Abstract
Plant adaptations for defense against herbivory vary both among species and among genotypes. Moreover, numerous forms of within-plant variation in defense, including ontogeny, induction, and seasonal gradients, allow plants to avoid expending resources on defense when herbivores are absent. We used an 18-year-old cottonwood common garden composed of Populus fremontii, Populus angustifolia, and their naturally occurring F1 hybrids (collectively referred to as “cross types”) to quantify and compare the relative influences of three hierarchical levels of variation (between cross types, among genotypes, and within individual genotypes) on univariate and multivariate phytochemical defense traits. Within genotypes, we evaluated ontogeny, induction (following cottonwood leaf beetle herbivory), and seasonal variation. We compared the effect sizes of each of these sources of variation on the plant defense phenotype. Three major patterns emerged. First, we observed significant differences in concentrations of defense phytochemicals among cross types, and/or among genotypes within cross types. Second, we found significant genetic variation for within-plant differences in phytochemical defenses: (a) based on ontogeny, levels of constitutive phenolic glycosides were nearly three times greater in the mature zone than in the juvenile zone within one cottonwood cross type, but did not significantly differ within another cross type; (b) induced levels of condensed tannins increased up to 65 % following herbivore damage within one cottonwood cross type, but were not significantly altered in another cross type; and (c) concentrations of condensed tannins tended to increase across the season, but did not do so across all cross types. Third, our estimates of effect size demonstrate that the magnitude of within-plant variation in a phytochemical defense can rival the magnitude of differences in defense among genotypes and/or cross types. We conclude that, in cottonwood and likely other plant species, multiple forms of within-individual variation have the potential to substantially influence ecological and evolutionary processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Genetic and phenotypic differences are generally greater (or assumed to be greater) between species than within species (Bolnick et al. 2003, 2011; Vellend 2006; Shipley 2007). However, the emerging field of community genetics has highlighted the extent to which the ecological impacts of genetic variation within a species can rival those among species (Whitham et al. 2006; Johnson and Stinchcombe 2007; Bailey et al. 2009). In the realm of plant–insect interactions, the ecological and evolutionary relevance of a third hierarchical level of defense, within-plant variation in defense is increasingly recognized (Kearsley and Whitham 1989; Lawrence et al. 2003; Boege and Marquis 2005; Donaldson et al. 2006; Barton 2007; Traw and Feeny 2008; Holeski et al. 2009; Axelsson et al. 2011; Moreira et al. 2012). However, much less is known about the relative contribution of within-plant versus inter-plant variation to defense phenotypes.
Common modes of within-plant variation in defense include variation due to ontogeny, induction, and seasonal variation (defined below). These modes of within-plant variation may occur independently of one another, may overlap, or may interact. Despite the likelihood that the modes interact or overlap, previous studies have generally examined each independently. This emphasis contrasts with recent decades of research on among-plant variation in defense, which have witnessed numerous studies on the interactive effects of biotic and abiotic factors on plant chemical composition.
Regardless of its mode, if within-plant phenotypic variation is genetically based, and on a scale rivaling differences between genotypes or species, then its effects are particularly relevant to studies of community genetics, ecology, and trait evolution. In this study, we used cottonwood phytochemical defense traits to explore patterns of genetic and phenotypic variation at three hierarchical levels. In a common garden environment, we compared the relative magnitude of effect of the traditionally examined sources of phenotypic variation (between cross types and among genotypes within cross types) to the magnitude of within-plant variation (ontogeny, induction, and seasonal variation) on defenses, and evaluated our results in the context of their ecological and evolutionary implications.
Juvenile and mature zones are regions of a plant that vary phenotypically from one another, often due to shoot developmental differences that result from canalized changes in gene expression in the meristem (Poethig 2010). A classic example of morphological variation between developmental zones is heteroblasty in Eucalyptus species (Wiltshire et al. 1998). Such variation that arises at least in part from genetic mechanisms is termed “ontogenetic”. Ontogenetic variation can be independent of the environment, as canalized gene activity in the meristems causes phenotypic changes in the tissues subsequently produced by the meristems (Poethig 1990, 2003, 2010; Holeski et al. 2009, 2010). Ontogenetic variation can also arise from modification of gene activity due to environmental cues. While less frequently documented than ontogenetic variation, variation between two developmental zones can also result entirely from environmental effects such as changes in water and nutrient relations or shading (Fortainer and Jonkers 1976; Kearsley and Whitham 1998). While this type of variation does not have a developmental basis sensu stricto, environmental variation can affect one developmental zone but not another. Regardless of its mechanistic underpinnings, spatial variation in defense between such developmental zones can have striking effects on the distribution, fitness, and diversity of associated herbivores (Kearsley and Whitham 1989, 1998; Waltz and Whitham 1997; Karban and Thaler 1999; Lawrence et al. 2003; Holeski et al. 2009).
Only when variation among developmental zones has an ontogenetic basis can selection by herbivores result in different evolutionary trajectories of defenses among developmental zones. Thus, for both ecological and evolutionary reasons, it is important to distinguish between ontogenetic and purely physiological modes of variation. For example, at the population level, heavy herbivory across developmental zones of a plant could lead to selection for plant-wide increased concentrations of a particular defense. In contrast, heavy herbivory in one developmental zone but not another (e.g., as by deer that browse only the lower juvenile zone of a tree) could create selection for increased defenses in the frequently damaged zone, relative to the non-damaged zone. Across generations, selection on defensive traits could change the ontogenetic trajectory of developmental zones to converge or diverge.
Induction, the most frequently documented mode of within-plant variation, is manifested as changes in levels of defense following herbivore damage (Adler and Harvell 1990). In contrast to the increasing interest in ontogenetic variation in constitutive defenses, patterns of defense induction within and across developmental zones following herbivory (i.e., developmental zone × herbivory interactions for a defense response, indicating that induction is developmental zone-dependent) remain largely unexplored. The occurrence and extent of induction can vary due to the resources available for allocation to defense, the tissue value of the material being eaten, the perceived probability of future herbivory, and processes such as leaf expansion that alter plant biomass (thus altering concentrations of phytochemicals; Tallamy and Raupp 1991; Koricheva 1999; Boege and Marquis 2005; Traw and Feeny 2008; Barton and Koricheva 2010). Many of these factors affecting the occurrence or strength of induction are likely to differ across plant developmental zones, altering patterns of induction in currently unpredictable ways.
