Abstract
Climate change has the potential to alter the genetic diversity of plant populations with consequences for community dynamics and ecosystem processes. Recent research focused on changes in climatic means has found evidence of decreased precipitation amounts reducing genetic diversity. However, increased variability in climatic regimes is also predicted with climate change, but the effects of this aspect of climate change on genetic diversity have yet to be investigated. After 10 years of experimentally increased intra-annual variability in growing season precipitation regimes, we report that the number of genotypes of the dominant C4 grass, Andropogon gerardii Vitman, has been significantly reduced in native tallgrass prairie compared with unmanipulated prairie. However, individuals showed a different pattern of genomic similarity with increased precipitation variability resulting in greater genome dissimilarity among individuals when compared to unmanipulated prairie. Further, we found that genomic dissimilarity was positively correlated with aboveground productivity in this system. The increased genomic dissimilarity among individuals in the altered treatment alongside evidence for a positive correlation of genomic dissimilarity with phenotypic variation suggests ecological sorting of genotypes may be occurring via niche differentiation. Overall, we found effects of more variable precipitation regimes on population-level genetic diversity were complex, emphasizing the need to look beyond genotype numbers for understanding the impacts of climate change on genetic diversity. Recognition that future climate change may alter aspects of genetic diversity in different ways suggests possible mechanisms by which plant populations may be able to retain a diversity of traits in the face of declining biodiversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global climate change is predicted to result in changes in the mean and variability of precipitation regimes (IPCC 2007), both of which will result in chronic alterations in resources (Smith et al. 2009). These novel changes in resources and environmental conditions are predicted to alter diversity within populations (Lovejoy and Hannah 2005). There is the potential, however, for intraspecific genetic diversity to respond differently to shifts in climatic means compared to increased climatic variability. Indeed, the effects of increased variability of precipitation regimes on ecosystems are predicted to be greater than changes in mean annual precipitation (Heisler and Weltzin 2006). Changes in precipitation amounts (positive or negative) results primarily in altered resource means (e.g., reduced mean soil moisture). On the other hand, increased intra-annual variation in precipitation, which is expected to be manifested as larger but fewer rainfall events (IPCC 2007), not only results in reductions in resource means but also in increased resource variability (e.g., pulses versus deficits in water availability; e.g., Knapp et al. 2002). This increase in resource variability and reduction in means will be driven by two mechanisms. First, larger rainfall events penetrate farther into the soil, which allows for deeper soil layers to be recharged, but potentially results in more water being lost to ground water. Second, longer periods between rainfall events result in shallow soil layers becoming drier (Knapp et al. 2008).
Changes in resources, such as soil moisture, could impose differing selective pressures on populations of species (Etterson 2008). At the plant population level, intra-specific genetic diversity is expected to decrease with changes in climatic means, as a result of directional selection for traits that allow individuals to withstand the altered conditions (Hewitt and Nichols 2005; Jump and Penuelas 2005; Thomas 2005; Reusch and Wood 2007; Gienapp et al. 2008; Hoffmann and Willi 2008; Jump et al. 2009). Recent studies altering mean climatic conditions have found support for this hypothesis (Gienapp et al. 2008; Jump et al. 2008). In contrast, with more variable precipitation patterns, there is the potential for genetic diversity to either decrease or increase. With the latter, niche breadth may actually be increased in space and time due to the variety of ways that resources can be altered, resulting in diversifying selection for genotypes capable of coping with different aspects of the altered environmental conditions. Critically, tests of the effects of increased precipitation variability on intraspecific genetic diversity are lacking in the literature.
Our objective with this study was to examine whether a decade of increased variability in precipitation patterns has resulted in altered genetic variation of the dominant C4 grass, Andropogon gerardii Vitman, in intact, native tallgrass prairie. The focus on a single dominant species is highly relevant, because there is mounting evidence that both genotype number and genetic diversity within dominant species can affect community properties and ecosystem function (Wimp et al. 2004; Vellend and Geber 2005; Bangert et al. 2006; Crutsinger et al. 2006; Whitham et al. 2006). For example, positive relationships have been observed between the number of genotypes of dominant species in monoculture and productivity (Reusch et al. 2005; Crutsinger et al. 2006) and the genetic diversity of cottonwood trees and diversity of associated insect communities (Wimp et al. 2004; Bangert et al. 2006). Moreover, studies have found that the number of genotypes in stands of seagrass affects ecosystem resistance to and recovery from disturbance (Hughes and Stachowicz 2004; Reusch et al. 2005). Collectively, these studies suggest that understanding the responses of intraspecific genetic diversity in dominant plant species is critical for predicting how ecosystems will respond to future climate change.
Using an on-going climate change experiment—the Rainfall Manipulation Plots (RaMPs) located in northeastern Kansas—we assessed the effects of increased variability in precipitation regimes on two aspects of genetic diversity for populations of A. gerardii: (1) genotype richness, and (2) genomic (dis)similarity among individuals. Recent studies have focused primarily on genotype richness (Hughes and Stachowicz 2004; Reusch et al. 2005; Crutsinger et al. 2006), but such measures do not consider the extent to which individuals’ genomes may differ from one another (Avolio et al. 2012). The degree of genomic similarity (or dissimilarity) between individuals may be an indicator of fine-scale trait variation within populations, which is not necessarily captured with number or composition of genotypes.
