Abstract
Top predators are declining globally, in turn allowing populations of smaller predators, or mesopredators, to increase and potentially have negative effects on biodiversity. However, detection of interactions among sympatric predators can be complicated by fluctuations in the background availability of resources in the environment, which may modify both the numbers of predators and the strengths of their interactions. Here, we first present a conceptual framework that predicts how top-down and bottom-up interactions may regulate sympatric predator populations in environments that experience resource pulses. We then test it using 2 years of remote-camera trapping data to uncover spatial and temporal interactions between a top predator, the dingo Canis dingo, and the mesopredatory European red fox Vulpes vulpes and feral cat Felis catus, during population booms, declines and busts in numbers of their prey in a model desert system. We found that dingoes predictably suppress abundances of the mesopredators and that the effects are strongest during declines and busts in prey numbers. Given that resource pulses are usually driven by large yet infrequent rains, we conclude that top predators like the dingo provide net benefits to prey populations by suppressing mesopredators during prolonged bust periods when prey populations are low and potentially vulnerable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dominant, or top predators, are important in structuring many ecological communities and maintaining biodiversity in lower trophic levels (Johnson et al. 2007). One extensively studied process that drives this pattern is the depredation of herbivore populations; this in turn reduces impacts on primary producers (Ripple and Beschta 2012). Another, more recently studied, process occurs when top predators regulate populations of smaller predators, or mesopredators, and in turn reduce their impacts on small prey (Ritchie et al. 2012). As top-predator populations have declined around the world due to habitat destruction and persecution from humans, populations of mesopredators have increased, often with negative effects on diversity (Estes et al. 2011; Ripple et al. 2014). The mesopredator-release hypothesis predicts that, if present, dominant predators will suppress their subordinate counterparts but, if removed, the mesopredators will be ‘released’ from competition or direct interference; increases in their numbers then may lead to increased impacts on small prey (Prugh et al. 2009).
Despite this simple theoretical framework, confirmation of the mechanisms that underpin top- and mesopredator relationships is often difficult to obtain. This is because predator densities are often low and interactions are consequently difficult to observe, and because interactions between top and subordinate predators may not be constant. In particular, bottom-up processes such as the productivity of prey populations often vary over time, and will potentially influence the timing and strength of predator relationships (Elmhagen and Rushton 2007). Understanding how bottom-up processes affect interactions among predators is a central question in ecology, as limited empirical evidence is sometimes used to argue both for and against reintroductions of top predators or even to avoid preserving them (Fleming et al. 2012; Johnson et al. 2013). The effects of fluctuating productivity may be most profound and pervasive in environments that experience large yet short-lived resource pulses, as these pulses can allow prey populations temporarily to escape predator regulation and also decouple interactions between the predators (Letnic and Dickman 2010). Resource-pulse environments are globally widespread (Yang et al. 2010), but are most conspicuous in deserts and rangelands. These environments also are frequently arenas for conflict between shepherds and their livestock and top predators.
Environments that experience resource pulses from rapid increases in primary productivity, such as from flood rains, show dramatic ‘booms’ and ‘busts’ in populations of primary consumer organisms (Caughley 1987; Dickman et al. 1999, 2010; Greenville et al. 2013). Even though resource pulses may be infrequent, many consumers efficiently exploit them; for example, populations of some mammals increase 10–60 fold within 6 months via elevated reproduction and immigration (Dickman et al. 1999, 2010). This produces different phases in their population cycles, with long busts, or periods of low numbers, and short-lived booms followed by rapid declines (Fig. 1). The decline phase is of particular interest for prey populations as it can be associated with high per capita predation because of a lag in the numerical response of the predators (Spencer et al. 2014), which often peak during the decline phase (e.g. Fig S1). By contrast, the increase phase of small mammals occurs quickly and inevitably when predator numbers are low; per capita predation then is likely to be low and to have little or no effect on prey populations. Arid environments, covering 40 % of the global land area (Ward 2009), often exhibit such dynamics in consumer populations due to rainfall unreliability (Dickman et al. 2010; Van Etten 2009).
Population dynamics of prey in resource-pulse environments. ‘Booms’, or population irruptions, arise from large resource pulses that are stimulated by events such as flooding rainfall. ‘Declines’ represent subsequent, sometimes brief, periods of negative increase, while ‘busts’ are often long periods when resources and prey numbers are relatively low
Here, we firstly outline a conceptual framework that predicts how predator interactions vary in resource-pulse environments, and then present an empirical test of the predictions using predators and prey in central Australia as a model system. For simplicity, we depict relationships between predators as being linear, but note that true relationships could take other forms. We assume that dominant predators exert their effects via interference competition or intraguild killing (Moseby et al. 2012), or by driving partitioning of space and time, and use changes in prey populations as a measure of bottom-up processes.