Finally, seasonal variation consists of progressive changes in traits across a growing season (Darrow and Bowers 1997). Foliar phytochemistry often changes in a predictable manner across a growing season, and can influence herbivore performance (Hwang and Lindroth 1998; Osier et al. 2000a). Seasonal variation in foliar phytochemical concentrations likely has both genetic and environmental components, and is a reflection of changing resource allocation priorities (e.g., among growth, reproductive, and defense functions) as leaves age (Darrow and Bowers 1997). Seasonal variation in phytochemistry could be selected for by a number of factors that affect the relative costs and benefits of defense, such as resource availability or temporal variation in herbivore population densities (Darrow and Bowers 1997; Jamieson and Bowers 2010). Previous studies of variation in plant secondary compounds have demonstrated genetic variation for seasonal gradients. For example, in quaking aspen (Populus tremuloides), the magnitude of within-year seasonal variation in some phytochemicals varied among aspen genotypes grown in a common garden (time × genotype interaction; Osier et al. 2000b). Such interactions indicate that genotypes may experience temporally divergent herbivory regimes and populations containing these genotypes may evolve in response to different selection pressures.
We used an 18-year-old cottonwood common garden, composed of clonally-replicated genotypes of two cottonwood species and their naturally occurring F1 hybrids (collectively referred to as cross types), to characterize patterns of multivariate and univariate phytochemical defense at three levels of organization: among cross types, within cross types (among genotypes), and within individuals. We address the following questions: (1) Do levels of multivariate and univariate defenses vary: (a) among cross types and/or genotypes, (b) between juvenile and mature developmental zones, (c) before and after herbivory, and (d) temporally (early and late season)? (2) Does the variation in 1b, c, or d (if present) have a genetic basis; i.e., does it differ at either the cross type or genotypic level within the common garden environment? (3) Of the five types of variation examined (among cross types and/or genotypes, between developmental zones, before and after herbivory, and between seasonal timepoints), what is the relative magnitude of each?
Materials and methods
We used clonally-replicated cottonwood genotypes, grown in a common garden, to characterize patterns of defense of two cottonwood species and their F1 hybrids. The trees used in this study were part of an 18-year-old common garden with randomly chosen and distributed genotypes at the Ogden Nature Center (ONC) in Ogden, Utah, USA (Lamit et al. 2011). These trees were propagated from cuttings of trees growing along the Weber River in Utah and established in the ONC garden in 1991 (Martinsen et al. 2001). Fremont cottonwood (P. fremontii) is found at <1,300 m elevation on the Weber River, narrowleaf cottonwood (P. angustifolia) is found at >1,470 m, and naturally occurring F1 hybrids of the two species are found at an intermediate elevation where both species overlap (Keim et al. 1989).
In cottonwood (Populus spp.), shoot developmental age is a function of its degree of separation from the root crown (Lillie 1927; fig. 1 in Kearsley and Whitham 1998). Shoots in the chronologically oldest regions of a plant (closest to the root crown) will express the most developmentally young (“juvenile”) phenotypes and are not reproductive, while the youngest regions of the plant (i.e., upper canopy) express the developmentally oldest phenotypes and are reproductively mature (Poethig 1990, 2003). Previous work in cottonwood has shown that some developmental aspects of its constitutive defense have a stable, genetically-programmed (i.e., ontogenetic) basis and can have a pronounced effect on herbivore richness and abundance (Kearsley and Whitham 1989, 1998; Waltz and Whitham 1997; Rehill et al. 2006; Holeski et al. 2009). The link between ontogeny and herbivore community characteristics can be indirect, mediated through the effects of ontogeny on the presence and abundance of a few herbivores (Kearsley and Whitham 1989, 1998; Waltz and Whitham 1997; Martinsen et al. 1998).
Our experiment consisted of seven narrowleaf genotypes, seven F1 hybrid genotypes, and five Fremont genotypes. Each genotype consisted of 3–12 clonally replicated trees. Genotypes and replication within a genotype were limited by the sample size available in the common garden. Within a genotype, we arbitrarily assigned each tree to one of four treatment categories: mature zone control, mature zone herbivory, juvenile zone control, or juvenile zone herbivory. Any single genotype with four or fewer replicates was assigned only two treatments (control and herbivory, within a single developmental zone). To assess variation between developmental zones in early season constitutive levels of phytochemistry, in mid-May we collected leaves from the designated treatment zone within each experimental tree prior to the initiation of treatment. We merged control samples from another experiment (all leaves collected in the same way and at the same time across studies) into our early season statistical analysis, which increased our sample size (these added samples were from genotypes that did not overlap with our “experimental” genotypes, and were used only in early season constitutive measurements; i.e., could not be and were not used in our calculations of induction). Prior research with Populus indicated that removal of leaves by clipping at the base of the petiole does not induce measurable changes in phytochemistry (Mattson and Palmer 1988). We flagged the branches from which leaves were collected, and control trees were re-sampled later in the season on an adjacent branch to assess seasonal variation in phytochemistry within juvenile and mature zones.
Cottonwood leaf beetle larval damage
The cottonwood leaf beetle (Chrysomela confluens; Coleoptera: Chrysomelidae) is a foundation insect herbivore on cottonwood, as its experimental removal is shown to result in a 120 % increase in insect species richness and a 75 % increase in abundance relative to trees with beetles (Waltz and Whitham 1997). Cottonwood leaf beetles specialize on tissue containing phenolic glycosides such as salicin, which they convert to salicylaldehyde and secrete as a defense against predators (Pasteels et al. 1983; Kearsley and Whitham 1992). Adult cottonwood leaf beetles emerge in April, feed on developing leaves, mate, and lay eggs (a single female may lay multiple clutches of up to 40 eggs). First and second stadium larvae skeletonize leaves, while later instars commonly consume the whole leaf blade, including secondary veins, and can completely defoliate small trees (Floate and Whitham 1994). Larval development is generally complete by mid-July, when the new adults feed briefly before dropping to the ground to overwinter. We collected C. confluens eggs in mid-May from a site (Horseshoe Bend) in the cottonwood hybrid zone of the Weber River. We divided each clutch into two parts, removed leaf material from around the eggs, and placed the eggs on our experimental trees within 2 days of collection.