We hypothesized that the altered environmental conditions associated with more variable precipitation regimes (i.e., reduction in average soil moisture and increased soil moisture variability; Fay et al. 2011) would affect the abundance and distribution of A. gerardii, whereby individuals of some genotypes would be better able to persist over others. We are invoking an asexual reproduction mechanism through which the change in genotypic structure may occur. Those genotypes capable of tolerating the more variable environmental conditions would expand vegetatively, alongside the contraction and eventual loss of genotypes not favored by the altered conditions. A. gerardii is long-lived perennial that reproduces primarily vegetatively through extensive belowground bud banks that create a dense matrix of tillers (Weaver 1954; Benson and Hartnett 2006). Although genets may be long-lived, previous research has shown that loss and gain of individual tillers (ramets) within and between years can be substantial (Benson and Hartnett 2006). Therefore, despite the relatively short timeframe of the experiment relative to the lifespan of genets of A. gerardii, we expected that rapid bud turnover could result in aboveground tiller frequencies that reflected the relative success of a genotype under the altered precipitation regime. In species that reproduce asexually through vegetative spread, changes in the frequency of genotypes reflects the relative fitness of genotypes (Pan and Price 2001), and the persistence of some genotypes over others can lead to changes in genetic diversity (Stuefer et al. 2009). Thus, we were confident that if the genetic diversity was being altered by the increased precipitation variability, we would be able to detect it in the number of genotypes and the genomic dissimilarity of individuals within populations subjected to the altered precipitation patterns.
Materials and methods
RaMPs experimental design
The RaMPs experiment was initiated in 1998 at the Konza Prairie Biological Station in northeastern Kansas, USA. The experiment is located in an annually burned (in April), intact native tallgrass prairie site dominated by A. gerardii (Fay et al. 2000). The experiment consists of 12 fixed-roof shelters, each 9 × 14 m in size, which cover a 6 × 6 m experimental area that is divided into four 2 × 2 m subplots. One of these subplots is left as a control, one is used in a grazing experiment, and two are warmed by 2 °C. For our purposes, we sampled only from the control subplots. Each shelter allows for the collection and re-application of rainfall during the growing season, April–October (Fay et al. 2000). Six of the RaMPs receive ambient rainfall (ambient treatment) where collected rainfall is immediately applied. The remaining six RaMPs receive the same quantity of rainfall overall but applied in an altered pattern (altered treatment), consisting of a 50 % increase in the intervals between rainfall events. This results in a statistically extreme and highly variable rainfall regime where rainfall events are much larger in size but fewer in frequency than ambient patterns, and the periods between rainfall events are longer (Knapp et al. 2002; Smith 2011). Such alterations in precipitation regimes are predicted to occur with climate change in the central Great Plains of the US (IPCC 2007). At the end of the growing season, both the altered and ambient plots have received the same amount of rainfall overall. The altered precipitation patterns result in a significant reduction in soil moisture by 14 % over 10 years and increases soil moisture variability by 18 % (Fay et al. 2011), which are the key drivers of community and ecosystem responses to increased variability in precipitation (Knapp et al. 2002; Fay et al. 2011). The altered precipitation treatment has never resulted in prolonged deficits in soil moisture conditions that are lethal for all the plants (A. Knapp, personal communication).
Study design
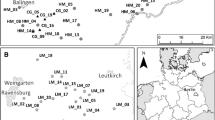
To assess the impacts of altered precipitation patterns on intraspecific genetic variation of A. gerardii, we compared the genetic diversity of the ambient and altered RaMP control subplots to unmanipulated prairie, hereafter referred to as the reference plots. We compared two aspects of genetic diversity, genotype richness and genomic dissimilarity. We used the reference plots because the genotype richness and genomic dissimilarity of A. gerardii populations were unknown at the start of the experiment. We assumed that genetic structure between the RaMPs and reference plots was similar before the RaMPs experiment was initiated. We believe this is a valid assumption as the reference plots were located directly adjacent (within 10 m; Fig. 1) to the RaMPs plots and had the same soil type, drainage, and plant community, and because the entire area was treated similarly prior to initiation of the RaMPs experiment. For these comparisons, we established 12, 2 × 2 m reference plots in an identical spatial array as the RaMPs (Fig. 1). We controlled for variation in distance among the treatment plots by comparing the ambient and altered plots to their spatially respective reference plots (Fig. 1). We did this because A. gerardii reproduces predominately asexually and therefore proximity of plots to one another could affect overlap genotypic composition.
Experimental set-up showing location of the Rainfall Manipulation Plots (RaMPs) experiment (filled squares) and the unmanipulated reference plots (open squares). Because the altered precipitation and ambient treatment plots are not uniformly distant from one another in the RaMPs experiment, we assigned the reference plots to an identical treatment array as the RaMPs (gray vs. black squares)
We determined genotype richness and genomic dissimilarity at two scales: the plot scale (n = 6), which is representative of the plant neighborhood scale, and at the treatment scale, where all six RaMPs or reference plots within a treatment were pooled. The latter allowed us to assess changes in diversity at the population scale, where such changes are likely to be manifest first. Lastly, we examined whether the two aspects of genetic diversity of A. gerardii correlated with plant community properties and aboveground net primary productivity (ANPP) for the native tallgrass prairie reference plots only, because ANPP could not be collected from the RaMP control subplots.