Conceptual models
Dominant–subordinate predator interactions can be influenced by top-down or bottom-up processes, or both processes together, in resource-pulse environments. If top-down processes are most influential, then the top predator will have a negative effect on subordinate predators, regardless of the prey population phase (boom, bust or decline) (Fig. 2a). For example, wolves (Canis lupus) generally suppress coyote (Canis latrans) numbers across different habitat types due to interference competition (Fedriani et al. 2000; Merkle et al. 2009). A scenario based on only bottom-up processes will result in both top- and subordinate predators increasing (or decreasing) together, as the prey base booms or declines (Fig. 2b). Finally, predator populations may be structured by both top-down and bottom-up processes (Elmhagen and Rushton 2007). As resource pulses drive an irruption in the prey base (Fig. 1), populations of both dominant and subordinate predators will increase; however, in the bust and decline phases of prey populations when prey is scarce, the dominant predator will likely have negative effects on subordinate predators owing to increased levels of competition or direct intraguild killing (Fig. 2c).
Conceptual models of dominant and subordinate predator interactions in resource-pulse environments. Three processes are possible: a top-down only, b bottom-up only, and c both top down and bottom up. Linear relationships are shown for simplicity, but non-linear relationships may be possible. Scale represents abundance
Our conceptual models aim to capture the major effects of dominant on subordinate predators in resource-pulse environments, but do not preclude the possibility that other interactions might occur. For example, interactions are likely between predators and other species in most ecological systems, and interaction strengths may vary depending on which species co-occur (Billick and Case 1994). In addition, the influence of bottom-up effects may change over time, with decreases in prey availability arising due to behavioural changes (e.g. increased predator avoidance), habitat changes (e.g. increases in cover due to plant growth after a resource pulse), or changes in resources other than prey (Power 1992). However, while such effects may modify the slopes or shapes of our conceptual models, we assume that their form is robust owing to the strong interference relationships that characterize most interactions between predator species (Palomares and Caro 1999).
Case study
Central Australia has experienced widespread extinctions of its mammal fauna (Smith and Quin 1996) and now the most abundant mammalian predators are the dingo (Canis dingo; body mass 15–25 kg) (Crowther et al. 2014), invasive red fox (Vulpes vulpes; 3.5–7.5 kg) and feral cat (Felis catus; 3.8–4.4 kg). The dingo is the continent’s mammalian top predator and thought to be important in structuring populations of both mesopredators, red fox and feral cat; strong suppressive effects have been documented on populations of the fox, and weaker effects on the feral cat (Brook et al. 2012; Letnic et al. 2011, 2012; Letnic and Koch 2010). All three predators have high dietary overlap in central Australia, with rodents often representing 70–95 % by occurrence in their respective diets, and there is also some evidence of direct killing by dingoes of red foxes and feral cats (Cupples et al. 2011; Mahon 1999; Moseby et al. 2012; Pavey et al. 2008). Interactions between the red fox and feral cat are largely unknown. However, high dietary overlap and evidence of predation on feral cats by the red fox suggest that interference competition and perhaps intraguild predation may take place (Molsher 1999; Paltridge 2002).
Flooding rains in 2010 stimulated a resource pulse across central Australia and resulted in dramatic irruptions of small rodents in 2011 (Greenville et al. 2012, 2013). Predator populations also increased in the wake of this resource pulse; their populations usually respond within 12 months of flood rains (Letnic and Dickman 2006; Pavey et al. 2008). Here, we track the activity of dingoes, foxes and cats from the pre-irruption bust phase, through the rodent prey pulse, the subsequent decline and into the next bust period to test our conceptual models (Fig. 2a–c). Because of the cryptic nature of the predators, we use 2 years of data obtained from remote cameras to track temporal changes in their populations (Vine et al. 2009). If top-down forces predominate, we expect that dingoes will exert suppressive effects at all times (Fig. 2a). If bottom-up forces predominate, predators should respond only to the abundance of their prey (Fig. 2b). If top-down and bottom-up processes are both important in shaping the activity or demographic performance of the sympatric mesopredators (Fig. 2c), we predict that:
-
1.
Top-down forcing of dingoes on mesopredators (red fox and feral cat) will be manifest only in the bust and decline phases of the predators’ shared prey.
-
2.
Populations of the mesopredators will increase from bottom-up processes, with the red fox increasing more than the feral cat if it is the more dominant of the two.
In addition to these population-level interactions, we predict that the red fox and feral cat will have different daily activity times to the dominant dingo to avoid potential encounters, and that the daily activity of the dingo will coincide most closely with that of its rodent prey.