Ten to twenty eggs were attached to a leaf on a single branch of each “herbivory” tree with a small piece of tape. Because our goal was to standardize the amount of leaf area removed by the leaf beetle larvae, rather than measure leaf palatability or leaf beetle performance, we applied more eggs to mature zones than to juvenile zones (leaves from the mature zone are known to be less palatable to cottonwood leaf beetle larvae; Kearsley and Whitham 1989). Herbivory and control branches were free of herbivore damage and were enclosed with bags sewn from No-see-um mesh to keep leaf beetle larvae localized on the treatment branch and to exclude other herbivores. We minimized environmental factors, such as light, within and across treatments [e.g., branches within a particular zone were sampled from the same height, 1.5 m from the ground (juvenile zone) or 7 m from the ground (mature zone)]. Control and herbivory treatments were done on the tips of branches within each juvenile and mature zone.
Nineteen days after treatments were initiated (mid-June; beetles were still feeding), we collected leaves from “control” and “herbivore” treatment bags (5–15 leaves were collected per bag) for chemical analysis. We collected two types of leaves from “herbivore” bags: (1) herbivore-damaged, and (2) undamaged leaves adjacent to herbivory leaves (and within the mesh enclosure). Within the herbivory category, cottonwood leaf beetle larvae removed, on average, 40 % of leaf material across the collected leaves. We measured induction in damaged leaves or nearby undamaged leaves (these results were later combined; see Statistical analysis section); it is unknown whether cottonwood leaf beetles cause systemic induction in these cottonwoods. To ascertain whether the magnitude of chemical induction was related to the amount of leaf damage, we regressed induction of each phytochemical onto the proportion and/or amount of leaf material removed (for the herbivore-damaged leaves only). Results showed that induction was not significantly related to the extent of foliar damage (L. Holeski, unpublished data).
Leaf collection and chemical analyses
Leaves from the control and herbivore-damaged branches were removed at the base of the petiole and flash frozen using dry ice. In the laboratory, leaves were subsequently freeze-dried, finely ground using a Wiley mill (mesh size #40), and stored at −20 °C. We analyzed leaf samples for nitrogen, an index of protein, as well as condensed tannins and phenolic glycosides (salicin, salicortin, and HCH-salicortin). We refer to nitrogen as a defense trait, as low levels of nitrogen can lead to decreases in the amount of herbivory that a plant experiences (barring compensatory feeding; Berenbaum 1995; Wold and Marquis 1997). Nitrogen was quantified using a Thermo Finnigan Flash 1112 elemental analyzer (Thermo Finnigan, San Jose, CA, USA). Condensed tannins and phenolic glycosides are the main secondary metabolites in Populus (Palo 1984; Lindroth et al. 1987; Rehill et al. 2005, 2006). To assess condensed tannin content, we used the acid butanol assay (Porter et al. 1986) with purified narrowleaf cottonwood condensed tannins as standards Rehill et al. (2006). We assessed phenolic glycosides (salicortin, salicin, and HCH-salicortin) using capillary HPLC (see supplementary information), with purified cottonwood phenolic glycosides as standards. Salicin standard was obtained from Sigma-Aldrich, while salicortin and HCH-salicortin were purified from Fremont and narrowleaf tissue in our laboratory. We report the results of each assay as percent (%) dry weight and “total phenolic glycosides” as the sum of its individual components (salicin, salicortin, HCH-salicortin).
Statistical analysis
Phytochemical data for herbivore-damaged leaves did not differ significantly from data for adjacent, undamaged leaves within individual mesh bags. Thus, for simplicity of presentation, we merged the two categories into a single “herbivory” category in our final analyses.
Multivariate analysis
To characterize broad patterns in tree phytochemistry, we merged the univariate chemical traits of each tree (C:N ratio, and condensed tannin and phenolic glycoside concentrations) into a multivariate defense phenotype. Trees with missing values for any chemical trait were excluded from this analysis (117 trees had complete data). We examined late-season variation in the multivariate defense phenotype among cross types, genotypes nested within cross type, ontogeny (juvenile and mature zones), and induction (control and herbivore-damaged treatments). In a separate analysis, we examined variation between the multivariate defense phenotypes during early season and late season collection dates. We created a Euclidean distance-based similarity matrix and used nonmetric multidimensional scaling (NMDS) to visualize the data (Kruskal 1964; Bangert et al. 2006). Nonmetric multidimensional scaling is commonly used in ecological studies because it employs nonparametric methods that do not require normally distributed data and it provides robust results that are easy to interpret (Clark and Warwick 2001; McCune and Grace 2002). Two-dimensional stress levels across several NMDS runs were consistently less than 0.15, suggesting the data fit the ordination well. Data points near each other in the NMDS plots are more similar than points further apart. The error bars on NMDS plots represent the variance of the NMDS scores in the x and y directions across tree replicates (e.g., for each cross type). NMDS axes do not have associated quantitative units.