AFLP protocol and analysis
We used amplified fragment length polymorphism (AFLP; Vos et al. 1995) to assess the genotypic structure and genomic dissimilarity of A. gerardii. The AFLP technique has been used in a variety of ecological studies to perform genome scans inferring selection by determining whether certain loci have different allele frequencies with specific environmental conditions (Jump et al. 2006). However, using this method to infer past selection has limitations (Reusch and Wood 2007), and therefore we restricted our use of the AFLP technique just to assigning individuals to genotypes and to determine the relatedness between individuals (see below). In August 2007, we collected leaf tissue from 40 tillers in a uniform spatial array within each 2 × 2 m RaMP control subplot and corresponding reference plot (total = 960 individual tillers sampled, 240 individuals per treatment). This sampling density has been shown to be adequate for capturing the majority of genotypes within a plot of this size (Avolio et al. 2011). Collected tissue was then stored in silica gel until DNA was extracted.
For the AFLP molecular work, we followed the methods of (Avolio et al. 2011). AFLP chromatograms were analyzed with GeneMarker software (v.1.7; SoftGenetics, State College, PA, USA). In total, 103 polymorphic AFLP loci, ranging from 81 to 375 bp in length, were scored in our analysis to create a presence/absence matrix for all individuals sampled. A low degree of genetic differentiation between genotypes, which we expected from sampling within a single population, could lead to a higher error rate (Holland et al. 2008); our error rate was 7.6 %, based on the number of loci for which 14 technical replicates had a different banding pattern divided by the total number of loci. While this error rate is higher than error rates obtained by manual scoring of peaks, it is well within the range of published error rates, 5–18 %, when using automatic scoring of a larger number of samples (Lasso 2007; Holland et al. 2008; Whitlock et al. 2008; Arrigo et al. 2009).
We used the presence/absence matrix of all individuals sampled to assign individuals to a particular genotype with Genodive v.20b17 (Meirmans and Van Tienderen 2004). We used the clonal distance model to calculate differences between individuals. The clonal distance model assumes clonal reproduction and calculates distances based on the number of mutations taken to transform an individual of one genotype into another genotype. In long-lived clonal plants, each genotype is often not a unique multilocus genotype (Douhovnikoff and Dodd 2003; Lasso 2007; Mock et al. 2008; Reusch and Bostrom 2011). To distinguish between different genotypes, we first analyzed a histogram of clonal distance values of 109 individuals which were collected from the field as 39 clones, with individuals confirmed as clones because they were physically connected via rhizomes (Douhovnikoff and Dodd 2003; Lasso 2007). This histogram has two clearly defined peaks, where the first peak represents the similarity values between clonal individuals and the second peak the similarity values between unrelated individuals (Douhovnikoff and Dodd 2003; Meirmans and Van Tienderen 2004). We used this histogram to determine the threshold to use when assigning individuals to the same genotype. A cut-off of 12 (clonal distances range from 1 to 100), correctly grouped the 109 individuals into their respective known clones (Fig. S1). We used the same cutoff of 12 with the clonal model when assigning each of the sampled individuals to particular genotypes. We then calculated genotype richness (number of genotypes) at both the plot and treatment level.
We used a mean pairwise distance (MPD; sensu Webb et al. 2002) using a Dice dissimilarity index as our measure of genomic similarity. MPD was originally developed for community ecology for assessing phylogenetic diversity of co-occurring species in a given community. Phylogenetic diversity of a community is defined as the average time separating all co-occurring species relative to a regional species pool from which the community is drawn. For clonal species, measuring genomic dissimilarity using MPD can be thought of in the same way. To calculate MPD, we first created a dendrogram based on a pairwise Dice dissimilarity matrix of all individuals (Fig. S2). Using this dendrogram, we then calculated the mean pairwise distance separating all co-occurring individuals in each plot for the plot scale analyses or in each treatment (across six plots within a treatment) for the treatment scale analyses, relative to the regional genotype pool of all individuals. If co-occurring individuals within a plot have genomes (AFLP profiles) that are similar to each other, the MPD of the plot will be lower compared with a plot where individuals’ genomes were less similar to one another.
Field data collection
Percent aerial cover (nearest 1 %) was estimated for each species separately within each of the four 1 × 1 m2 quadrats located within plots in June and August 2008 for the reference plots only. Maximum cover estimates for each species were averaged across the four quadrats and used to calculate plant community richness, evenness, and Shannon’s diversity. Aboveground biomass was clipped at ground level from four 0.1 m2 subplots randomly located within each reference plot in late August 2008 (after the species composition measurements), dried at 60 °C for ~48 h, and then weighed. Because the site is burned each spring, this provides a direct measure of aboveground net primary production (ANPP).
Statistical analyses
A significance level of α ≤ 0.05 was used for all statistical analyses. All analyses were performed in R (R Core Development Team) except for the SIMPER and PERMANOVA (Clarke 1993) analyses which were performed in Primer (v.6; Clarke and Gorley 2006). In R, the vegan package (Oksanen et al. 2012) was used to calculate pair-wise Dice dissimilarities among all individuals sampled to create the regional pool dendogram (Fig. S2), and the picante package (Kembel et al. 2009) was used for calculating distance measures (MPD) between all individuals within plots or treatments. Genotype structure was analyzed using SIMPER and PERMANOVA to assess whether there were differences between the altered, ambient, and reference plots in their genotype composition, using a Bray–Curtis similarity matrix. We excluded rare genotypes, those consisting of only one individual from these analyses. The PERMANOVA tested whether there was greater or reduced genotypic compositional similarity among all six plots in the altered and ambient plots compared with their spatially respective reference plots. The SIMPER analysis identified which genotypes typified plots—which led to plots being more similar within a treatment—and which genotypes best differentiated between the ambient, altered, or reference plots—which led to plots being less similar among treatments.