Materials and methods
Study region
The study region occupies 8,000 km2 in the north-eastern Simpson Desert in central Australia, and covers three properties: Ethabuka Reserve, Carlo Station and Cravens Peak Reserve. Dune fields comprise 73 % of the region, with smaller areas consisting of clay pans, rocky outcrops and gibber flats. Sand dunes run parallel in a north–south direction aligned with the prevailing southerly wind. Dunes typically reach 10 m in height and are 0.6–1 km apart (Dickman et al. 2010). Vegetation in the interdune swales and on dune sides is predominantly spinifex grassland (Triodia basedowii) with small stands of gidgee trees (Acacia georginae), woody Acacia shrubs or mallee eucalypts; low-lying clay pans fill with water temporarily after heavy rain.
During summer, daily temperatures usually exceed 40 °C and minima in winter fall below 5 °C (Dickman et al. 2010). Highest rainfall occurs in summer, but heavy rains can fall locally or regionally at any time in the year. Long-term weather stations in the study area are at Glenormiston (1890–2011), Boulia (1888–2011) and Birdsville (1954–2011), and have median annual rainfalls of 186 mm (n = 122 years), 216.2 mm (n = 124 years), and 153.1 mm (n = 58 years), respectively (Greenville et al. 2012).
Remote cameras
To survey the predators and their rodent prey, we placed 25 remote cameras (24 Moultrie i40 and one Reconyx RapidFire) 1–10 km apart next to access tracks in spinifex habitat in the interdune swales, as animals—especially the predators—frequently use these tracks (Mahon et al. 1998). Two years of continuous monitoring were required to capture any lag (up to 12 months, see above) in changes in predator numbers following irruptions of prey (Fig. S1). Cameras were mounted atop 1.5-m-high metal stakes, and angled at ~10° so the field of view covered the track; their locations were assumed to be independent (spatial autocorrelation, Moran’s I—dingo I = 0.15, P = 0.22; red fox I = 0.31, P = 0.14; feral cat I = 0.33, P = 0.06). Cameras were active from April 2010 to April 2012 and downloaded 3–4 times a year. Each photograph was tagged with the site name, camera identification number, download trip, moon phase, species and number of individuals recorded, and the tags written to the exif data of each file (jpeg) using EXIFPro 2.0 (Kowalski and Kowalski 2012). EXIFPro 2.0 was used to database the photographs and export the exif data as a text file for analysis. To ensure independence, a delay of 1 min was programmed on-camera between each trigger, and multiple photographs of the same presumptive individual (photographs taken <2 min apart) were removed prior to analysis. This resulted in a total of at least 3 min between photographs. Histograms were inspected for each species to confirm that this was an appropriate breakpoint (Fig. S2).
The major prey of the three predator species, rodents, irrupted across all sites in the study region after flood rains in 2010 (Greenville et al. 2012, 2013). Population phases were defined as bust (April–December 2010), boom (January–September 2011) and decline (October 2011–April 2012) from inspection of camera-trapping records (Fig. S3) and also from a concurrent live-trapping study (see Greenville et al. 2012), as small mammals may have lower detection rates compared to the larger predators. The numbers of photographs for each species (dingo, red fox, feral cat and rodents—all species combined) were pooled for each of these phases.
Data analyses
To test our predictions, we used negative binomial generalised linear models to compare numbers of photographs of the three predators in the bust, boom and decline phases, and the predator × phase interaction. The negative binomial distribution was chosen instead of the Poisson distribution as the count data showed signs of over-dispersion (Zuur 2009). Due to camera failures, wildfire or memory cards filling up before downloads, cameras sometimes had different sampling effort. To calculate sampling effort per camera, we recorded every day each camera was active over the 2-year period. Summing these records yielded a total of 10,260 active camera days and nights. An offset for number of days each camera was active was used in our models to account for unequal camera effort. Analysis of deviance was used to test the effects and followed a χ2 distribution (Zuur 2009). Analyses used MASS 7.3–16 package (Venables and Ripley 2002), in R 2.14.1 (R Core Team 2013).
To detect differences in activity times of the dingo, the mesopredators and prey (rodents), the time stamps for photographs of each predator and for rodents were pooled for each phase. Activity times followed a circular distribution over 24 h. To test for differences in activity times of predators within and between phases, and for any coincidence of activity times between the predators and prey, we used ANOVAs with a likelihood ratio test that followed the χ2 distribution (Cordeiro et al. 1994). To estimate confidence intervals for mean activity times, bootstrapping with 1,000 replicates was used. Analysis used the Circular 0.4–3 package (Agostinelli and Lund 2013) in R 2.14.1 (R Core Team 2013).
