Abstract
Natural enemies and environmental factors likely both influence the population cycles of many forest-defoliating insect species. Previous work suggests precipitation influences the spatiotemporal patterns of gypsy moth outbreaks in North America, and it has been hypothesized that precipitation could act indirectly through effects on pathogens. We investigated the potential role of climatic and environmental factors in driving pathogen epizootics and parasitism at 57 sites over an area of ≈72,300 km2 in four US mid-Atlantic states during the final year (2009) of a gypsy moth outbreak. Prior work has largely reported that the Lymantria dispar nucleopolyhedrovirus (LdNPV) was the principal mortality agent responsible for regional collapses of gypsy moth outbreaks. However, in the gypsy moth outbreak-prone US mid-Atlantic region, the fungal pathogen Entomophaga maimaiga has replaced the virus as the dominant source of mortality in dense host populations. The severity of the gypsy moth population crash, measured as the decline in egg mass densities from 2009 to 2010, tended to increase with the prevalence of E. maimaiga and larval parasitoids, but not LdNPV. A significantly negative spatial association was detected between rates of fungal mortality and parasitism, potentially indicating displacement of parasitoids by E. maimaiga. Fungal, viral, and parasitoid mortality agents differed in their associations with local abiotic and biotic conditions, but precipitation significantly influenced both fungal and viral prevalence. This study provides the first spatially robust evidence of the dominance of E. maimaiga during the collapse of a gypsy moth outbreak and highlights the important role played by microclimatic conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pathogen ecology is of increasing interest, especially with regard to increases in occurrence and potential changes in the geographic distributions of emergent infectious diseases (Jones et al. 2008; Lafferty 2009; Keesing et al. 2010). Diseases caused by fungi and their relatives have had significant impacts on plant populations, such as causing epidemics leading to the Irish potato famine and the decimation of chestnut trees in eastern North America and elm trees in North America and Europe (Fisher et al. 2012). Until recently, fungal pathogens were not a well-known cause of epizootics in animal populations. However, a number of animal species are currently under threat from emerging fungal diseases such as chytridiomycosis in amphibians (Collins 2013), white-nose syndrome in bats (Frick et al. 2010), and colony collapse disorder in bees (Cameron et al. 2011). Since 1989, an emergent fungal entomopathogen has established and spread in North American gypsy moth [Lymantria dispar (L.)] populations (Hajek 1999). High-density, or outbreak, populations of caterpillars feeding on foliage of forest trees have frequently been known to experience synchronous population collapses due to high levels of pathogen infection (Myers and Corey 2013).
Many insect species prone to outbreaks exhibit cyclical population behavior characterized by rapid population growth followed by population collapse. Outbreak cycles in these species are often synchronous over large areas. Spatial synchrony of population fluctuations is generally thought to occur either as a result of the dispersal of individuals or due to spatially synchronous fluctuations in weather or other environmental factors (Liebhold et al. 2004). In forest insect pests, the latter hypothesis appears to better explain observations of synchronous population fluctuations over large distances (Peltonen et al. 2002; Liebhold et al. 2012). For example, Haynes et al. (2013) found that the most likely driver of synchrony in gypsy moth outbreaks was precipitation, which could synchronize gypsy moth populations directly by affecting gypsy moth survival or reproduction, producing a Moran effect, or indirectly through effects on pathogens, predators, or oak masting. Climatic (e.g., moisture levels, temperature, etc.) and physiographic (e.g., soil type, slope, etc.) conditions can directly or indirectly drive pathogen transmission (Agrios 2005; Sacks et al. 2003; Cushman and Meentemeyer 2008; Shapiro-Ilan et al. 2012), thus influencing levels of disease. In gypsy moth populations, a baculovirus, Lymantria dispar nucleopolyhedrovirus; LdNPV), has long been known to cause epizootics that result in outbreak collapse (Alalouni et al. 2013; McManus and Csóka 2007). The impact of precipitation has been suggested to indirectly influence the collapse of host populations by increasing the dispersal of this virus (D’Amico and Elkinton 1995). However, pathogen infection rates can also be influenced by biological factors including host density, pathogen density, and the occurrence of competitors, predators, or parasites (Holt and Dobson 2006).
After years of controversy, it is generally now agreed that combinations of exogenous (or density-independent) and endogenous (or density-dependent) factors drive population dynamics (Royama 1992; Berryman 1999; Turchin 2003). Interactions between these factors are often complex, making it difficult to delineate the relative influences of individual factors, although large temporal and spatial ecological studies can lead to a greater understanding of these relationships (Brown et al. 2001). The present study was developed initially to evaluate the effects of environmental factors on major natural enemies of the gypsy moth in areas where gypsy moth outbreaks occur. Fortuitously, we conducted this study during the final year of a spatially widespread collapse of a gypsy moth outbreak. In doing so, we present the most spatially robust examination of the role of pathogens and parasitoids in a gypsy moth outbreak collapse. We conducted this study at a total of 63 geographically and environmentally diverse sites in 4 US states, encompassing an area of ≈72,300 km2 (Fig. 1; Appendix S1 in the Electronic supplementary material, ESM). Based on prior analysis of gypsy moth outbreaks (Haynes et al. 2013), we hypothesized that environmental moisture levels would be associated with the activities of the two dominant pathogens. We also tested whether additional environmental and biological variables could potentially be associated with prevalence of larval pathogens and parasitoids. In addition, we analyzed the spatial dynamics of the natural enemies of gypsy moth larvae, including their interactions.
Materials and methods
Study system
The gypsy moth was introduced into North America (outside of Boston, MA, USA) in 1869, and has since spread such that it now occupies a range from Nova Scotia to Wisconsin, and Ontario to Virginia (Tobin et al. 2012). Although gypsy moth populations are innocuous and barely noticed in most years, spatially synchronous outbreaks occur at fairly regular cycles in some regions (Appendix S1 of the ESM; Haynes et al. 2009; AEH, unpublished data). At low gypsy moth population densities, predatory small mammals are thought to be the most important natural enemies, while outbreaking populations generally collapse due to entomopathogens (Elkinton and Liebhold 1990; Hajek 1999; Dwyer et al. 2004). An entomopathogenic virus, LdNPV, that was accidentally introduced before 1907 has generally been considered the most abundant and effective natural enemy in outbreaking gypsy moth populations, responsible for epizootics in defoliating populations that result in population crashes (Alalouni et al. 2013; McManus and Csóka 2007). Other natural enemies, predominantly parasitoids, were introduced purposefully for classical biological control, but only a few of these species became well established, and all generally have low impact (Fuester et al. 2013).