For the plot-level analyses of genotype richness and genomic dissimilarity, we performed separate paired t tests between the RaMPs ambient and altered plots and their spatially respective reference plots. For the treatment scale, statistical comparison of genotype richness was not possible (n = 1). Instead, we conducted a net relatedness index (NRI) analysis (Webb 2000) to assess whether MPD of the ambient and altered treatments and the reference plots differed from a random distribution of MPD values. The null distribution of MPD values was created by 999 reshuffles of the regional pool of individuals in the dendrogram (Fig. S2), effectively randomizing the Dice dissimilarity distances. A significant positive NRI value indicates that co-occurring individuals were more genetically similar to each other than expected by chance and are clustered on the dendrogram. A significant negative NRI value indicates that co-occurring individuals are more genetically dissimilar from each than expected by chance and therefore over dispersed on the dendrogram. Lastly, we assessed relationships between plant community diversity and ANPP for the reference plots by correlating these community and ecosystem properties with plot level MPD.
Results
For all the plots sampled, we found 316 unique genotypes. Genotypes occurred in inter-mixed stands (Fig. S3), and average genotype richness was 15.0 (±1.2 SE) per 2 × 2 m plot. A large number of rare genotypes (consisting of only one individual) were found (Fig. S3). However, in most cases, these rare genotypes clustered with other genotypes (Fig. S2), suggesting they are not actually genetically novel but instead have very similar genomes to larger multi-member genotypes.
At the plot scale, there were on average 49 % fewer genotypes in the altered plots compared with their corresponding reference plots (Table 1); however, genotype richness did not differ significantly between the ambient RaMP treatment plots and their corresponding reference plots. At the treatment scale (across the six plots per treatment), there were 113 and 95 genotypes found in the ambient and altered reference plots, respectively. The RaMP plots were found to have fewer genotypes than the reference plots, with 52 and 75 genotypes in the altered and ambient treatment plots, respectively. Consequently, the number of effective genotypes (genotype richness normalized by the number of individuals) was reduced by 55 % in the altered treatment plots, versus a 19 % reduction in the ambient RaMPs, relative to their respective reference plots. Thus, although there were fewer genotypes in the RaMPs overall, the greatest reduction of genotypes at the treatment scale was observed in the altered precipitation plots.
The reduction of the number of genotypes at the treatment scale in the altered precipitation treatment RaMPs appears to be driven primarily by a reduction in the number of rare genotypes (i.e., shorter tail of the rank–abundance distribution; Fig. 2), as well as an increase in the abundance of a few genotypes when compared to ambient or reference plots (Fig. 2). These changes in the number and abundance of genotypes resulted in the altered RaMP plots being more similar in their genotype composition when compared to their corresponding reference plots (PERMANOVA analysis, p = 0.038). In contrast, the ambient RaMP plots, which did not exhibit as large of change in genotype numbers and abundances, were not more similar in their genotypic composition than their corresponding reference plots (p = 0.342). The findings of the SIMPER analysis also reflect this difference, as the altered RaMP plots were more similar in their genotype composition (19 %) than the ambient plots (10 %) or reference plots (8 %). Furthermore, the SIMPER analysis revealed that two genotypes—G1 and G4, which accounted for 21.6 and 16.5 % of individuals sampled, respectively—were contributing most to similarity among the altered plots (Fig. 2; Table 3). Although G1 was found across all three treatments, it had relatively greater abundance in the altered plots. In turn, these genotypes, along with G2, contributed most to the differences between the altered and ambient RaMP plots, whereas G1 and G4 contributed most to the differences between the altered RaMPs and reference plots. Only one genotype, G2, contributed most of the differences between the ambient and reference plots (Table 3).
Rank–abundance of genotypes in the RaMPs experiment a ambient and b altered treatment plots and the respective c ‘ambient’ and d ‘altered’ unmanipulated reference plots. The four most abundant genotypes are labeled (G1–G4; see Table 3)
At the plot scale, we found no difference between genetic dissimilarity, as measured by MPD of individuals between the RaMPs and reference plots, for either the altered or ambient treatments (Table 1). At the treatment scale, however, the NRI analysis revealed positive NRI values for the ambient RaMPs and both the altered and ambient RaMP and reference plots, which were significantly different from the null NRI, suggesting individuals in these treatments are more closely related to each other than expected by chance (Table 2). NRI of the altered RaMPs treatment, however, was not significantly different from the null NRI (Table 2), suggesting that the genetic dissimilarities among individuals in this treatment were different than what was observed in the ambient RaMPs and unmanipulated prairie. This difference was further exemplified by a shift in the mode of MPD values in the altered plots towards higher values (Fig. 3). The shift towards higher MPD values was not caused by a greater range of MPD values; the range of MPD values was actually greater in the ambient plots (0.011–0.602) compared with the altered plots (0.051–0.513).
The genomic dissimilarity (MPD) of A. gerardii in the reference plots was not correlated with measures of plant community structure (Shannon’s diversity r = −0.143, p = 0.658; richness r = 0.188, p = 0.558; evenness r = −0.348, p = 0. 268). However, genomic dissimilarity of A. gerardii was positively correlated with aboveground primary productivity (ANPP; r = 0.638; p = 0.026; Fig. 4). Genotypic richness did not correlate with measures of plant community structure or ecosystem productivity (data not shown).