Results
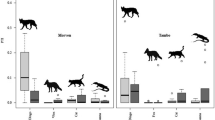
In total, 1,247 independent photographs of the three predator species were captured over the period of study, and comprised 626 images of feral cats, 238 of dingoes, 383 of red foxes, and 483 of rodents. Four species of rodents were identified (long-haired rat Rattus villosissimus, sandy inland mouse Pseudomys hermannsburgensis, spinifex hopping mouse Notomys alexis and house mouse Mus musculus) and their images pooled. In general, photographs of rodents showed the presence of multiple individuals and photographs of predators had only one individual, but each photograph was considered an event. There was a significant interaction between the dingo and population phase of prey with the number of photographic records of the two species of mesopredators (Tables 1, S1). In the boom phase, records of the dingo and red fox were positively associated, but in the bust and decline phases records of the fox decreased with increasing dingo activity (Fig. 3a). In contrast, as dingoes increased, there was a decrease in the number of feral cat records in all phases (Fig. 3b). The red fox and feral cat increased together in all phases, with cats increasing at a progressively faster rate during bust conditions (Fig. 3c). To assess the effect of the outliers in Fig. 3, they were removed and the tests re-run. Both analyses yielded significant results, thus indicating that the outliers were not unduly important in driving the observed relationships (Table S2).
Interactions between prey population phase (bust, boom and decline) and the effects of dominant predators on subordinate predators: a dingo–red fox, b dingo–feral cat, and c red fox–feral cat, represented by numbers of photographic images from 2 years of continuous remote-camera trapping in the Simpson Desert, central Australia. Log numbers are shown on the y-axis. Solid and dashed lines are predicted values from a generalised linear models
Mean daily activity times (24 h clock, 95 % confidence intervals) for dingoes, red foxes and feral cats in the bust phase were 02:12 (0:32–03:50), 23:23 (22:31–0:17) and 23:59 (23:16–0:44), respectively (Fig. 4a). For the boom phase, respective mean activity times were 02:03 (0:58–03:11), 23:42 (22:49–0:38) and 0:18 (23:46–0:52) (Fig. 4b) and for the decline phase 02:47 (01:56–03:38), 0:40 (23:47–01:29) and 0:52 (0:11–01:36) (Fig. 4c). Daily activity times differed between the three predator species in the bust (χ 2 = 12.95, P = 0.002, df = 2), boom (χ 2 = 12.22, P = 0.002, df = 2) and decline phases (χ 2 = 14.06, P = 0.001, df = 2), but there was no difference in each predator’s activity time between phases: dingo (χ 2 = 1.12, P = 0.57, df = 2), red fox (χ 2 = 4.09, P = 0.13, df = 2), and feral cat (χ 2 = 3.71, P = 0.16, df = 2). The red fox showed some activity by day in the boom and decline phases compared to the bust, the dingo showed some daytime activity in all phases, but feral cats were entirely nocturnal (Fig. 4a–c).
Daily activity patterns of the dingo, red fox, feral cat and rodents (prey) during a bust, b boom and c decline phases of the prey population, Simpson Desert, central Australia. Arrows represent mean activity times and grey boxes frequency (square-root) of observations recorded from 2 years of continuous remote-camera trapping
Rodents were active only at night, with mean activity times (24 h clock, 95 % confidence intervals) for bust, boom and decline population phases being 23:36 (22:09–0:59), 23:31 (23:07–23:58) and 0:46 (0:11–01:17), respectively. Activity times differed between phases (χ 2 = 9.209, P = 0.01, df = 2), shifting to early morning in the decline phase (Fig. 4a–c). There was no difference between the mean activity times of the red fox and rodents for any population phase (bust χ 2 = 0.08, P = 0.8, df = 1; boom χ 2 = 0.17, P = 0.7, df = 1; decline χ 2 = 0.04, P = 0.8, df = 1). The activity of feral cats coincided with that of rodents during the bust (χ 2 = 0.27, P = 0.3, df = 1) and decline phases (χ 2 = 0.05, P = 0.8, df = 1), but not in the boom phase (χ 2 = 5.8, P = 0.02, df = 1). Dingoes differed in mean activity time from rodents in all population phases (bust χ 2 = 5.6, P = 0.02, df = 1; boom χ 2 = 22.6, P < 0.001, df = 1; decline χ 2 = 14.7, P < 0.001, df = 1).
Discussion
The results broadly support our initial predictions, and show that interactions between dominant and subordinate predators can shift markedly during the course of a bottom-up resource pulse. Bottom-up processes are important in structuring populations of sympatric predators in other systems (Elmhagen and Rushton 2007; Jaksic et al. 1997; White 2008), suggesting that such effects may be general. In the present study, top-predator suppression was most obvious in the consistently negative relationship between the dingo and feral cat, but was evident also in the negative relationship between dingoes and foxes in the bust and decline phases of the rodent populations. As predicted for this latter pair, foxes evidently escaped suppression by dingoes only during boom conditions when rodents were very abundant. Although the relationship between fox and cat populations was positive in each of the three population phases of their prey, the relatively greater increase in cat numbers during bust conditions when foxes were scarce is consistent with the interpretation that the cat is the subordinate predator in the desert system. We interpret shifts in pairwise interactions between the predators below.