We used permutational multivariate analysis of variance (PERMANOVA), a nonparametric permutation-based method of testing ANOVA experimental designs, to assess differences in phytochemical community composition (Anderson 2001a, b; McArdle and Anderson 2001; programs PRIMER 6 and PERMANOVA + (PRIMER-E, Plymouth, UK)). We used PERMANOVA because it does not require multivariate normality as does MANOVA, it retains the flexibility and robustness of the NMDS procedure, and it correctly partitions data for complex experimental designs (e.g., nested, unbalanced designs) (Anderson 2001a, b; McArdle and Anderson 2001; Anderson et al. 2008). PERMANOVA calculates distance-based pseudo-F statistics in a manner analogous to how MANOVA calculates F statistics. Data labels are shuffled repeatedly across groups (i.e., trees) to obtain the pseudo-F values. P values are generated for each test statistic by examining the proportion of pseudo-F values that are greater than or equal to the pseudo-F value obtained with the original data. The three cross types (Fremont, F1 hybrids, narrowleaf), two zones (juvenile and mature), and two induction treatments (herbivore-damaged vs. control) were all fixed factors in the analysis. Genotype was included as a random factor nested within cross type. None of the interactions among fixed factors were significant, so we removed them from the final analyses. In addition to the PERMANOVA to test for main effects, we ran a PERMANOVA pairwise test for each main factor individually. We did this to determine which cross types and genotypes (nested within a cross type) were the most different from each other and to calculate effect sizes for cross type, genotype, juvenile and mature zones, and herbivore induction. We calculated multivariate effect size by determining the distance between the centroids in two dimensions for each analysis.
Univariate analysis
We log-transformed the condensed tannin data prior to univariate analysis to satisfy statistical assumptions of normality. Sample sizes listed in the following text are the maximums; missing data for some traits for some samples led to slightly different sample sizes across univariate analyses. Early season constitutive phytochemistry was assessed using pre-experimental leaf collections, and included all of the experimental trees (both controls and those that would undergo herbivory treatment, n = 149 [n mature = 73 (35 NL, 30 F1, 8 Fremont); n juvenile = 76 (34 NL, 25 F1, 17 Fremont)], allowing us to increase our sample size and statistical power. Late season constitutive phytochemistry was assessed at the end of the experiment, using only experimental control trees, n = 27 [n mature = 12 (6 NL, 4 F1, 2 Fremont); n juvenile = 15 (7 NL, 5 F1, 3 Fremont)]. Induction within each species and ontogeny was calculated at the genotype level, as the late “herbivory” mean for a particular phytochemical minus the late season control mean, n = 40 [n mature = 17 (10 NL, 6 F1, 1 Fremont); n juvenile = 23 (10 NL, 8 F1, 5 Fremont)]. Positive values for induction thus indicate an increase in phytochemical concentration following herbivory.
We used a series of general linear model ANOVAs (with Type III sums of squares) to evaluate differences in univariate defenses among and within cross types and ontogeny (Minitab 14, State College, PA, USA). Cross type, ontogeny, and herbivore induction were all fixed factors, and genotype was nested within cross type and was a random variable. Genotypes with unbalanced data (only one individual representing a factor) could not be used in the univariate analysis and were removed. This resulted in the use of four narrowleaf genotypes, four F1 genotypes, and two Fremont genotypes throughout the late season univariate analysis. Non-significant interaction terms were removed from subsequent analyses (Sokal and Rohlf 1995). We used Pearson product-moment correlation tests to evaluate the relationship between constitutive and induced concentrations for each phytochemical. As each of our tests had an easily distinguishable a priori question driving it, we did not employ multiple test corrections (Moran 2003).
We calculated Hedges-d, with corresponding 95 % confidence intervals, for: (1) early season constitutive phytochemistry, and (2) late season constitutive and induced phytochemistry (Nakagawa and Cuthill 2007). Hedges-d is an effect size statistic designed to measure the standardized magnitude of difference between two variables or groups (Hedges and Olkin 1985; Gurevitch and Hedges 2001). We used the first Hedges-d calculations to evaluate the effect sizes of cross type (narrowleaf vs. Fremont), genotype (two most divergent genotypes within each cross type; thus treated as a fixed factor in the effect size analyses) and ontogeny (juvenile vs. mature zone) within each cross type on constitutive, early season phytochemistry. We used the second Hedges-d calculations to evaluate the effect sizes of cross type, as well as ontogeny, herbivore induction, season, and genotype within each cross type on late season phytochemistry.
Results
Multivariate defense phenotypes
Multivariate defense phenotypes differed significantly among cross types, genotypes nested within each cross type, between juvenile and mature zones within genotypes and cross types, between herbivory treatments, and between early and late season collections (Fig. 1; Table 1). For example, Fig. 1 shows that, when all defense traits are analyzed together in ordination space, all three cross types differ significantly from one another. Note that F1 hybrids are intermediate between their parental species but closer to pure narrowleaf cottonwood suggesting dominance effects as also described by Dungey et al. (2000). Variation among genotypes and cross types indicates genetic variation in their multivariate defense phenotypes.
Nonmetric multidimensional scaling (NMDS) ordination plot comparing the multivariate defense phenotype of three cottonwood cross types, narrowleaf (NL), Fremont, and their F 1 hybrids. Each point represents the mean of the NMDS scores of multiple trees sampled for each cross type. Standard error bars represent the within-defense phenotype variance for each axis of the two dimensional solution [±1 standard error (SE)]. Axes have no inherent meaning. We tested for significance using PERMANOVA
While ontogeny and herbivore damage had significant overall effects, we did not detect genetic variation for within-plant variation in the multivariate defense phenotypes (only main effects were significant in Table 1). Ontogeny (juvenile and mature zones) varied in a consistent manner among cross types (non-significant ontogeny × cross type interaction; p > 0.28). Cross types, genotypes, and ontogeny responded in a fundamentally similar manner to damage (i.e., there were no significant interactions between these independent variables; all p values >0.28). The effect sizes (±1 SD) of cross type (3.06 ± 0.79), genotype nested within cross type (narrowleaf 3.02 ± 0.73; F1 2.44 ± 0.75; Fremont 2.06 ± 0.76), ontogeny (2.25 ± 1.03), herbivore induction (2.28 ± 1.02), and season (2.34 ± 0.98) were not significantly different from one another, indicating that each independent factor had similar magnitudes of effect on the multivariate defense phenotypes.