Discussion
Previous research has demonstrated that changes in climate means can decrease genetic diversity; however, the effects increased variability in precipitation regimes on genetic diversity has not been investigated. Our study is the first to show that a decade of increased intra-annual variability in precipitation regimes impacts two important aspects of genetic diversity—genotype richness as well as genomic dissimilarity among individuals. Similar to other studies, we found a significant reduction in the number of genotypes of A. gerardii at both the plot and treatment scales with altered precipitation patterns. Additionally, however, we found that genomic dissimilarity was greater in the altered RaMPs than the reference plots and ambient RaMPs at the treatment scale. We observed greater MPD values overall for the altered treatment (Fig. 3) and significantly lower MPD values for the ambient treatment and reference plots than expected by chance (Table 3).
There are several mechanisms that could have led to the reduction in genotype richness in the altered precipitation treatment. The first is that there may be fewer ramets overall in the populations subjected to a decade of altered precipitation patterns. However, this does not appear to be the case as the number of A. gerardii tillers did not differ significantly between the altered and ambient treatments (30.6 ± 3.3 vs. 37.8 ± 9.8 tillers per 0.1 m2, respectively; p = 0.505). Alternatively, the altered precipitation treatment plots may have had a different composition of genotypes and reduced genotype richness at the start of the experiment. However, there are several lines of evidence to suggest that both the altered and ambient RaMPs plots had a similar number of genotypes as the unmanipulated reference plots prior to initiation of treatments. First, the reference plots share many of the same genotypes as the RaMP plots (Fig. S2). Second, the number and composition of genotypes found in directly adjacent RaMPs and reference blocks—the RaMPs were established as three spatial blocks—are similar, suggesting that the site in which the RaMPs experiment was established was not depauperate at the start of the experiment (Fig. S3).
It appears, instead, that the primary mechanism driving the reduction in genotype richness is the increase in abundance of a few genotypes along with the concomitant loss of rarer genotypes (Fig. 2). The three most abundant genotypes in the altered treatment (G1, G4, and G9) accounted for 48 % of all individuals sampled, considerably higher than was found in either the ambient treatment (33 %) or the reference plots (24 %). The increased abundance of genotypes G1 and G4 was also observed at the plot scale (Fig. S3), where, in many of the replicate plots, these genotypes had greater abundance, indicating a pattern of selection for these genotypes that is being repeated across the landscape. It is important to note that the ambient RaMPs plots also tended to have fewer genotypes than the reference plots, with the reduction in genotype richness primarily driven by loss of rarer genotypes. This suggests that the shelters alone could be having an effect on genotype richness in both the ambient and altered RaMPs, potentially as a result of the altered microclimate conditions created by the RaMP shelters (reduced evapotranspiration and light; Fay et al. 2000). However, mean richness and genomic dissimilarity among individuals for the ambient RaMPs was not significantly different from the reference plots. Thus, any potential shelter effects on genetic structure appear to be minor when compared to the effects of the altered precipitation treatment.
The difference in genotype richness and genomic dissimilarity between the altered and the ambient treatments and their respective reference plots appears to be the result of ecological sorting of genotypes. Ecological sorting—a concept taken from phylogenetic community ecology (sensu Ackerly 2003)—is the result of ecological interactions between individuals and species over time. In this context, ecological sorting is inferred by looking at the relationships among species using phylogenetic distance, where time separating any two species is proportional to the amount of evolutionary and genetic divergence, and presumably trait differences, between species (Felsenstein 1985). There are often two mechanisms of ecological sorting invoked: habitat filtering and niche differentiation. If species in a habitat are more closely related to one another (clustered on a dendrogram), habitat filtering is expected to be the primary ecological sorting force. In this case, species with similar phenotypes survive best under the dominant environmental conditions resulting in greater niche overlap (Webb et al. 2002). Alternatively, if species within a habitat are more distantly related to one another (over-dispersed on a dendrogram), then competitive exclusion is expected to be the primary ecological sorting force. Here, species are occupying unique niches relative to one another, resulting in reduced niche overlap or niche differentiation (Webb et al. 2002). Although this framework was developed for differences between species, the same mechanisms could hold for individuals within populations of a species, assuming that the amount of genomic differences separating one genotype or individual from another is related to the amount of trait differences between the genotypes or individuals.
In the altered treatment, we observed a shift in the genomic dissimilarity distribution towards greater MPD values. This shift, however, cannot be confirmed as niche differentiation because we did not observe negative NRI values. Instead, we found no difference between the null NRI and the mean NRI of altered treatment. In contrast, we observed positive NRI values for the reference and ambient treatments that were significantly different from the null NRI, indicating that habitat filtering may be occurring in these treatments. The habitat filtering processes occurring in the reference and the ambient RaMP treatments do not appear to be occurring in the altered plots. Rather, a different type of ecological sorting appears to be occurring there. It is likely that we are observing the early stages of niche differentiation in the altered plots, as individuals in the altered plots were less related to one another than if they had not undergone 10 years of climate manipulation.