Top-down forcing of foxes by dingoes was most obvious during periods of rodent decline and bust. Competition and intraguild killing of subordinate by dominant predators are most likely when prey is scarce (Donadio and Buskirk 2006), and suggest that top-down processes may generally dominate at these times. In an instructive recent study, however, Moseby et al. (2012) showed that dingoes kill but do not eat foxes upon encounter with them, suggesting that the interaction is one of extreme interference competition. If this is correct, it is relevant to ask why dingoes do not maintain top-down forcing of foxes during prey irruptions.
In the first instance, while dingoes and foxes often show large overlaps in diet, dingoes hunt and kill larger prey if these are available (Cupples et al. 2011; Spencer et al. 2014). If larger prey, such as kangaroos, disperse after rain and become available to dingoes, this may reduce competition for shared small prey between the two canid species and allow foxes to escape suppression. While possible, this explanation seems unlikely. Kangaroos and other large potential prey usually show limited responses even to large pulses of primary productivity in spinifex grasslands and remain present but at consistently low density (Letnic and Dickman 2006); the dominant vertebrates are small mammals, reptiles and birds. Populations of some reptile species respond after rainfall, although breeding takes place only during the austral spring and summer, and sexual maturity occurs after 11–12 months (Greenville and Dickman 2005; James 1991). Thus reptile populations respond slowly to rainfall events compared to rodent populations. Many species of birds exhibit population irruptions after large rainfall events (Tischler et al. 2013), but this prey type represents only 6–12 % of the diets of the three predators (Cupples et al. 2011; Pavey et al. 2008).
Secondly, the negative correlation between dingoes and the smaller predators may be due to the cat and red fox responding more quickly to increases and decreases in prey compared to the dingo, while the dingo switches to larger alternative prey as small mammals become scarce. However, the red fox is usually scarce in the study region and moves into the sand dune environment from more mesic areas after large rainfall events (Letnic and Dickman 2006). Thus the arrival and subsequent breeding of red foxes corresponds to the boom and decline phases of the rodents. Cats are persistent in the region but, as kangaroos and larger prey species show limited population responses to rainfall in the study region, prey switching by dingoes does not seem likely.
Thirdly, rain-stimulated increases in primary productivity lead to increased cover of ground-level vegetation in the study system (Dickman et al. 1999), and this in turn may provide foxes with sufficient shelter to escape detection by dingoes. Fourthly, and perhaps most plausibly, bottom-up pulses of productivity may allow such large increases in rodent populations that the benefits of ready access to abundant food for dingoes exceed the costs of interference. In arid Australia, rapid 10- to 60-fold increases in rodent populations occur after rain (Dickman et al. 1999, 2010) and these alone may be enough to reduce top-down effects, at least between the dominant and subordinate canid predators. Elsewhere in arid Australia, Lundie-Jenkins et al. (1993) found that competition between dingoes and mesopredators was alleviated during irruptions of rodents and locusts.
In contrast to the temporal shifts in interactions between the canid predators, dingoes suppressed populations of feral cats in all phases of the prey boom and bust cycle, suggesting that top-down effects generally predominate. As the smallest predators in the study system, cats could be expected to be subordinate to both the dingo and the fox. Dingoes kill cats quickly if they encounter them (Moseby et al. 2012), and dietary evidence confirms that foxes also may kill and eat cats, albeit infrequently (Molsher 1999; Paltridge 2002). Despite this, the significant phase interaction in the comparison of cat and dingo abundance suggests that cats may experience some alleviation in top-down forcing during boom compared to bust periods (Fig. 3b). If cats share rodent prey with both canids and also must avoid encounters with foxes in dingo-free space, this could explain why cats may alleviate top-down forcing during boom periods but not escape it altogether. The increase in cat abundance relative to that of the fox during bust periods, when this canid predator is often very scarce (Fig. 3c), suggests further that cats may escape suppression by foxes but not by dingoes during these times.
We also expected the predators to alter their times of daily activity in relation to each other and to their rodent prey, and our results here provide more insight into the interactions we observed. In the first instance, irrespective of the population phase of the rodents, the red fox and feral cat showed peak activity around the middle of the night some 2 h before peak activity of the dingo. Although all three predator species showed some activity during all the hours of darkness, this staggering in activity time between the mesopredators and the top predator would potentially reduce the likelihood of encounters (Brook et al. 2012). The lack of difference in activity times of the fox and cat is harder to reconcile if a dominant-subordinate relationship exists between them. During the boom phase, however, coinciding activity with the peak activity times of prey would likely yield energetic benefits that offset the risks of mutual encounter. A similar explanation holds for the lack of temporal partitioning among the large carnivores of Africa (Cozzi et al. 2012). During the decline and bust phases, when prey populations are low, it is possible that the two mesopredators were active at the same times but in different places, but insufficient observations were obtained across all the camera stations to test this.