Univariate defense phenotypes
Early season constitutive phytochemistry
Variation among genotypes and cross types
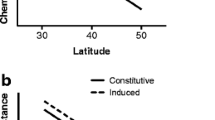
Early season constitutive levels of condensed tannins significantly differed among cross types within both juvenile and mature zones (F 2,48 = 39.6, p < 0.001; F 2,51 = 23.53, p < 0.001). Narrowleaf produced the highest levels of condensed tannins, followed by the F1 and Fremont (Fig. 2, panel 1a). The highest overall concentrations of condensed tannins were in the mature zone of narrowleaf, which had levels 32-times higher than either zone of Fremont, and in the juvenile zone of narrowleaf, which had concentrations 20-times higher than Fremont. Levels of condensed tannins also significantly differed among genotypes nested within cross types in the juvenile zone (F 17,48 = 2.68, p = 0.007). Genotype generally had a stronger effect on condensed tannin levels than did cross type (Fig. 3a; note exception in mature zone of narrowleaf and F1s).
1 Early season constitutive levels of condensed tannins, total phenolic glycosides, and nitrogen (% dw is % dry weight) in cottonwood foliage. 2 Late season levels of condensed tannins, total phenolic glycosides, and nitrogen in control and herbivore-damaged cottonwood foliage (% dry weight). Error bars ±1 SE
a Hedges-d values representing effect sizes of cross type, genotype, and juvenile/mature zones (ontogeny) on early season (May) constitutive levels of condensed tannins, total phenolic glycosides, and nitrogen. From left to right in each graph, the effect of cross type is illustrated for both the juvenile and mature zones, the effect of ontogeny is illustrated for each cross type, and the effect of genotype is illustrated within each cross type and developmental zone. b Hedges-d values representing the effect sizes of cross type, juvenile/mature zones, genotype, herbivory, and season on mid-June levels of condensed tannins, total phenolic glycosides, and nitrogen. From left to right in each graph, the effects of cross type, ontogeny and genotype are illustrated as in (a). The effects of herbivory and season are illustrated as are those of genotype (split by cross type and developmental zone). In (b), missing data for the effect of genotype (F1 mature zone, Fremont both developmental zones) and herbivory (Fremont mature zone) is a result of small sample sizes. Stars significant effect sizes (those with 95 % confidence intervals that do not overlap zero)
Constitutive levels of total phenolic glycosides differed significantly among cross types within the juvenile zone, but not in the mature zone (Fig. 2, panel 1b; F 2,55 = 4.96, p = 0.018; F 2,52 = 0.80, p = 0.469). In the juvenile zone, phenolic glycoside concentrations across species followed a pattern similar to condensed tannin concentrations (narrowleaf highest and more than 3-times greater than Fremont; F1s intermediate to the parent species). In the mature zone, phenolic glycoside concentrations in the F1 were greater than in the two parental species (Fig. 2, panel 1b). Patterns of total phenolic glycoside concentration were driven by patterns of salicortin concentrations, as it was the most prevalent phenolic glycoside within and among cross types (Supplementary data, Fig. 1S).
Total phenolic glycoside concentrations differed among genotypes within each cross type in both the mature and juvenile zones (F 17,55 = 2.54, p = 0.005; F 14,52 = 3.28, p = 0.001). Hedges-d values indicated that, as with condensed tannin levels, genotype generally had the strongest effect on total phenolic glycoside concentration, although cross type identity also had a significant effect in the juvenile zone (Fig. 3a).
Finally, constitutive concentrations of nitrogen did not differ among cross types within zones (Fig. 2, panel 1c; juvenile zone F 2,56 = 0.36, p = 0.702; mature zone F 2,56 = 0.13, p = 0.882), but did differ significantly among genotypes (nested within cross type) within each zone (juvenile zone F 17,56 = 2.81, p = 0.002; mature zone F 14,56 = 3.73, p < 0.001). Likewise, genotype was the only factor that significantly influenced nitrogen concentration (Fig. 3a).
Ontogenetic variation between juvenile and mature zones
Phenotypic expression of individual defense traits varied among zones, and did so consistently across genotypes within each cross type (no significant genotype × zone interactions). Levels of condensed tannins differed between zones in narrowleaf but not F1 hybrids or Fremont cottonwoods (Fig. 2, panel 1a; F 1,52 = 8.43, p = 0.001; F 1,46 = 3.63, p = 0.063; F 1,13 = 2.01, p = 0.180), with concentrations higher in the mature zone than in the juvenile zone.
Total phenolic glycoside concentrations differed between zones in the narrowleaf and F1s, but not in Fremont (Fig. 2, panel 1b; F 1,56 = 32.68, p < 0.001; F 1,46 = 19.05, p < 0.001; F 1,15 = 2.06, p = 0.171). Individual phenolic glycosides followed much the same pattern (Supplementary data, Table 1S). In contrast to condensed tannins, total phenolic glycoside concentrations within each cross type were highest in the juvenile zone. For phenolic glycosides, the mean difference in concentration between the juvenile and mature zones within narrowleaf (a ≥ twofold difference) was of a similar magnitude to the differences between the other cross types (Figs. 2, panel 1b; 3a).
Constitutive concentrations of nitrogen did not differ between zones within each cross type (Fig. 2, panel 1c; F 1,60 = 0.32, p = 0.573; F 1,46 = 0.80, p = 0.376; F 1,16 = 1.37, p = 0.259). Overall, the effect size of ontogeny on early season constitutive phytochemistry was smaller than that of genotype or cross type (Fig. 3a).
Late season phytochemistry
Induction following herbivory
We found significant induction in some univariate defenses following foliar damage by herbivores. In each instance of significant induction in condensed tannins or nitrogen, condensed tannin concentrations increased in response to herbivory, while nitrogen concentrations decreased (Fig. 2, panels 2a, c). Condensed tannins and nitrogen were more often induced in response to herbivory than were phenolic glycosides; we found changes in one or both of these constituents within the mature zone of each cross type (Fig. 2, panels 2a, c; Table 2). Levels of condensed tannins in the mature zone of the F1s more than doubled following herbivory, while those in the mature zone of narrowleaf increased by 65 %.