The altered precipitation patterns modify the soil moisture in a variety of ways, and mechanistically we envision two ways the altered conditions could lead to niche differentiation among individuals in the population via ecological sorting, by: (1) increasing soil moisture variability in time, and (2) increasing soil moisture variability in space. First, the more variable soil moisture environment over time could result in multiple pressures, including tolerating extended low soil moisture periods, responding to pulses in water availability after large rainfall events, and tolerating waterlogged soil conditions after extreme rainfall events. Thus, there are potentially a greater number of niches to be occupied by genotypes, and different genotypes that can better tolerate each specific novel environmental condition would persist. Second, greater spatial variability in soil moisture could also be an important ecological sorting force. The larger rainfall events in the altered treatment allow water to permeate deeper into the soil profile, thus creating a greater soil moisture gradient between shallow (0–15 cm) and deeper soils (15–30 cm), where the majority (70 %) of plant roots occur in this system (Knapp et al. 2002). In the altered plots, there is on average a greater soil moisture difference between these two depths (deep vs. shallow soil) when compared with the ambient treatment and unmanipulated prairie (0.122 ± 0.0011, 0.082 ± 0.0013, and 0.084 ± 0.0013, respectively, p < 0.001). Ecological sorting could be favoring genotypes that are better able to withstand the reduced soil moisture at shallow depths while also selecting for genotypes that have a higher proportion of total root biomass in deep roots.
While we cannot differentiate between the different mechanisms, the process of niche differentiation appears to best explain the patterns of ecological sorting of genotypes that we observed under altered precipitation. Previous research on clonal plants has shown that spatially or temporally heterogeneous environmental conditions can enable the coexistence of genotypes with a wider range of traits, maintaining genetic diversity through microhabitat differences (Burdon 1980). For niche differentiation to occur, there needs to be phenotypic differences among genotypes, and there are several lines of evidence to suggest that this is the case. First, a preliminary assessment of the phenotypes of the two most common genotypes in the altered precipitation treatment, G1 and G4, indicates that they differ in key traits associated with growth, including height and photosynthetic rates (Avolio, unpublished data). Second, these two genotypes are dispersed from one another on the dendrogram (Fig. S2). Additionally, in 2008, we investigated whether genotypes with more divergent genomes in the reference plots had correspondingly more divergent phenotypes, measured by specific leaf area (SLA; see supporting lnformation, Fig. S4). Using the Mantel function in the Ecodist package (Goslee and Urban 2007), we found a significant but weak correlation between genome dissimilarity and phenotype dissimilarity (p < 0.001, r = 0.116) (Fig. S4). Other studies using AFLPs to investigate multivariate phenotypic differences between genotypes or cultivars of grass species have found similar significant positive relationships between genetic diversity and phenotypic diversity (Ramakrishnan et al. 2004; Beyene et al. 2006; Noli et al. 2008). Thus, although additional studies are clearly needed and are currently underway (Avolio, unpublished data), these data suggest that individuals of A. gerardii with more dissimilar genomes have correspondingly more dissimilar phenotypes. Ultimately, the expectation is that ecological sorting via the mechanism of niche differentiation will result in diversifying selection over time within the population.
The alterations in genotype richness and composition and in genomic dissimilarity among individuals of A. gerardii that we observed with the more variable precipitation treatment could have implications for community diversity and ecosystem functioning, given that A. gerardii contributes disproportionately to ANPP and community dynamics in this system (Smith and Knapp 2003). We found genomic dissimilarity of A. gerardii was not correlated with any of the measures of plant community structure, which is in agreement with other studies that have found a lack of correlation between intra-specific genetic diversity of a dominant plant species with community diversity (Odat et al. 2004; Helm et al. 2009; Silvertown et al. 2009). In contrast, we found a significant positive correlation between genomic dissimilarity and ANPP. To our knowledge, our study is the first to show a correlation between genomic dissimilarity of a dominant species with ANPP for a diverse, intact plant community. Although causality remains to be examined, this pattern provides additional support for niche differences (complementarity) among genotypes (Reusch et al. 2005; Crutsinger et al. 2006) and emphasizes the importance of considering genomic dissimilarity of individuals in dominant plant species populations for ecosystem functioning.
Overall, our study suggests that global climate change predictions of increased precipitation variability and more extreme precipitation regimes should be further incorporated into current theories of the effect of climate change on biodiversity. Increased intra-annual variability associated with climate change may create conditions for ecological sorting via niche differentiation and ultimately diversifying selection to act, as there is potentially a greater range of microhabitat niche space to be occupied through time and space (Vavrek et al. 1996). This phenomenon, however, is likely possible only for plant species with large populations and relatively high initial genetic diversity. The rapid genetic response that we observed has been shown for other plant species exposed to human-influenced disturbances (Jump et al. 2008; Helm et al. 2009), evidence that rapid genetic changes have the potential to occur and may have immediate ecological effects and consequences for evolution (Thompson 2009). Ultimately, our study suggests that, even with a reduced number of genotypes, some populations may be able to retain a diversity of traits and the potential to maintain ecosystem functioning and adapt to future climate change.