We had expected also that the daily activity of the dingo would coincide most closely with that of its rodent prey, but this expectation was not met. Instead, fox, and then cat, activity showed the strongest coincidence in timing with the activity of rodents, with mean dingo activity peaking at least 2 h after the equivalent peak for rodents. For foxes and cats the benefits of focussing their activity when prey is most active may, as noted, exceed the potential risks of occasional mutual encounter. For the dingo, however, the relative delay in activity may suggest that, while it spends some time foraging for rodents, it has exclusive access to larger albeit rarer prey, such as kangaroos, that are easier to hunt at or just after dawn. Two pieces of evidence support this interpretation. Firstly, the dingo was more active during the early hours of daylight than the smaller predators (Fig. 4). Secondly, large mammals, especially red kangaroos, comprise 23–44 % of the diet of the dingo by frequency of occurrence in the study area, compared to just 3–4 % of the diet of the fox (Cupples et al. 2011; Spencer et al. 2014). By contrast, the respective representation of small mammals in the diets of these predators is 54 and 86 % (Cupples et al. 2011).
Our observations are broadly consistent with the idea that top-down forcing constrains populations of mesopredators in our study system, and that this is most obvious during periods of low prey activity. However, alternative possibilities need to be addressed before any further implications can be drawn. Most importantly, as this is an observational study, we cannot be certain that we are uncovering causal relationships between the predators. For example, changes in plant cover between boom and bust phases of prey may provide the mesopredators with different food or cover resources at these times. An increase in plant cover during the boom phase is the most obvious of these changes but, as noted above, does not readily explain why only foxes may exploit it to escape top-down suppression. There are, alternatively, other predators in the study system; these potentially could introduce indirect effects that mask the relationships between the dingo and the two smaller mammalian predators (Billick and Case 1994). For example, the goannas Varanus giganteus and Varanus gouldii and wedge-tailed eagle Aquila audax hunt rodents and larger prey (Aumann 2001; Pianka et al. 2004). However, all these other predators are diurnal (Aumann 2001; Pianka et al. 2004) and probably do not interact in any direct way with the three largely nocturnal mammalian predators. Habitat shifts by the dingo across the prey phases may lead to negative associations between dingoes and the mesopredators. For example, in the rodent bust phase, populations of the red fox and cat decline, but dingoes may be able to shift their foraging to alternative habitats with larger prey. Further research is required to test this possibility. In view of the high dietary overlap of the mammalian predators in our study system (Cupples et al. 2011; Mahon 1999), the extreme interference effects of dingoes on the two mesopredators (Moseby et al. 2012), and evidence of top-down suppression by dingoes in other habitats (Colman et al. 2014; Johnson and VanDerWal 2009; Letnic et al. 2011; Letnic and Koch 2010), it seems reasonable to conclude that our camera-based observations reflect real shifts in top predator-mesopredator interactions.
In summary, our results suggest that, in the resource-pulse conditions that characterize much of the Australian environment, mesopredator suppression by dingoes is not constant but is most marked in the bust and decline phases of prey populations (Fig. 5). The presence of the top predator may provide particularly strong net benefits to small native prey species at these times when they are at low numbers and perhaps at their most vulnerable to additive predator impacts and stochastic extinction risk (Sinclair et al. 1998). Given that resource pulses are often infrequent and ephemeral, as they are when driven by flood rains in arid and semi-arid environments, we suggest that top predators like the dingo will generally benefit small prey by suppressing mesopredators during the prolonged bust periods when prey populations are low.