The gypsy moth fungal pathogen Entomophaga maimaiga, an acute pathogen that kills Lymantria dispar (gypsy moth) larvae, was first found to be established in North America in New England during the exceptionally rainy spring of 1989 (Andreadis and Weseloh 1990; Hajek et al. 1990). It was hypothesized that the abundant rainfall in 1989 facilitated the dramatic epizootics that occurred (Elkinton et al. 1991), even though it remains unknown how, where, or when this pathogen from Japan initially became established in North America (Nielsen et al. 2005). Between 1989 and 1992, E. maimaiga spread, probably both on its own and with limited human assistance (i.e., resting spores were introduced to a limited number of areas where this fungus had not been found) (Elkinton et al. 1991; Hajek et al. 1995, 1996). Following establishment, E. maimaiga persists in areas after epizootics as resting spores that remain in the soil for many years (Hajek et al. 2000). During the spring, infective conidia are actively discharged from resting spores, or from cadavers of insects killed by the fungus, and these conidia infect new hosts.
The activity of LdNPV is dependent on host density (Dwyer 1994; Liebhold et al. 2013), while E. maimaiga activity has usually (Liebhold et al. 2013; Hajek 1999)—but not always (Weseloh and Andreadis 1992)—been reported to be density independent. Entomophaga maimaiga and LdNPV can co-infect hosts (Malakar et al. 1999) and are frequently both present in higher density L. dispar populations (AEH, unpublished data; Reilly et al. 2014).
Study sites
Cooperators in Maryland (MD), Pennsylvania (PA), Virginia (VA), and West Virginia (WV) established 63 study sites, encompassing an area of ≈72,300 km2, in the winter of 2008–2009 (Fig. 1; Appendix S1 in the ESM). All sites except for the most southern site (outside of Blacksburg, VA) were considered infested with gypsy moths according to county quarantine records (USA Code of Federal Regulations, Title 7, Chapter III, Part 301) since at least 2002, and most had previously experienced at least one gypsy moth outbreak before this study was conducted (Appendix S1 in the ESM). Entomophaga maimaiga was known to be established throughout the study area as early as 1992 (Hajek et al. 1996).
Across this area of the mid-Atlantic US the most recent occurrence of gypsy moth populations increasing toward outbreak densities began in 2006, and 2009 ultimately represented the final year of this outbreak cycle (Fig. 2). To facilitate the collection of larvae in 2009, sites were selected in part based on gypsy moth abundance in 2008, given that larvae are difficult to find where densities are low. Sites were also specifically chosen to represent different ecological regions (Fig. 1), and included the Coastal Plain (4 sites), Piedmont (10), Blue Ridge Mountains (10), Northern Ridge and Valley (26), Allegheny Mountains (8), and the unglaciated Allegheny Plateau (5) (McNab et al. 2007). Of the 63 sites, larvae were ultimately collected from 57 sites.
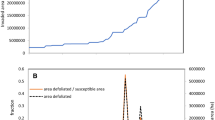
Time series of the area (km2) of gypsy moth, Lymantria dispar, defoliation based upon gypsy moth defoliation maps from aerial surveys (Liebhold et al. 1997; USDA Forest Service 2013a, b) during 1997–2010 of the states corresponding to the locations of our sampling sites. The most recent outbreak in this area occurred from 2006 to 2009. The gray shaded region represents the time during which we conducted our sampling, and underscores that our sampling was conducted during the final year of a spatially widespread gypsy moth outbreak
Additional site characteristics
To evaluate local drivers of larval mortality, study sites were georeferenced and linked to a suite of biological and abiotic (weather, soil, and topographic) variables in ArcGIS 10 (ESRI, Redlands, CA). Biological variables included (1) whether or not the site was within an area in which gypsy moth defoliation (as determined through aerial detection surveys; USDA Forest Service 2013a, b) occurred at least once during the 5 years prior to the study (2004–2008), (2) the gypsy moth egg mass density prior to egg hatch in 2009, and (3) the basal area of the gypsy moth’s preferred host tree species at each site, according to Morin et al. (2005); preferred host tree species include those in the genera Quercus, Betula, Crataegus, Populus, Salix, and Tilia (Liebhold et al. 1995). The average maximum and minimum monthly temperatures (March–June 2009) and monthly precipitation (March–June 2009) for each site were obtained from the PRISM Climate Group, Oregon State University (2011). Site elevation, aspect, and slope were derived from the 30-m USGS National Elevation Data (Gesch 2007; Gesch et al. 2002). Soil attributes (available water capacity and soil texture measured as % sand) for each site were obtained from the Soil Survey Geographic (SSURGO) database (USDA Natural Resources Conservation Service 2012).
Biological sampling
During all sampling efforts, personnel wore disposable shoe covers that were discarded between sites so that soil-borne E. maimaiga inoculum would not be transferred among sites. The density of gypsy moth egg masses was estimated prior to egg hatch in 2009, and then again during the winter of 2009–2010. Egg mass density was estimated based on counts from four 0.01-ha plots at each study site, following standard protocols (Liebhold et al. 1994).
Soil samples were taken at each study site prior to the 2009 gypsy moth egg hatch to estimate E. maimaiga resting spore density. At the center of each site, soil was collected from around five dominant trees (usually Quercus spp.) as follows: samples of the top 3 cm of soil from within 10 cm of the base of each tree were collected from the north and south sides of each tree. Resting spores were counted in soil samples using a modified method of discontinuous Percoll density gradient centrifugation to derive a count/g dry soil for each site (Hajek et al. 2012).