References
Ackerly DD (2003) Community assembly, niche conservatism, and adaptive ecolution in changing environments. Int J Plant Sci 164:S165–S184
Arrigo N, Tuszynski JW, Ehrich D, Gerdes T, Alvarez N (2009) Evaluating the impact of scoring parameters on the structure of intra-specific genetic variation using RawGeno, an R package for automating AFLP scoring. BMC Bioinformatics 10:1–14
Avolio ML, Chang CC, Smith MD (2011) Assessing fine-scale genotypic structure of a dominant species in native grasslands. Am Midl Nat 165:211–224
Avolio ML, Beaulieu JM, Lo E, Smith MD (2012) Measuring genetic diversity in ecological studies. Plant Ecol 213:1105–1115
Bangert RK, Turek RJ, Rehill B, Wimp GM, Schweitzer JA, Allan GJ, Bailey JK, Martinsen GD, Keim P, Lindroth RL, Whitham TG (2006) A genetic similarity rule determines arthropod community structure. Mol Ecol 15:1379–1391
Benson EJ, Hartnett DC (2006) The role of seed and vegetative reproduction in plant recruitment and demography in tallgrass prairie. Plant Ecol 187:163–177
Beyene Y, Botha A, Myburg AA (2006) Genetic diversity in traditional Ethiopian highland maize accessions assessed by AFLP markers and morphological traits. Biodivers Conserv 15:2655–2671
Burdon JJ (1980) Intraspecific diversity in a natural-population of Trifolium-Repens. J Ecol 68:717–735
Clarke KR (1993) Nonparametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Clarke, KR, Gorley, RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E, Plymouth
Crutsinger GM, Collins MD, Fordyce JA, Gompert Z, Nice CC, Sanders NJ (2006) Plant genotypic diversity predicts community structure and governs an ecosystem process. Science 313:966–968
Douhovnikoff V, Dodd RS (2003) Intra-clonal variation and a similarity threshold for identification of clones: application to Salix exigua using AFLP molecular markers. Theor Appl Genet 106:1307–1315
Etterson J (2008) Evolution in response to climate change. In: Carroll SP, Fox CW (eds) Conservation biology: evolution in action. Oxford University Press, Oxford, pp 145–163
Fay PA, Carlisle JD, Knapp AK, Blair JM, Collins SL (2000) Altering rainfall timing and quantity in a mesic grassland ecosystem: design and performance of rainfall manipulation shelters. Ecosystems 3:308–319
Fay PA, Blair JM, Smith MD, Nippert JB, Carlisle JD, Knapp AK (2011) Relative effects of precipitation variability and warming on tallgrass prairie ecosystem function. Biogeosciences 8:3053–3068
Felsenstein J (1985) Confidence-limits on phylogenies—an approach using the bootstrap. Evolution 39:783–791
Gienapp P, Teplitsky C, Alho JS, Mills JA, Merila J (2008) Climate change and evolution: disentangling environmental and genetic responses. Mol Ecol 17:167–178
Goslee SC, Urban DL (2007) The ecodist package for dissimilarity-based analysis of ecological data. J Stat Softw 22:1–19
Heisler JL, Weltzin JF (2006) Variability matters: towards a perspective on the influence of precipitation on terrestrial ecosystems. New Phytol 172:189–192
Helm A, Oja T, Saar L, Takkis K, Talve T, Pärtel M (2009) Human influence lowers plant genetic diversity in communities with extinction debt. J Ecol 97:1329–1336
Hewitt GM, Nichols RA (2005) Genetic and evolutionary impacts of climate change. In: Lovejoy TE, Hannah LJ (eds) Climate change and biodiversity. Yale University Press, New Haven, pp 176–192
Hoffmann AA, Willi Y (2008) Detecting genetic responses to environmental change. Nat Rev Genet 9:421–432
Holland BR, Clarke AC, Meudt HM (2008) Optimizing automated AFLP scoring parameters to improve phylogenetic resolution. Syst Biol 57:347–366
Hughes AR, Stachowicz JJ (2004) Genetic diversity enhances the resistance of a seagrass ecosystem to disturbance. Proc Natl Acad Sci USA 101:8998–9002
IPCC (2007) Climate Change 2007: The physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Jump AS, Penuelas J (2005) Running to stand still: adaptation and the response of plants to rapid climate change. Ecol Lett 8:1010–1020
Jump AS, Hunt JM, Martinez-Izquierdo JA, Penuelas J (2006) Natural selection and climate change: temperature-linked spatial and temporal trends in gene frequency in Fagus sylvatica. Mol Ecol 15:3469–3480
Jump AS, Penuelas J, Rico L, Ramallo E, Estiarte M, Martinez-Izquierdo JA, Lloret F (2008) Simulated climate change provokes rapid genetic change in the Mediterranean shrub Fumana thymifolia. Glob Change Biol 14:637–643
Jump AS, Marchant R, Penuelas J (2009) Environmental change and the option value of genetic diversity. Trends Plant Sci 14:51–58
Kembel S, Ackerly D, Blomberg S, Cowan P, Helmus M, Morlon H, Webb CO (2009) Picante: R tools for integrating phlogenies and ecology. R package version 05-3
Knapp AK, Fay PA, Blair JM, Collins SL, Smith MD, Carlisle JD, Harper CW, Danner BT, Lett MS, McCarron JK (2002) Rainfall variability, carbon cycling, and plant species diversity in a mesic grassland. Science 298:2202–2205
Knapp AK, Beier C, Briske DD, Classen AT, Luo Y, Reichstein M, Smith MD, Smith SD, Bell JE, Fay PA, Heisler JL, Leavitt SW, Sherry R, Smith B, Weng E (2008) Consequences of more extreme precipitation regimes for terrestrial ecosystems. Bioscience 58:811–821
Lasso E (2007) The importance of setting the right genetic distance threshold for identification of clones using amplified fragment length polymorphism: a case study with five speices in the tropic plant genus Piper. Mol Ecol Notes 8:74–82