Phenolic glycoside concentrations were more canalized than were condensed tannins or nitrogen; we observed an induction in total phenolic glycosides in only the juvenile zone of Fremont (where levels of total phenolic glycosides were almost tripled following herbivory; Fig. 2, panel 2b; Table 2; see Supplementary data, Tables 2S and 3S for individual phenolic glycosides). Despite these differences in the degree of induction of particular phytochemicals across cross types, we found no significant cross type × treatment or genotype × treatment interactions for any of the univariate response variables.
Seasonal variation
We found seasonal variation in some, but not all, univariate phytochemicals within a particular cross type and juvenile versus mature zone (Supplementary data, Table 4S). Concentrations of total phenolic glycosides and nitrogen tended to decrease over time, while those of condensed tannins increased (except in Fremont; Fig. 2). We found variation among cross types in their seasonal patterns in condensed tannin concentration (F 2,5 = 4.39; p = 0.04), and nitrogen (F 2,5 = 4.41; p = 0.05) in the juvenile zone, but not in total phenolic glycosides. Cross types and genotypes did not differ in their seasonal response within the mature zone. Genotypes nested within cross type did not vary in their seasonal response with respect to any of the univariate phytochemicals.
Changes in defense traits following herbivory can either enhance or offset seasonal patterns of these defenses. The most pronounced examples of these outcomes were in the concentrations of total phenolic glycosides in the narrowleaf juvenile zone, and in the nitrogen levels across zones and cross types. Total phenolic glycoside concentrations decreased sharply over time in the juvenile zone of narrowleaf (i.e., compare Fig. 2, panel 2b with the control plants in Fig. 2, panel 1b); induction of these phytochemicals following herbivory offset some of this seasonal reduction (Fig. 2, panel 2b). In contrast, the reduction in nitrogen concentration following herbivory accelerated the normal seasonal reduction in nitrogen (Fig. 2, panels 1c, 2c).
Relative effects on univariate defenses
Neither cross type nor genotype consistently had the largest effect size on univariate phytochemistry (Fig. 3). Rather, a mosaic of within-plant factors frequently trumped the effects of these among-plant forms of variation. For example, in the mature developmental zone, concentrations of total phenolic glycosides and nitrogen were influenced more strongly by induction and seasonal variation than by cross type identity (Fig. 3b; some of these effect sizes were significant while some were non-significant trends). Within Fremont, we had insufficient statistical power to calculate effect size of genotypes on foliar chemistry. However, the effect sizes of ontogeny on levels of total phenolic glycosides and nitrogen were larger than the effect size of cross type (Fig. 3b).
Discussion
Genetic and phenotypic variation within a species can result from and be a cause of evolutionary and ecological processes (Whitham et al. 2006; Hughes et al. 2008). In addition to variation between species and among genotypes within a species, numerous forms of within-plant variation in defense, including ontogenetic variation among zones, induction following herbivory, and seasonal gradients, allow plants to avoid expending resources on defense when herbivores are absent (Adler and Harvell 1990; Simms and Fritz 1990). While within-plant sources of variation in defenses are increasingly documented (Suomela and Ayres 1994; Lawrence et al. 2003; Boege and Marquis 2005; Barton and Koricheva 2010), few studies have investigated within-plant variation with experiments also designed to detect genetic variation in these traits (Agrawal et al. 1999; Lawrence et al. 2003; Barton 2007; Holeski 2007; Holeski et al. 2009). Here, we demonstrate genetic variation within and/or among species (or cross types) in two modes of within-plant variation, while also quantifying the effect sizes of this variation on plant defense phenotypes.
Genetic variation in defense
Our results demonstrate that the constitutive, multivariate defense phenotype varies in a predictable, genetically-based manner among cottonwood cross types (Fig. 1; Table 1). Similarly, we found significant variation at the cross type and/or genotype level for each constitutive, univariate defense that we examined; in many instances, the effect of genotype on a univariate defense was greater than the effect of cross type (Fig. 3a). These constitutive phytochemistry results support and extend previous work on constitutive phytochemical patterns in the cottonwood system (Bangert et al. 2006; Rehill et al. 2006).
We found genetic variation within and/or among cross types for two modes of within-plant variation (ontogeny and seasons) in univariate defenses. We did not, however, find genetic variation for within-plant variation in the multivariate phenotype, possibly due to its incorporation of multiple univariate traits, some of which did not display genetic variation in within plant defense when considered in the univariate analysis. Differences between the multivariate and univariate results could also be a function of power differences between the two types of analysis.
While we found induction of the multivariate defense phenotype and of several univariate defense traits following cottonwood leaf beetle herbivory, we did not find differential induction responses among cross types or genotypes (no cross type × treatment or genotype × treatment interactions). This implies that, insofar as we could detect in our study, genotypes and cross types will respond to herbivory in ecologically and evolutionarily similar ways. Experiments with greater replication will be required for a robust test of differential induction among cross types or genotypes.
We examined plant defenses from an herbivore perspective (concentrations), rather than from the plant perspective (quantity of a defense produced). Thus, we cannot comment on the evolutionary processes (such as trade-offs between defense production and allocation of resources to growth) that may have led to the observed patterns. Regardless of the processes that led to current patterns of defensive concentrations, the presence of genetic variation for defense at three levels of organization, along with significant effect sizes at each level, suggest that each may be important for both ecological interactions and evolutionary trajectories.
There are several notable differences between our results and those from previous work in the cottonwood system. First, a study of foliar induction in resprout growth of beaver-felled cottonwoods found significant increases in nitrogen and phenolic glycoside concentrations in resprout tissue (Martinsen et al. 1998). This pattern is in contrast to the decreases in nitrogen and/or increases in phenolic glycosides following cottonwood leaf beetle herbivory in our study. A likely explanation for this difference is that the induction response is influenced by the type of damage (e.g., leaf vs. stem/trunk removal) or herbivore (e.g., mammal vs. insect; Karban and Baldwin 1997; Agrawal 2000). Second, the patterns of early-season constitutive levels of phenolic glycosides observed in our study differed in rank order among species from those in a prior study in the same common garden (Rehill et al. 2006). We found phenolic glycosides in narrowleaf > F1 hybrids > Fremont, whereas Rehill et al. (2006) observed F1 hybrids > narrowleaf = Fremont. The reason for this difference is not clear, but could be due to differences in tree age or environmental factors between the two studies (Bailey et al. 2006; Donaldson et al. 2006; Donaldson and Lindroth 2008).