During the early spring of 2009, cooperators checked gypsy moth larval development weekly. Larval collections were begun when fourth instars were present and continued until pupation. Cooperators collected either 30 larvae or the number of larvae <30 that could be found during 1 h during each weekly collection. Due to variability in population levels, development rates and mortality, larvae were collected from 1 to 6 times per site (median = 3 times) (Appendix S1 in the ESM). All collected larvae (N = 2,935 across all sites) were reared in 29-ml clear plastic cups on a high wheat germ artificial diet (Bell et al. 1981) at 20–23 °C for 30 days. Any larvae that died within the first 15 days were monitored daily over the next 3 days to detect production of conidia by E. maimaiga. For larvae that died due to parasitism, parasitoids (exclusively Hymenoptera or Tachinidae) were reared and identified (Simons et al. 1974). All dead larvae after 10 days at room temperature were stored at 4 °C until they were dissected and smears were observed microscopically (200–400×) to detect occlusion bodies of Lymantria dispar nucleopolyhedrovirus (LdNPV) and E. maimaiga resting spores (Hajek and Tobin 2011).
Data analysis
Due to the low numbers of larvae often found during the final weeks of larval collection, we summed data across sample dates for each site to calculate the proportion of gypsy moth larvae killed by E. maimaiga, LdNPV or parasitoids. There were 54 larvae (of 2,935) with co-infections of E. maimaiga and LdNPV, 19 with co-infections of LdNPV and a parasitoid, and 9 with co-infections of E. maimaiga and a parasitoid. Due to the small number of occurrences of these co-infections, larvae with co-infections were included in the calculations of the proportions of larvae killed by each mortality agent.
We estimated spatial autocorrelation in larval mortality due to E. maimaiga, LdNPV, or larval parasitoids (combining all parasitoid species due to low numbers of individual species), and in the density of E. maimaiga resting spores (number of spores/g dry soil). We also estimated the spatial autocorrelation in gypsy moth egg mass densities (numbers per ha) during the outbreak (i.e., egg mass sampling in 2009 prior to hatch) and after the collapse of the outbreak (i.e., egg mass sampling during the winter of 2009–10). Egg mass densities were transformed using log10 + 1 to normalize the distribution. We also estimated the spatial autocorrelation in precipitation during the month of May, which corresponds to the month in which gypsy moths almost exclusively undergo larval development in this region. We estimated autocorrelation based on spatial nonparametric covariance functions using the R (R Development Core Team 2013) package “ncf” (Bjørnstad and Falck (2001). Significance of the covariance function was assessed based on bootstrap resampling using 500 iterations. We ascertained significance using the 25th and 75th percentiles of the bootstrapped distribution. We used these percentiles, as opposed to 95 % confidence limits (Efron and Tibshirani 1993), to denote significance because a major motivation was to determine if spatial autocorrelation in mortality agents should be considered in subsequent hypothesis testing. Thus, in using the 25th and 75th percentiles, we were more likely to observe significant spatial autocorrelation in mortality agents, which renders our final statistical models more conservative. To evaluate the possibility of competition among mortality agents, we also estimated spatial cross-correlations for all pairwise combinations of mortality agents based on the spatial nonparametric covariance function described above. In this latter case, we used the stricter 95 % confidence limits, based upon 500 bootstrapped replications, to ascertain significant cross-correlation.
We then examined the effects of local abiotic (Table 1) and biotic variables on the proportions of gypsy moth larvae killed by E. maimaiga, LdNPV, or parasitoids, using spatial lag models (Anselin 1988). We also assessed whether these sources of larval mortality seemed to influence gypsy moth density by examining the relationships between the proportions of larvae killed by each natural enemy and the change in egg mass density from 2009 to 2010 (log10[density in 2010] − log10[density in 2009]). A spatial lag model provides tests of relationships between a spatially autocorrelated response variable and various predictor variables and is used when the autocorrelation is thought to arise largely through direct dependence of the value of the response variable in a given location on its values in neighboring locations (Anselin 1988). We expected some degree of spatial dependence in mortality sources due to natural enemy dispersal. In a spatial lag model, spatial dependence is modeled by including a spatially lagged measure of the response variable (e.g., proportion killed by LdNPV in neighboring locations) as a predictor variable.
The spatial lag variables were calculated as the mean proportions of larvae killed by each enemy type (E. maimaiga, LdNPV, and parasitoids) within a fixed radius of each focal site. The length of the radius was the distance over which the spatial autocovariance function (see above) of the respective dependent variable was >0, i.e., the maximum distance at which spatial autocorrelation was detected. To minimize heterogeneity of variance and to improve the normality of residuals from the spatial lag models, proportions of larvae killed by E. maimaiga and LdNPV were transformed using log10(proportion killed +1), and proportions killed by parasitoids, resting spore density, and lagged resting spore density were square root-transformed.
Each spatial lag model, in addition to the abiotic variables listed in Table 1, included three biotic predictor variables that could influence the development of gypsy moth outbreaks: the density of gypsy moth egg masses (egg masses ha−1), the basal area of the gypsy moth’s preferred host tree species (m2 ha−1), and a binary variable for the presence or absence of defoliation by gypsy moths in the previous 5 years. In addition, E. maimaiga resting spore density was added as a predictor variable to the model examining larval mortality due to E. maimaiga to examine the contribution of this reservoir life stage to infection rates. We also considered an additional spatial lag model to examine influences of the abiotic variables (Table 1) defoliation within the previous 5 years and host tree density on the density of E. maimaiga resting spores. To avoid multicollinearity of predictor variables, variables with variance inflation factors >10 were not considered in any model. To meet this criterion, some variables were combined (i.e., maximum temperatures were averaged across all 4 months), while some predictor variables (minimum temperatures and ecoregion) were not considered in any model. We chose to analyze effects of maximum temperatures instead of minimum temperatures because of recent evidence of adverse effects of high spring and summer temperatures on gypsy moth populations in the southern portion of the gypsy moth’s range in North America (Tobin et al. 2014).
Results
Host and pathogen densities
In spring 2009 prior to egg hatch, gypsy moth egg mass density averaged (±SE) 3,941.2 (618.6) per ha across sites, and egg masses were found at 89 % of the sites sampled. Densities of the subsequent generation dropped dramatically, as the average egg mass density, measured in the fall of 2009, was 14.6 (5.2) per ha. Furthermore, by the fall of 2009, no gypsy moth egg masses were found in 82 % of the sites. Therefore, our larval samples were all taken during the final year of a gypsy moth outbreak when populations were collapsing.