Lovejoy TE, Hannah LJ (2005) Climate change and biodiversity. Yale University Press, New Haven
Meirmans PG, Van Tienderen PH (2004) GENOTYPE and GENODIVE: two programs for the analysis of genetic diversity of asexual organisms. Mol Ecol Notes 4:792–794
Mock KE, Rowe CA, Hooten MB, Dewoody J, Hipkins VD (2008) Clonal dynamics in western north American aspen (Populus tremuloides). Mol Ecol 17:4827–4844
Noli E, Teriaca MS, Sanguineti MC, Conti S (2008) Utilization of SSR and AFLP markers for the assessment of distinctness in durum wheat. Mol Breeding 22:301–313
Odat N, Jetschke G, Hellwig FH (2004) Genetic diversity of Ranunculus acris L. (Ranunculaceae) populations inferred in relation to species diversity and habitat type in grassland communities. Mol Ecol 13:1251–1257
Oksanen J, Kindt R, Legendre P, O’Hara B, Simpson GL, Stevens HH (2012) vegan: Community ecology package. R package version 111-4
Pan JJ, Price JS (2001) Fitness and evolution in clonal plants: the impact of clonal growth. Evol Ecol 15:583–600
Ramakrishnan AP, Meyer SE, Waters J, Stevens MR, Coleman CE, Fairbanks DJ (2004) Correlation between molecular markers and adaptively significant genetic variation in Bromus tectorum (Poaceae), an inbreeding annual grass. Am J Bot 91:797–803
Reusch TBH, Bostrom C (2011) Widespread genetic mosaicism in the marine angiosperm Zostera marina is correlated with clonal reproduction. Evol Ecol 25:899–913
Reusch TBH, Wood TE (2007) Molecular ecology of global change. Mol Ecol 16:3973–3992
Reusch TBH, Ehlers A, Hammerli A, Worm B (2005) Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proc Natl Acad Sci USA 102:2826–2831
Silvertown J, Biss PM, Freeland J (2009) Community genetics: resource addition has opposing effects on genetic and species diversity in a 150-year experiment. Ecol Lett 12:165–170
Smith MD (2011) The ecological role of climate extremes: current understanding and future prospects. J Ecol 99:651–655
Smith MD, Knapp A (2003) Dominant species maintain ecosystem function with non-random species loss. Ecol Lett 6:509–517
Smith MD, Knapp AK, Collins SL (2009) A framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecology 90:3279–3289
Stuefer JF, Anten NPR, de Kroon H, Peters JL, Smit-Tiekstra A, Vermeulen PJ, During HJ (2009) Genotypic selection shapes patterns of within-species diversity in experimental plant populations. J Ecol 97:1020–1027
Thomas C (2005) Recent evolutionary effects of climate change. In: Lovejoy TE, Hannah LJ (eds) Climate change and biodiversity. Yale University Press, New Haven, pp 75–88
Thompson JN (2009) Which ecologically important traits are most likely to evolve rapidly? Oikos 118:1281–1283
Vavrek MC, McGraw JB, Yang HS (1996) Within-population variation in demography of Taraxacum officinale: maintenance of genetic diversity. Ecology 77:2098–2107
Vellend M, Geber MA (2005) Connections between species diversity and genetic diversity. Ecol Lett 8:767–781
Vos P, Hogers R, Bleeker M, Reijans M, Vandelee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) Aflp—a new technique for DNA-fingerprinting. Nucleic Acids Res 23:4407–4414
Weaver JE (1954) North American prairie. Johnsen, Lincoln
Webb CO (2000) Exploring the phylogenetic structure of ecological communities: an example for rain forest trees. Am Nat 156:145–155
Webb CO, Ackerly DD, McPeek MA, Donoghue MJ (2002) Phylogenies and community ecology. Annu Rev Ecol Syst 33:475–505
Whitham TG, Bailey JK, Schweitzer JA, Shuster SM, Bangert RK, Leroy CJ, Lonsdorf EV, Allan GJ, DiFazio SP, Potts BM, Fischer DG, Gehring CA, Lindroth RL, Marks JC, Hart SC, Wimp GM, Wooley SC (2006) A framework for community and ecosystem genetics: from genes to ecosystems. Nat Rev Genet 7:510–523
Whitlock R, Hipperson H, Mannarelli M, Butlin RK, Burke T (2008) An objective, rapid and reproducible method for scoring AFLP peak-height data that minimizes genotyping error. Mol Ecol Resour 8:725–735
Wimp GM, Young WP, Woolbright SA, Martinsen GD, Keim P, Whitham TG (2004) Conserving plant genetic diversity for dependent animal communities. Ecol Lett 7:776–780
Acknowledgments
We thank A. Knapp, J. Blair, S. Collins and a number of research technicians, graduate students and undergraduates for their assistance with and maintenance of the RaMPs project, notably P. O’Neal. We also thank G. Caccone and the DNA analysis facility for support with the AFLP analysis. Thanks to C. Chang, T. Schreck, T. Nelson and S. Koerner for assistance in the field, and E. Lo for assisting with analysis of the genetic data. Lastly, we thank A. Knapp, S. Collins, G. Caccone, D. Post, C. Chang, and anonymous reviewers for comments on an earlier version of the manuscript. This research was supported by the US Department of Energy’s Program for Ecosystem Research (#DE-FG02-04ER63892), the USDA CSREES Ecosystem Studies Program, the NSF Ecosystems Program, and the NSF Long-Term Ecological Program at Konza Prairie. The experiment complies with the current laws of the United States of America where the experiment was performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Bryan Foster.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Avolio, M.L., Beaulieu, J.M. & Smith, M.D. Genetic diversity of a dominant C4 grass is altered with increased precipitation variability. Oecologia 171, 571–581 (2013). https://doi.org/10.1007/s00442-012-2427-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2427-4