Complex dominant-subordinate predator interactions in a the boom phase and b the bust or decline phases of their prey. Line weights represent relative interaction strengths. Dashed lines represent indirect interactions. This model predicts that in times when the prey population is most vulnerable to predation from the mesopredators (bust and decline phases), the top predator has a net positive effect on prey
References
Agostinelli C, Lund U (2013) R package ‘circular’: circular statistics (version 0.4–3). https://r-forge.r-project.org/projects/circular/
Aumann T (2001) Habitat use, temporal activity patterns and foraging behaviour of raptors in the south-west of the Northern Territory, Australia. Wildl Res 28:365–378
Billick I, Case TJ (1994) Higher order interactions in ecological communities: what are they and how can they be detected? Ecology 75:1529–1543
Brook LA, Johnson CN, Ritchie EG (2012) Effects of predator control on behaviour of an apex predator and indirect consequences for mesopredator suppression. J Appl Ecol 49:1278–1286
Caughley G (1987) Ecological relationships. In: Caughley G, Shepherd N, Short J (eds) Kangaroos: their ecology and management in the sheep rangelands of Australia. Cambridge University Press, New York, pp 159–187
Colman NJ, Gordon CE, Crowther MS, Letnic M (2014) Lethal control of an apex predator has unintended cascading effects on forest mammal assemblages. Proc R Soc B: Biol Sci 281
Cordeiro G, Paula G, Botter D (1994) Improved likelihood ratio tests for dispersion models. Int Stat Rev 62:257–274
Cozzi G, Broekhuis F, McNutt JW, Turnbull LA, Macdonald DW, Schmid B (2012) Fear of the dark or dinner by moonlight? Reduced temporal partitioning among Africa’s large carnivores. Ecology 93:2590–2599
Crowther MS, Fillios M, Colman N, Letnic M (2014) An updated description of the Australian dingo (Canis dingo Meyer, 1793). J Zool (in press)
Cupples JB, Crowther MS, Story G, Letnic M (2011) Dietary overlap and prey selectivity among sympatric carnivores: could dingoes suppress foxes through competition for prey? J Mammal 92:590–600
Dickman CR, Mahon PS, Masters P, Gibson DF (1999) Long-term dynamics of rodent populations in arid Australia: the influence of rainfall. Wildl Res 26:389–403
Dickman CR, Greenville AC, Beh C-L, Tamayo B, Wardle GM (2010) Social organization and movements of desert rodents during population “booms” and “busts” in central Australia. J Mammal 91:798–810
Donadio E, Buskirk SW (2006) Diet, morphology, and interspecific killing in carnivora. Am Nat 167:524–536
Elmhagen B, Rushton SP (2007) Trophic control of mesopredators in terrestrial ecosystems: top-down or bottom-up? Ecol Lett 10:197–206
Estes JA et al (2011) Trophic downgrading of planet Earth. Science 333:301–306
Fedriani JM, Fuller TK, Sauvajot RM, York EC (2000) Competition and intraguild predation among three sympatric Carnivores. Oecologia 125:258–270
Fleming PJS, Allen BL, Ballard G-A (2012) Seven considerations about dingoes as biodiversity engineers: the socioecological niches of dogs in Australia. Aust Mammal 34:119–131
Greenville AC, Dickman CR (2005) The ecology of Lerista labialis (Scincidae) in the Simpson Desert: reproduction and diet. J Arid Environ 60:611–625
Greenville AC, Wardle GM, Dickman CR (2012) Extreme climatic events drive mammal irruptions: regression analysis of 100-year trends in desert rainfall and temperature. Ecol Evol 2:2645–2658
Greenville AC, Wardle GM, Dickman CR (2013) Extreme rainfall events predict irruptions of rat plagues in central Australia. Aust Ecol 38:754–764
Jaksic FM, Silva SI, Meserve PL, Gutierrez JR (1997) A long-term study of vertebrate predator responses to an El Nino (ENSO) disturbance in western South America. Oikos 78:341–354
James CD (1991) Annual variation in reproductive cycles of scincid lizards (Ctenotus) in Central Australia. Copeia 1991:744–760
Johnson CN, VanDerWal J (2009) Evidence that dingoes limit abundance of a mesopredator in eastern Australian forests. J Appl Ecol 46:641–646
Johnson CN, Isaac JL, Fisher DO (2007) Rarity of a top predator triggers continent-wide collapse of mammal prey: dingoes and marsupials in Australia. Proc R Soc B: Biol Sci 274:341–346
Johnson CN, Ritchie EG et al (2013) The dingo and biodiversity conservation: response to Fleming et al. (2012). Aust Mammal 35:8–14
Kowalski M, Kowalski M (2012) EXIFPro 2.0, California
Letnic M, Dickman CR (2006) Boom means bust: interactions between the El Niño/Southern Oscillation (ENSO), rainfall and the processes threatening mammal species in arid Australia. Biodivers Conserv 15:3847–3880
Letnic M, Dickman CR (2010) Resource pulses and mammalian dynamics: conceptual models for hummock grasslands and other Australian desert habitats. Biol Rev 85:501–521
Letnic M, Koch F (2010) Are dingoes a trophic regulator in arid Australia? A comparison of mammal communities on either side of the dingo fence. Aust Ecol 35:167–175
Letnic M et al (2011) Does a top predator suppress the abundance of an invasive mesopredator at a continental scale? Glob Ecol Biogeogr 20:343–353