A total of 2,935 larvae (instars 4–6) were sampled from 57 sites, as no larvae could be found at 6 of the 63 sites. Site-specific proportions of larvae infected with the entomopathogens E. maimaiga (1,559 larvae) or LdNPV (170 larvae), co-infected by these two pathogens (54 larvae), parasitized (199 larvae), or containing no natural enemies (953 larvae) generally demonstrated the dominance of E. maimaiga (Fig. 3). Entomophaga maimaiga was the most abundant natural enemy; it was detected at all 57 sites, and >50 % of larvae died from E. maimaiga infections at >50 % of sites. Moreover, E. maimaiga was a more dominant mortality factor than all other sources of larval mortality combined at 30 (of the 57) sites. In contrast, the viral pathogen was detected at 38.6 % of sites, averaged 6.3 % infection (SE = 1.2) across all sites, and was less abundant than E. maimaiga at every site. Parasitoids, as a group, were present at 33.3 % of sites and the parasitism rate averaged 7.2 % (SE = 1.4). The principal parasitoids were the braconid Cotesia melanoscela (104 larvae parasitized) and the tachinid Parasetigena silvestris (55 larvae), although Phobocampe unicincta (19 larvae) and Compsilura concinnata (15 larvae) were also detected. Six larvae were parasitized by parasitoids that were not successfully identified.
Spatial dependence in mortality agents
We detected significant spatial autocorrelation in gypsy moth egg mass densities during the outbreak as well as after the collapse of the outbreak (Fig. 4a, b). We also noted significant spatial autocorrelation in May precipitation (Fig. 4d), and in the proportions of larvae killed by E. maimaiga, LdNPV, and parasitoids (Fig. 4e, g, h). Significance was based on the 25th and 75th percentiles of the bootstrapped distribution to intentionally lower the statistical bar for deciding which variables should be included in a spatial lag model, which then becomes a more conservative method for denoting variable significance. The ranges of spatial dependence (e.g., the distance between sampling locations over which the spatial autocorrelation was >0) for egg mass density during the outbreak, May precipitation, and mortality due to E. maimaiga, LdNPV, and parasitoids were 59.9, 114.4, 72.5, 100.5, and 64.9 km, respectively. The last three range estimates suggest relatively strong spatial autocorrelation in all mortality agents during this final year of a gypsy moth outbreak cycle. The density of E. maimaiga resting spores per g of soil at each site was also spatially autocorrelated, with a range of spatial dependency of 69.1 km (Fig. 4f).
Spatial autocorrelation (±25th and 75th percentiles) of a gypsy moth egg mass densities (number per ha) during the outbreak in 2009; b egg mass densities after the 2009 outbreak had collapsed; c the change in egg mass densities from 2009 to 2010; d May precipitation; e the proportion of gypsy moth larvae killed by E. maimaiga, f the density of E. maimaiga resting spores (spores per gram of dry soil); g the proportion of gypsy moth larvae killed by LdNPV; and h the proportion of gypsy moth larvae killed by parasitoids. In each, the “range” refers to the range of spatial dependence, as defined by the spatial lag distance at which the spatial autocorrelation estimate =0
We only detected significant spatial cross-correlation (when using 95 % confidence intervals) between the proportion of larvae killed by E. maimaiga and parasitoids (Fig. 5). Higher proportions of mortality due to E. maimaiga at a site were associated with lower proportions of mortality due to parasitoids, and the range of this significantly negative spatial cross-correlation extended to 47.5 km (95 % CI 24.6, 88.0 km).
Spatial cross-correlation (±95 % confidence intervals) between a E. maimaiga and parasitoids; b E. maimaiga and LdNPV; and c LdNPV and parasitoids. The “range” refers to the range of spatial dependence, as defined by the lag distance at which the spatial autocorrelation estimate = 0; only in the interaction between E. maimaiga and parasitoids did we detect significant, and negative, spatial cross-correlation
Effects of site characteristics
Local rates of larval mortality due to E. maimaiga increased significantly with gypsy moth egg mass density, precipitation in May, and the spatial lag variable representing the mean rate of E. maimaiga-induced larval mortality within the surrounding 72.5 km (Table 2). Precipitation in March, however, was negatively associated with E. maimaiga-induced larval mortality. Densities of E. maimaiga resting spores per g of soil were positively associated with precipitation in May, but showed the opposite relationship with precipitation in June (Table 3). Resting spore densities also decreased significantly with increasing slope. There was a significant positive relationship between local resting spore densities and the respective spatial lag variable (mean resting spore density within the surrounding 69.1 km).
Mortality of larvae caused by LdNPV increased with gypsy moth egg mass density, April and June precipitation, available water supply in the soil, basal area of preferred gypsy moth host trees, and the presence of defoliation in the previous outbreak (Table 2). In contrast, there was a negative relationship between LdNPV-induced mortality and precipitation in May. The spatial lag variable (mean proportion of larvae killed by LdNPV within the surrounding 100.5 km) in the LdNPV model was not significant.
The local risk of parasitism increased significantly with the proportion of larvae killed by parasitoids in the surrounding 64.9 km (Table 2). However, parasitism was not correlated with any site characteristics.
Roles of natural enemies in gypsy moth decline
From 2009 to 2010, there was a marked decline in gypsy moth egg mass density (no. ha−1) at our study sites, with density declining by an average (SD) of 3,866.5 (2,479.6). The change in egg mass density displayed significant positive spatial autocorrelation (Fig. 4c), and autocorrelation was >0 over distances up to 50.1 km. Based on a spatial lag model, we found that the change in egg mass density (log10[density in 2010] − log10[density in 2009]) declined significantly with the proportions of larvae killed by E. maimaiga and parasitoids, but there was no relationship between the decline of egg mass densities and the proportion killed by LdNPV (Table 4). Finally, there was a significant positive relationship between change in egg mass density and the spatial lag variable (mean change in egg mass density within the surrounding 50.1 km).
Discussion
We present the most spatially robust study to date on the role of the emergent fungal entomopathogen E. maimaiga in the collapse of outbreaking North American gypsy moth populations. Entomophaga maimaiga was the dominant source of mortality in the synchronous 2009 collapse of a regional gypsy moth outbreak, largely replacing the viral pathogen (LdNPV) which previously played this role (Elkinton and Liebhold 1990; Campbell and Podgwaite 1971; Doane 1970; Campbell 1963). In addition, the severity of the gypsy moth population crash tended to increase with the prevalence of E. maimaiga and larval parasitoids, but not LdNPV (Table 4). It is interesting that we detected a relationship between larval parasitoids and the severity of the crash, but given that they killed far fewer larvae than E. maimaiga (Fig. 3), parasitoids could not have been as influential as E. maimaiga in the gypsy moth population crash.
