández R, Carlquist S (2007) Logistic regression in comparative wood anatomy: tracheid types, wood anatomical terminology, and new inferences from the Carlquist and Hoekman southern Californian data set. Bot J Linn Soc 154:331–351. doi:10.1111/J.1095-8339.2007.00667.X
Rosell JA, Gleason SM, Méndez-Alonzo R, Chang Y, Westoby M (2014) Bark functional ecology: evidence for tradeoffs, functional coordination, and environment producing bark diversity. New Phytol 201:486–497. doi:10.1111/nph.12541
Roth I (1981) Structural patterns of tropical barks. Gebruder Borntraeger, Berlin
Ruzin SE (1999) Plant microtechnique and microscopy. Oxford University Press, New York
Saveyn A, Steppe K, Ubierna N, Dawson TE (2010) Woody tissue photosynthesis and its contribution to trunk growth and bud development in young plants. Plant Cell Environ 33:1949–1958. doi:10.1111/j.1365-3040.2010.02197.x
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Meth 9:671–675
Scholz FG, Bucci SJ, Goldstein G, Meinzer FC, Franco AC, Miralles-Wilhelm F (2007) Biophysical properties and functional significance of stem water storage tissues in Neotropical savanna trees. Plant Cell Environ 30:236–248. doi:10.1111/j.1365-3040.2006.01623.x
Srivastava LM (1964) Anatomy, chemistry and physiology of bark. Int Rev For Res 1:203–277
Stubben C, Milligan B (2007) Estimating and analyzing demographic models using the popbio package in R. J Stat Softw 22:1–23
Swenson NG, Enquist BJ (2008) The relationship between stem and branch wood specific gravity and the ability of each measure to predict leaf area. Am J Bot 95:516–519. doi:10.3732/Ajb.95.4.516
Teskey RO, Saveyn A, Steppe K, McGuire MA (2008) Origin, fate and significance of CO2 in tree stems. New Phytol 177:17–32. doi:10.1111/j.1469-8137.2007.02286.x
Uhl C, Kauffman JB (1990) Deforestation fire susceptibility and potential tree responses to fire in the Eastern Amazon Brazil. Ecology 71:437–449. doi:10.2307/1940299
Warton DI, Wright IJ, Falster DS, Westoby M (2006) Bivariate line-fitting methods for allometry. Biol Rev 81:259–291. doi:10.1017/S1464793106007007
Warton DI, Duursma RA, Falster DS, Taskinen S (2012) smatr 3—an R package for estimation and inference about allometric lines. Methods Ecol Evol 3:257–259. doi:10.1111/j.2041-210X.2011.00153.x
Webb CO, Ackerly DD, Kembel SW (2008) Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 24:2098–2100
Wikstrom N, Savolainen V, Chase MW (2001) Evolution of angiosperms: calibrating the family tree. Proc R Soc 268:2211–2220
Williamson GB, Wiemann MC (2010) Measuring wood specific gravity correctly. Am J Bot 97:519–524. doi:10.3732/Ajb.0900243
Wittmann C, Pfanz H (2008) General trait relationships in stems: a study on the performance and interrelationships of several functional and structural parameters involved in corticular photosynthesis. Physiol Plant 134:636–648. doi:10.1111/j.1399-3054.2008.01165.x
Wright IJ, et al. (2004) The worldwide leaf economics spectrum. Nature 428:821–827. doi:10.1038/nature02403
Acknowledgments
This work was supported by Consejo Nacional de Ciencia y Tecnología grant no. 237061, Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (UNAM) grant no. IA201415, a Young Scientist Award from the MAB program (Unesco) awarded to J. A. R., the Australian Research Council through a Laureate Fellowship awarded to M. W., and the Daintree Rainforest Observatory. We thank Mark Olson, Sherwin Carlquist, Wade Tozer, Yvonne Chang, Nicole Vella, Debra Birch, Sean Gleason, Andrew Ford, Peter Byrnes, Andrew Thompson, Julia Cooke, Marina Scalon, Alicia Cook, Christopher Blackman, Ashleigh Brice, Michael Brand, Lindsay Hutley, Martha García, Enrique García, Jorge Vega, and Antonio Lot for their kind help with field work and helpful discussions. We thank two anonymous reviewers whose comments greatly helped improve the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Allan T. G. Green.
Electronic supplementary material
Below is the link to the Electronic Supplementary Material.
Rights and permissions
About this article
Cite this article
Rosell, J.A., Castorena, M., Laws, C.A. et al. Bark ecology of twigs vs. main stems: functional traits across eighty-five species of angiosperms. Oecologia 178, 1033–1043 (2015). https://doi.org/10.1007/s00442-015-3307-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3307-5