Letnic M, Ritchie EG, Dickman CR (2012) Top predators as biodiversity regulators: the dingo Canis lupus dingo as a case study. Biol Rev 87:390–413
Lundie-Jenkins G, Corbett L, Phillips C (1993) Ecology of the rufous hare-wallaby, Lagorchestes hirsutus Gould (Marsupialia: Macropodidae) in the Tanami Desert, Northern Territory. III. Interactions with introduced mammal species. Wildl Res 20:495–511
Mahon PS (1999) Predation by feral cats and red foxes and the dynamics of small mammal populations in arid Australia. Ph.D. thesis, University of Sydney, Sydney
Mahon PS, Banks PB, Dickman CR (1998) Population indices for wild carnivores: a critical study in sand-dune habitat, south-western Queensland. Wildl Res 25:11–22
Merkle JA, Stahler DR, Smith DW (2009) Interference competition between gray wolves and coyotes in Yellowstone National Park. Can J Zool 87:56–63
Molsher RL (1999) The ecology of feral cats, Felis catus, in open forest in New South Wales: interactions with food resources and foxes. Ph.D. thesis, University of Sydney, Sydney
Moseby KE, Neilly H, Read JL, Crisp HA (2012) Interactions between a top order predator and exotic mesopredators in the Australian rangelands. Int J Ecol, Article No. 250352
Palomares F, Caro TM (1999) Interspecific killing among mammalian carnivores. Am Nat 153:492–508
Paltridge R (2002) The diets of cats, foxes and dingoes in relation to prey availability in the Tanami Desert, Northern Territory. Wildl Res 29:389–403
Pavey CR, Eldridge SR, Heywood M (2008) Population dynamics and prey selection of native and introduced predators during a rodent outbreak in arid Australia. J Mammal 89:674–683
Pianka ER, King DR, King RA (2004) Varanoid lizards of the world. Indiana University Press, Indiana
Power ME (1992) Top-down and bottom-up forces in food webs: do plants have primacy? Ecology 73:733–746
Prugh LR et al (2009) The rise of the mesopredator. Bioscience 59:779–791
R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ripple WJ, Beschta RL (2012) Trophic cascades in Yellowstone: the first 15 years after wolf reintroduction. Biol Conserv 145:205–213
Ripple WJ et al (2014) Status and ecological effects of the world’s largest carnivores. Science 343:1241484
Ritchie EG, Elmhagen B, Glen AS, Letnic M, Ludwig G, McDonald RA (2012) Ecosystem restoration with teeth: what role for predators? Trends Ecol Evol 27:265–271
Sinclair ARE, Pech RP, Dickman CR, Hik D, Mahon P, Newsome AE (1998) Predicting effects of predation on conservation of endangered prey. Conserv Biol 12:564–575
Smith AP, Quin DG (1996) Patterns and causes of extinction and decline in Australian conilurine rodents. Biol Conserv 77:243–267
Spencer EE, Crowther MS, Dickman CR (2014) Diet and prey selectivity of three species of sympatric mammalian predators in Central Australia. J Mammal (in press)
Tischler M, Dickman CR, Wardle GM (2013) Avian functional group responses to rainfall across four vegetation types in the Simpson Desert, Central Australia. Aust Ecol 38:809–819
Van Etten EJB (2009) Inter-annual rainfall variability of arid Australia: greater than elsewhere? Aust Geogr 40:109–120
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Vine SJ et al (2009) Comparison of methods to detect rare and cryptic species: a case study using the red fox (Vulpes vulpes). Wildl Res 36:436–446
Ward D (2009) The biology of deserts. Oxford University Press, New York
White TCR (2008) The role of food, weather and climate in limiting the abundance of animals. Biol Rev 83:227–248
Yang LH, Edwards KF, Byrnes JE, Bastow JL, Wright AN, Spence KO (2010) A meta-analysis of resource pulse–consumer interactions. Ecol Monogr 80:125–151
Zuur AF (2009) Mixed effects models and extensions in ecology with R. Springer, New York, London
Acknowledgments
We thank Bush Heritage Australia and G. Woods for allowing access to the properties in the study region; members of the Desert Ecology Research Group, N. Hills, D. Nelson and G. Madani for valuable assistance in the field, and V. Nguyen for statistical advice. A. C. G. was supported by an Australian Postgraduate Award and Paddy Pallin Grant, Royal Zoological Society of NSW. Funding support for G. W. and C. D. was provided by the Australian Research Council and by the Australian Government’s Terrestrial Ecosystems Research Network (http://www.tern.gov.au), an Australian research infrastructure facility established under the National Collaborative Research Infrastructure Strategy and Education Infrastructure Fund—Super Science Initiative through the Department of Industry, Innovation, Science, Research and Tertiary Education.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ilpo Kojola.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Greenville, A.C., Wardle, G.M., Tamayo, B. et al. Bottom-up and top-down processes interact to modify intraguild interactions in resource-pulse environments. Oecologia 175, 1349–1358 (2014). https://doi.org/10.1007/s00442-014-2977-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-2977-8