Although larval mortality due to E. maimaiga was generally high, and everywhere more frequent than LdNPV-induced mortality, mortality attributed to both pathogens exhibited geographic variation associated with environmental conditions. Surprisingly, numerous variables included in analyses, such as slope and the percentage of sand in the soil, were rarely or never significant (Tables 2, 3). However, precipitation occurring in months when larvae were active (April–June) was generally positively associated with mortality due to both E. maimaiga and LdNPV, and available water supply of the soil was positively associated with prevalence of LdNPV (Table 2). Thus, environmental moisture was generally associated with higher rates of pathogen-induced mortality. Similarly, Haynes et al. (2013) found that region-wide synchrony of gypsy moth population cycles increased with the synchrony of precipitation. One hypothesis for this relationship is that precipitation synchronizes gypsy moth populations through its influence on pathogen transmission. Our study supports this hypothesis by demonstrating that geographic variation in pathogen-induced mortality of gypsy moth larvae is largely associated with environmental moisture. Furthermore, the dominance of E. maimaiga over other sources of larval mortality during a gypsy moth population crash suggests that any contributions of pathogens to the synchrony of gypsy moth populations may be more a function of E. maimaiga than LdNPV.
Although precipitation was associated with both E. maimaiga and LdNPV, these relationships varied by month. Entomophaga maimaiga infections were positively associated with precipitation in May, when earlier instars would be present, but negatively associated in March, before egg hatch. This is the second study to show that weather conditions before gypsy moth egg hatch are associated with subsequent E. maimaiga infection levels (Hajek and Tobin 2011). Other studies have also demonstrated repeatedly that when E. maimaiga is active (the fungus is inactive 10 months of the year), infection prevalence is positively related to moisture levels (Hajek 1999; Reilly et al. 2014). In contrast, LdNPV infections were positively associated with precipitation in April and June but not May. Previous studies have documented that LdNPV infections are positively associated with rainfall (D’Amico and Elkinton 1995) and are more prevalent during cold and wet weather (Alalouni et al. 2013). However, in our study, the prevalences of parasitoids and both pathogens were not associated with geographic variation in temperature.
Haynes et al. (2013) found that the spatial proximity of gypsy moth populations did not influence the synchrony of gypsy moth outbreaks after accounting for precipitation, which agrees with the limited dispersal ability in gypsy moth. Dispersal of certain natural enemies, however, may help explain the spatial patterns of mortality in gypsy moth larvae that we observed in this study. We found significant spatial autocorrelation in the prevalence of all three types of natural enemy (Fig. 4), and for E. maimaiga and parasitoids, the spatial lag model results (specifically the significance of spatial lag variables in Table 2) show that there was residual spatial autocorrelation that was not explained by the biotic and environmental predictor variables. We hypothesize that this unexplained spatial autocorrelation in E. maimaiga and parasitoid prevalence may, in part, be due to their dispersal. Conidia of E. maimaiga are actively ejected from larval cadavers after host death, and airborne conidia have been documented in association with temporally lagged environmental moisture (Hajek et al. 1999). Modeling E. maimaiga dispersal, Dwyer et al. (1998) and Weseloh (2003) suggested the occurrence of both shorter- and longer-distance E. maimaiga spread via airborne conidia, e.g., a long-distance spread of 121.5 km in 1 year was estimated. Results from the present study as well as studies by Weseloh (2004) and Tobin and Hajek (2012) suggest that E. maimaiga also has a substantial capacity for localized spread. Although we found spatial autocorrelation in viral prevalence (Fig. 4g), there was no significant residual spatial autocorrelation after accounting for effects of abiotic and biotic characteristics including host density and host density (Table 2). We hypothesize that the lack of unexplained spatial lag in LdNPV infection rates was likely due to limited dispersal by this virus. Although LdNPV does not actively disperse, it can be vectored by ballooning first instars that are infected, and potentially also by parasitoids (Dwyer and Elkinton 1995) and predators (Cunningham 1982). Although the means used to spread are not known, numerous nucleopolyhedroviruses (including LdNPV) have been documented dispersing over larger areas within 1 year (Fuxa 2004). However, gypsy moth is univoltine, with limited numbers of cycles of transmission each year (Woods et al. 1991), which could have reduced LdNPV dispersal in this study compared with nucleopolyhedroviruses infecting multivoltine hosts. Laboratory studies have demonstrated the possibility of very low levels of vertical transmission of LdNPV (Myers et al. 2000; Murray and Elkinton 1989), but because adult females cannot fly, dispersal of infected adult females would not lead to virus dispersal. It is possible that vectoring pathways for LdNPV were not effective during this study, or that virus dispersal occurred but that virus infections were overtaken due to the faster progression of E. maimaiga infections when co-infections occurred (Malakar et al. 1999).
In this large-scale study spanning the US mid-Atlantic region, we found no evidence of spatial density dependence in parasitism rates, but mortality of gypsy moth larvae due to LdNPV and E. maimaiga infection was greater in sites with high densities of larval hosts. Although these results are consistent with prior investigations of LdNPV-induced mortality (Liebhold et al. 2013; McManus and Csóka 2007), previous studies have produced mixed results regarding the density dependence of mortality due to E. maimaiga. In a study conducted on a smaller spatial scale, Liebhold et al. (2013) found that E. maimaiga-induced mortality was density independent across sites and three years. A literature review reported that, while density independence was more common (Hajek 1999), E. maimaiga infection was occasionally positively associated with spatial variation in host density (Weseloh and Andreadis 1992). Among natural enemies that actively search for hosts (e.g., parasitoids), a positive spatial density dependence in attack rates over small spatial scales can result from behavioral aggregation to high-density host populations (Rothman and Darling 1991). A spatial density dependence in attack rates over distances exceeding the dispersal ability of the natural enemy may, however, primarily result from differences in population dynamics (e.g., higher reproduction of natural enemies in areas with high-density host populations) among largely independent local populations (Walde and Murdoch 1988; Rothman and Darling 1991). In our study, the patterns of spatial autocorrelation in the densities of natural enemies (outlined in the previous paragraph) suggest that dispersal of E. maimaiga and parasitoids (but not LdNPV) among nearby sites may have been relatively common. Although E. maimaiga actively disperses, its dispersal is not directed to hosts, thus ruling out behavioral aggregation of this pathogen to high-density host populations. Therefore, the positive spatial density dependence in the prevalences of both LdNPV and E. maimaiga observed here may be due to local density dependence in the transmission of these pathogens. In addition, the ability to detect density dependence in the infection rates of a relatively mobile pathogen such as E. maimaiga may require the quantification of infection rates over large scales. Resolving the conditions under which the transmission of E. maimaiga is density dependent would represent an important advance in understanding the role of this pathogen in gypsy moth population dynamics.