Ecological implications
We show that within-plant variation in phytochemical defense can rival that among genotypes and/or species which corresponds with patterns of differential insect community composition between juvenile and mature zones, among cross types, and seasons (Waltz and Whitham 1997; Bangert et al. 2006; Wimp et al. 2007). Differences in defenses among genotypes within cross types also correspond to differences in arthropod and microbial communities (Shuster et al. 2006; Bailey et al. 2006; Schweitzer et al. 2008; Keith et al. 2010). Thus, we predict that in instances where the effect sizes of within-plant variation are similarly large, those sources of variation will also have pronounced effects on cottonwood-associated herbivore communities. These findings are also consistent with the genetic similarity rule of Bangert et al. (2006) and Barbour et al. (2009), in which they found that more genetically similar plants had more similar phytochemistry and more similar arthropod communities confirming that plant chemistry is genetically based and important at the community level.
Suggested framework for future studies
Our results highlight several questions important to address in future studies of plant defense (or other ecologically-relevant) traits. For example, what are the ecological and evolutionary causes and consequences of genetic variation in within-plant variation in defense? How commonly do effect sizes of within-plant variation rival those between genotypes or between species? How might factors such as plant life history (woody vs. herbaceous species, annual vs. perennial species), the role of a plant species in a community (foundation vs. non-foundation species) and climate change affect these patterns? These questions are straight-forward to address using common garden experiments and statistical techniques such as effect size calculations. Studies across an array of plant species and communities are necessary to identify broad patterns, and the relevance of such patterns to ecological and evolutionary processes.
References
Adler FR, Harvell CD (1990) Inducible defenses, phenotypic plasticity, and biotic environments. Trends Ecol Evol 5:407–410
Agrawal AA (2000) Specificity of induced resistance in wild radish: causes and consequences for two specialist and two generalist caterpillars. Oikos 89:493–500
Agrawal AA, Strauss SY, Stout MJ (1999) Costs of induced responses and tolerance to herbivory in male and female components of wild radish. Evolution 53:1093–1104
Anderson MJ (2001a) A new method for nonparametric multivariate analysis of variance. Aust Ecol 26:32–46
Anderson MJ (2001b) Permutation tests for univariate or multivariate analysis of variance and regression. Can J Fish Aquat Sci 58:626–639
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA + for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth
Axelsson EP, Hjältén J, Whitham TG, Julkunen-Tiitto R, Pilate G, Wennström A (2011) Leaf ontogeny interacts with Bt modification to affect innate resistance in GM aspens. Chemoecology 21:161–169
Bailey JK, Wooley SC, Lindroth RL, Whitham TG (2006) Importance of species interactions to community heritability: a genetic basis to trophic-level interactions. Ecol Lett 9:78–85
Bailey JK, Schweitzer JA, Ubeda F, Koricheva J, LeRoy CJ, Madritch MD, Rehill BJ, Bangert RK, Fischer DG, Allan GJ, Whitham TG (2009) From genes to ecosystems: a synthesis of the effects of plant genetic factors across levels of organization. Philos Trans R Soc Lond B 324:1607–1616
Bangert RK, Allan GJ, Turek RJ, Wimp GM, Meneses N, Martinsen GD, Keim P, Whitham TG (2006) A genetic similarity rule determines arthropod community structure. Mol Ecol 15:1379–1392
Barbour RC, O’Reilly-Wapstra JM, De Little DW, Jordan GJ, Steane DA, Humphreys JR, Bailey JK, Whitham TG, Potts BM (2009) A geographic mosaic of genetic variation within a foundation tree species and its community-level consequences. Ecology 90:1762–1772
Barton KE (2007) Early ontogenetic patterns in chemical defense in Plantago (Plantaginaceae): Genetic variation and trade-offs. Am J Bot 94:56–66
Barton KE, Koricheva J (2010) The ontogeny of plant defense and herbivory: characterizing general patterns using meta-analysis. Am Nat 175:481–493
Berenbaum MR (1995) Turnabout is fair play-secondary roles for primary components. J Chem Ecol 21:925–940
Boege K, Marquis RJ (2005) Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends Ecol Evol 20:441–448
Bolnick DI, Svanback R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML (2003) The ecology of individuals: incidence and implications of individual specialization. Am Nat 161:1–28
Bolnick DI, Amarasekare P, Araujo MS, Burger R, Levine JM, Novak M, Rudolf VHW, Schreiber SJ, Urban MC, Vasseur DA (2011) Why intraspecific trait variation matters in community ecology. Trends Ecol Evol 26:183–192
Clark KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E, Plymouth
Darrow K, Bowers MD (1997) Phenological and population variation in iridoid glycosides of Plantago lanceolata. Biochem Syst Ecol 25:1–11
Donaldson JR, Lindroth RL (2008) Effects of variable phytochemistry and budbreak phenology on defoliation of aspen during a forest tent caterpillar outbreak. Agric For Entomol 10:399–410
Donaldson JR, Stevens MT, Barnhill HR, Lindroth RL (2006) Age-related shifts in leaf chemistry of clonal aspen (Populus tremuloides). J Chem Ecol 32:1415–1429
Dungey HS, Potts BM, Whitham TG, Li HF (2000) Plant genetics affects arthropod community richness and composition: Evidence from a synthetic eucalypt hybrid population. Evolution 54:1938–1946
Floate KD, Whitham TG (1994) Aphid-ant interaction reduces chrysomelid herbivory in a cottonwood hybrid zone. Oecologia 97:215–221