With regard to the potential for competition among mortality agents, the only significant spatial cross-correlation between these agents was a negative association between E. maimaiga and parasitoids. Overall, parasitoids caused much lower rates of mortality than E. maimaiga, and parasitoid-induced mortality was especially low where E. maimaiga was more prevalent. Negative associations between E. maimaiga and gypsy moth parasitoids have also been reported for the parasitoid C. concinnata in North America (Hajek and Tobin 2011) and tachinids in Bulgaria (Georgiev et al. 2013). Negative associations between entomopathogens and parasitoids are not unique to the gypsy moth system (Shapiro-Ilan et al. 2012), but our finding appears to be unique in demonstrating potential competition among natural enemies through negative associations across space.
This study was conducted during the collapse of a regional gypsy moth outbreak. The “upward” phase in gypsy moth outbreaks may be promoted by low oak mast production, which subsequently decreases vertebrate predation on gypsy moth pupae (Elkinton et al. 1996; Liebhold et al. 2012). For the “downward” phase, epizootics of both E. maimaiga and LdNPV are known to drive the collapse of high-density populations, and both pathogens have stages that can remain dormant and thus facilitate their persistence, which is especially important between outbreaks. However, as we have shown, rates of infection by E. maimaiga and LdNPV are strongly influenced by local abiotic and biotic conditions. The infection rates by both pathogens were associated with precipitation and host density, but only LdNPV was affected by physiographic features. The fact that E. maimaiga kills hosts more quickly than LdNPV (Malakar et al. 1999), coupled with greater dispersal by E. maimaiga compared with LdNPV, could explain the apparent replacement of LdNPV by E. maimaiga as the major larval mortality agent during the collapse of gypsy moth outbreaks. Furthermore, the relative insensitivity of E. maimaiga to local physiographic conditions compared to LdNPV may have contributed to the rapid spread since 1989 (Hajek 1999) and consistently high prevalence of E. maimaiga throughout the gypsy moth’s range in North America.
References
Agrios G (2005) Plant pathology. Elsevier/Academic, San Diego
Alalouni U, Schädler M, Brandl R (2013) Natural enemies and environmental factors affecting the population dynamics of the gypsy moth. J Appl Entomol 137:721–738. doi:10.1111/jen.12072
Andreadis TG, Weseloh RM (1990) Discovery of Entomophaga maimaiga in North American gypsy moth, Lymantria dispar. Proc Natl Acad Sci USA 87:2461–2465
Anselin L (1988) Spatial econometrics: methods and models. Kluwer, Dordrecht
Bell RA, Owens CD, Shapiro M, Tardiff JR (1981) Mass rearing and virus production. In: Doane CC, McManus ML (eds) The gypsy moth: research towards integrated pest management. USDA For Serv Tech Bull 1584:599–655
Berryman AA (1999) Principles of population dynamics and their application. Stanley Thornes, Cheltenham
Bjørnstad O, Falck W (2001) Nonparametric spatial covariance functions: estimating and testing. Environ Ecol Stat 8:53–70
Brown JH, Whitham TG, Ernest MSK, Gehring CA (2001) Complex species interactions and the dynamics of ecological systems: long-term experiments. Science 293:643–650
Cameron SA, Lozier JD, Strange JP, Koch JB, Cordes N, Solter LF, Griswold TL (2011) Patterns of widespread decline in North American bumble bees. Proc Natl Acad Sci USA 108:662–667
Campbell RW (1963) The role of disease and desiccation in the population dynamics of the gypsy moth Porthetria dispar (L.) (Lepidoptera: Lymantriidae). Can Entomol 95:426–434
Campbell RW, Podgwaite JD (1971) The disease complex of the gypsy moth: I. Major components. J Invertebr Pathol 18:101–107
Collins JP (2013) History, novelty, and emergence of an infectious amphibian disease. Proc Natl Acad Sci USA 110:9193–9194
Cunningham JC (1982) Field trials with baculoviruses: control of forest insect pests. In: Kurstak E (ed) Microbial and viral pesticides. Marcel Dekker, New York, pp 335–386
Cushman JH, Meentemeyer RK (2008) Multi-scale patterns of human activity and the incidence of an exotic forest pathogen. J Ecol 96:766–776
D’Amico V, Elkinton JS (1995) Rainfall effects on transmission of gypsy moth (Lepidoptera: Lymantriidae) nuclear polyhedrosis virus. Environ Entomol 24:1144–1149
Doane CC (1970) Primary pathogens and their role in the development of an epizootic in the gypsy moth. J Invertebr Pathol 15:21–33
Dwyer G (1994) Density dependence and spatial structure in the dynamics of insect pathogens. Am Nat 143:533–562
Dwyer G, Elkinton JS (1995) Host dispersal and the spatial spread of insect pathogens. Ecology 76:1262–1275
Dwyer G, Elkinton JS, Hajek AE (1998) Spatial scale and the spread of a fungal pathogen of gypsy moth. Am Nat 152:485–494
Dwyer G, Dushoff J, Yee SH (2004) The combined effects of pathogens and predators on insect outbreaks. Nature 430:341–345
Efron B, Tibshirani RJ (1993) An introduction to the bootstrap. Chapman and Hall, London
Elkinton JS, Liebhold AM (1990) Population dynamics of gypsy moth in North America. Annu Rev Entomol 35:571–596
Elkinton JS, Hajek AE, Boettner GH, Simons EE (1991) Distribution and apparent spread of Entomophaga maimaiga (Zygomycetes: Entomophthorales) in gypsy moth (Lepidoptera: Lymantriidae) populations in North America. Environ Entomol 20:1601–1605
Elkinton JS, Healy WM, Buonaccorsi JP, Boettner GH, Hazzard AM, Smith HR, Liebhold AM (1996) Interactions among gypsy moths, white-footed mice, and acorns. Ecology 77:2332–2342
Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ (2012) Emerging fungal threats to animal, plant and ecosystem health. Nature 484:186–194
Frick WF, Pollock JF, Hicks AC, Langwig KE, Reynolds DS, Turner GG, Butchkoski CM, Kunz TH (2010) An emerging disease causes regional population collapse of a common North American bat species. Science 329:679–682
Fuester R, Hajek AE, Schaefer P, Elkinton JS (2013) Biological control of Lymantria dispar. In: Van Driesche RG, Reardon R (eds) The use of classical biological control to preserve forests in North America. FHTET-2013-02. USDA Forest Service, Forest Health Technology Enterprise Team, Morgantown, West Virginia, pp 49–82
Fuxa JR (2004) Ecology of insect nucleopolyhedroviruses. Agric Ecosys Environ 103:27–43
Georgiev G, Hubenov Z, Georgieva M, Mirchev P, Matova M, Solter LF, Pilarska D, Pilarski P (2013) Interactions between the introduced fungal pathogen Entomophaga maimaiga and indigenous tachinid parasitoids of gypsy moth Lymantria dispar in Bulgaria. Phytoparasitica 41:125–131
Gesch DB (2007) The national elevation dataset. In: Maune D (ed) Digital elevation model technologies and applications: the DEM user’s manual, 2nd edn. American Society for Photogrammetry and Remote Sensing, Bethesda, pp 99–118
Gesch D, Oimoen M, Greenlee S, Nelson C, Steuck M, Tyler D (2002) The National Elevation Dataset. Photogramm Eng Remote Sens 68:5–11
Hajek AE (1999) Pathology and epizootiology of the Lepidoptera-specific mycopathogen Entomophaga maimaiga. Microbiol Molec Biol Rev 63:814–835
Hajek AE, Tobin PC (2011) Introduced pathogens follow the invasion front of a spreading alien host. J Anim Ecol 80:1217–1226
Hajek AE, Humber RA, Elkinton JS, May B, Walsh SRA, Silver JC (1990) Allozyme and RFLP analyses confirm Entomophaga maimaiga responsible for 1989 epizootics in North American gypsy moth populations. Proc Natl Acad Sci USA 87:6979–6982
Hajek AE, Humber RA, Elkinton JS (1995) The mysterious origin of Entomophaga maimaiga in North America. Am Entomol 41:31–42
Hajek AE, Elkinton JS, Witcosky JJ (1996) Introduction and spread of the fungal pathogen Entomophaga maimaiga along the leading edge of gypsy moth spread. Environ Entomol 25:1235–1247
Hajek AE, Olsen C, Elkinton JS (1999) Dynamics of airborne conidia of the gypsy moth (Lepidoptera: Lymantriidae) fungal pathogen Entomophaga maimaiga (Zygomycetes: Entomophthorales). Biol Control 16:111–117
Hajek AE, Shimazu M, Knoblauch B (2000) Isolating Entomophaga maimaiga using resting spore-bearing soil. J Invertebr Pathol 75:298–300
Hajek AE, Plymale RC, Reilly JR (2012) Comparing two methods for quantifying soil-borne Entomophaga maimaiga resting spores. J Invertebr Pathol 111:193–195
Haynes KJ, Liebhold AM, Johnson DM (2009) Spatial analysis of harmonic oscillation of gypsy moth outbreak intensity. Oecologia 159:249–256
Haynes KJ, Bjørnstad ON, Allstadt AJ, Liebhold AM (2013) Geographical variation in the spatial synchrony of a forest-defoliating insect: isolation of environmental and spatial drivers. Proc R Soc B 280:20130112. doi:10.1098/rspb.2012.2373
Holt RD, Dobson AP (2006) Extending the principles of community ecology to address the epidemiology of host–pathogen systems. In: Collinge SK, Ray C (eds) Disease ecology: community structure and pathogen dynamics. Oxford University Press, Oxford, pp 6–27
Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P (2008) Global trends in emerging infectious diseases. Nature 451:990–993
Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, Holt RD, Hudson P, Jolles A, Jones KE, Mitchell CE, Myers SS, Bogich T, Ostfeld RS (2010) Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468:647–652
Lafferty KD (2009) The ecology of climate change and infectious diseases. Ecology 90:888–900
Liebhold AM, Thorpe K, Ghent J, Lyons DB (1994) Gypsy moth egg mass sampling for decision-making: a user’s guide. Publication NA-TP-04-94. USDA Forest Service, Morgantown
Liebhold AM, Gottschalk KW, Muzika RM, Montgomery ME, Young R, O’Day K, Kelley B (1995) Suitability of North American tree species to the gypsy moth: a summary of field and laboratory tests. Gen Tech Rpt NE-211. USDA Forest Service, Washington, DC
Liebhold AM, Gottschalk KW, Luzader ER, Mason DA, Bush R, Twardus DB (1997) Gypsy moth in the United States: an atlas. Gen Tech Rpt NE-233. USDA Forest Service, Washington, DC
Liebhold AM, Koenig WD, Bjørnstad ON (2004) Spatial synchrony in population dynamics. Annu Rev Ecol Evol Syst 35:467–490
Liebhold AM, Haynes KJ, Bjørnstad ON (2012) Spatial synchrony of insect outbreaks. In: Barbosa P, Letourneau DK, Agrawal AA (eds) Insect outbreaks revisited. Wiley, Chichester, pp 113–125
Liebhold AM, Plymale R, Elkinton JS, Hajek AE (2013) Emergent fungal entomopathogen does not alter density dependence in a viral competitor. Ecology 94:1217–1222
Malakar R, Elkinton JS, Hajek AE, Burand JP (1999) Within-host interactions of Lymantria dispar L. (Lepidoptera: Lymantriidae) nucleopolyhedrosis virus (LdNPV) and Entomophaga maimaiga (Zygomycetes: Entomophthorales). J Invertebr Pathol 73:91–100
McManus M, Csóka G (2007) History and impact of gypsy moth in North America and comparison to recent outbreaks in Europe. Acta Silvat Lignar Hungar 3:47–64
McNab WH, Cleland DT, Freeouf JA, Keys Jr JE, Nowacki GJ, Carpenter CA (2007) Description of ecological subregions: sections of the conterminous United States. Gen Tech Rpt WO-76B. USDA Forest Service, Washington, DC
Morin Jr RS, Liebhold AM, Luzader ER, Lister AJ, Gottschalk KW, Twardus DB (2005) Mapping host-species abundance of three major exotic forest pests. Res Pap NE-726. USDA Forest Service, Washington, DC
Murray K, Elkinton J (1989) Environmental contamination of egg masses as a major component of transgenerational transmission of gypsy moth nuclear polyhedrosis virus (LdMNPV). J Invertebr Pathol 19:662–665
Myers JH, Corey JS (2013) Population cycles in forest Lepidoptera revisited. Annu Rev Ecol Evol Syst 44:565–592
Myers JH, Malakar R, Cory JS (2000) Sublethal nucleopolyhedrovirus infection effects on female pupal weight, egg mass size, and vertical transmission in gypsy moth (Lepidoptera: Lymantriidae). Environ Entomol 29:1268–1272
Nielsen C, Milgroom MG, Hajek AE (2005) Genetic diversity in the gypsy moth fungal pathogen Entomophaga maimaiga from founder populations in North America and source populations in Asia. Mycol Res 109:941–950
Peltonen M, Liebhold AM, Bjørnstad ON, Williams DW (2002) Spatial synchrony in forest insect outbreaks: roles of regional stochasticity and dispersal. Ecology 83:3120–3129
PRISM Climate Group (2011) PRISM climate data. PRISM Climate Group, Oregon State University, Corvallis. http://prism.oregonstate.edu, created 10 October 2011
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reilly JR, Hajek AE, Liebhold AM, Plymale R (2014) The impact of Entomophaga maimaiga on outbreak gypsy moth populations: the role of weather. Environ Entomol 43:632–641
Rothman LD, Darling DC (1991) Spatial density dependence: effects of scale, host spatial pattern and parasitoid reproductive strategy. Oikos 62:221–230
Royama T (1992) Analytical population dynamics, 1st edn. Chapman and Hall, London
Sacks BN, Woodward DL, Colwell AE (2003) A long-term study of non-native-heartworm transmission among coyotes in a Mediterranean ecosystem. Oikos 102:478–490
Shapiro-Ilan DI, Bruck DJ, Lacey LA (2012) Principles of epizootiology and microbial control. In: Vega FE, Kaya HK (eds) Insect pathology, 2nd edn. Elsevier, Amsterdam, pp 29–72
Simons EE, Reardon RC, Ticehurst M (1974) Selected parasites and hyperparasites of the gypsy moth, with keys to adults and immatures. Handbook 540. USDA, Washington, DC
Tobin PC, Hajek AE (2012) Release, establishment, and initial spread of the fungal pathogen Entomophaga maimaiga in island populations of Lymantria dispar. Biol Control 63:31–39
Tobin PC, Bai BB, Eggen DA, Leonard DS (2012) The ecology, geopolitics, and economics of managing Lymantria dispar in the United States. Int J Pest Manag 53:195–210
Tobin PC, Gray DR, Liebhold AM (2014) Supraoptimal temperatures influence the range dynamics of a non-native insect. Divers Distrib 20:813–823
Turchin P (2003) Complex population dynamics: a theoretical/empirical synthesis. Princeton University Press, Princeton
USDA Forest Service (2013a) Gypsy moth digest. http://na.fs.fed.us/fhp/gm/. Accessed 28 May 2010
USDA Forest Service (2013b) Insect and disease detection surveys. http://www.fs.fed.us/foresthealth/technology/adsm.shtml. Accessed 28 September 2010
USDA Natural Resources Conservation Service (2012) Soil survey geographic (SSURGO) database. http://soildatamart.nrcs.usda.gov. Accessed 28 May 2010
Walde SJ, Murdoch WW (1988) Spatial density dependence in parasitoids. Annu Rev Entomol 33:441–466
Weseloh RM (2003) Short and long range dispersal in the gypsy moth (Lepidoptera: Lymantriidae) fungal pathogen, Entomophaga maimaiga (Zygomycetes: Entomophthorales). Environ Entomol 32:111–122
Weseloh RM (2004) Effect of conidial dispersal of the fungal pathogen Entomophaga maimaiga (Zygomycetes: Entomophthorales) on survival of its gypsy moth (Lepidoptera: Lymantriidae) host. Biol Control 29:138–144
Weseloh RM, Andreadis TG (1992) Epizootiology of the fungus Entomophaga maimaiga and its impact on gypsy moth populations. J Invertebr Pathol 59:133–141
Woods SA, Elkinton JS, Murray KD, Liebhold AM, Gould JR, Podgwaite JD (1991) Transmission dynamics of a nuclear polyhedrosis virus and predicting mortality in gypsy moth (Lepidoptera: Lymantriidae) populations. J Econ Entomol 84:423–430
Acknowledgments
We thank R. Rabaglia and R. Reardon (USDA Forest Service) for helping to conceive, assist, and design this study. This study was only possible due to extensive assistance from R. Tatman (MD Department of Agriculture), T. Marasco (PA Bureau of Forestry), C. Asaro (VA Department of Forestry), and R. Turcotte (USDA Forest Service, Morgantown, WV) and their teams. At Cornell, samples were diagnosed and data were collated by R. Plymale, S. Long, K. Ciccaglione, C. Fritzen, M. Garvey, M. Grambor, T. James, A. Navarro, and J. Tyvoll, and J. Nix counted resting spore densities in soil samples. We thank L. Blackburn (USDA Forest Service) and J. Walter (University of Virginia) for technical assistance, and A. Liebhold and two anonymous reviewers for comments on the manuscript. This study was funded by USDA Forest Service, Forest Health Protection USDA Forest Service grant #07-CA-11420004-152.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Wolf M. Mooij.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hajek, A.E., Tobin, P.C. & Haynes, K.J. Replacement of a dominant viral pathogen by a fungal pathogen does not alter the collapse of a regional forest insect outbreak. Oecologia 177, 785–797 (2015). https://doi.org/10.1007/s00442-014-3164-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-3164-7