Fortainer EJ, Jonkers H (1976) Juvenility and maturity of plants as influenced by their ontogenetical and physiological aging. Acta Hortic 56:37–43
Gurevitch J, Hedges LV (2001) Meta-analysis: combining results of independent experiments. In: Gurevitch J, and Scheiner SM (eds) Design and analysis of ecological experiments, Oxford University Press, Oxford, p 347–369
Hedges LV, Olkin I (1985) Statistical methods for meta-analysis. Academic, New York
Holeski LM (2007) Within and between generation phenotypic plasticity in trichome density of Mimulus guttatus. J Evol Biol 20:2092–2100
Holeski LM, Kearsley MJC, Whitham TG (2009) Separating ontogenetic and environmental determination of resistance to herbivory in cottonwood. Ecology 90:2969–2973
Holeski LM, Chase-Alone R, Kelly JK (2010) The genetics of phenotypic plasticity in plant defense: trichome production in Mimulus guttatus. Am Nat 175:391–400
Hughes AR, Inouye BD, Johnson MTJ, Underwood N, Vellend M (2008) Ecological consequences of genetic diversity. Ecol Lett 11:609–623
Hwang SY, Lindroth RL (1998) Consequences of clonal variation in aspen phytochemistry for late season herbivores. Ecoscience 5:508–516
Jamieson MA, Bowers MD (2010) Iridoid glycoside variation in the invasive plant Dalmation toadflax, Linaria dalmatica (Plantaginaceae), and sequestration by the biological control agent, Calophasia lunula. J Chem Ecol 36:70–79
Johnson MTJ, Stinchcombe JR (2007) An emerging synthesis between community ecology and evolutionary biology. Trends Ecol Evol 22:250–257
Karban, R, Baldwin IT (1997) How damage is perceived and signaled. In: Induced responses to herbivory, University of Chicago Press, Chicago, pp 12–46
Karban R, Thaler JS (1999) Plant phase change and resistance to herbivory. Ecology 80:510–517
Kearsley MJ, Whitham TG (1989) Developmental changes in resistance to herbivory: implications for individuals and populations. Ecology 70:422–434
Kearsley MJ, Whitham TG (1992) Guns and butter-A no cost defense against predation for Chrysomela confluens. Oecologia 92:556–562
Kearsley MJC, Whitham TG (1998) The developmental stream of cottonwoods affects ramet growth and resistance to galling aphids. Ecology 79:178–191
Keim P, Paige KN, Whitham TG, Lark KG (1989) Genetic analysis of an interspecific hybrid swarm of Populus: occurrence of unidirectional introgression. Genetics 123:557–565
Keith AR, Bailey JK, Whitham TG (2010) A genetic basis to community repeatability and stability. Ecology 11:3398–3406
Koricheva J (1999) Interpreting phenotypic variation in plant allelochemistry: problems with the use of concentrations. Oecologia 119:467–473
Kruskal JB (1964) Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 29:1–26
Lamit LJ, Bowker MA, Holeski LM, Naesborg RR, Wooley SC, Zinkgraf M, Lindroth RL, Whitham TG, Gehring CA (2011) Genetically-based trait variation within a foundation tree species influences a dominant bark lichen. Fungal Ecol 4:103–109
Lawrence R, Potts BM, Whitham TG (2003) Relative importance of plant ontogeny, host genetic variation, and leaf age for a common herbivore. Ecology 84:1171–1178
Lillie FR (1927) The gene and the ontogenetic process. Science 66:361–368
Lindroth RL, Hsia MTS, Scriber JM (1987) Seasonal patterns in the phytochemistry of 3 Populus species. Biochem Syst Ecol 15:681–686
Martinsen GD, Driebe EM, Whitham TG (1998) Indirect interactions mediated by changing plant chemistry: Beaver browsing benefits beetles. Ecology 79:192–200
Martinsen GD, Whitham TG, Turek RJ, Keim P (2001) Hybrid populations selectively filter gene introgression between species. Evolution 55:1325–1335
Mattson WJ, Palmer SR (1988) Changes in foliar minerals and phenolics in trembling aspen, Populus tremuloides, in response to artificial defoliation. In: Mattson WJ et al. (eds) Mechanisms of woody plant defenses against insects: search for pattern. Springer, New York, p 157–169
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: a comment of distance based redundancy analysis. Ecology 82:290–297
McCune B, Grace JB (2002) Analysis of ecological communities. MjM software design, Gleneden Beach
Moran MD (2003) Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 100:403–405
Moreira X, Zas R, Sampedro L (2012) Differential allocation of constitutive and induced chemical defenses in pine tree juveniles: a test of the optimal defense theory. PLoS ONE 7:e34006
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval, and statistical significance: a practical guide for biologists. Biol Rev 82:591–605
Osier TL, Hwang SY, Lindroth RL (2000a) Effects of phytochemical variation in quaking aspen Populus tremuloides clones on gypsy moth Lymantria dispar performance in the field and laboratory. Ecol Entomol 25:197–207
Osier TL, Hwang SY, Lindroth RL (2000b) Within- and between-year variation in early season phytochemistry of quaking aspen (Populus tremuloides Michx.) clones. Biochem Syst Ecol 28:197–208
Palo RT (1984) Distribution of birch (Betula spp.), willow (Salix spp.), and poplar (Populus spp.) secondary metabolites and their potential role as chemical defense against herbivores. J. Chem Ecol 10:499–520
Pasteels JM, Rowellrahier M, Braekman JC, Dupont A (1983) Salicin from host plant as precursor of salicylaldehyde in defensive secretion of Chrysomeline larvae. Physiol Entomol 8:307–314
Poethig RS (1990) Phase change and the regulation of shoot morphogenesis in plants. Science 250:923–930
Poethig RS (2003) Phase change and the regulation of developmental timing in plants. Science 301:334–336
Poethig RS (2010) The past, present, and future of vegetative phase change. Plant Physiol 154:541–544
Porter LJ, Hrstich LN, Chan BG (1986) The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 25:223–230
Rehill BJ, Clauss A, Wieczorek L, Whitham TG, Lindroth RL (2005) Foliar phenolic glycosides from Populus fremontii, Populus angustifolia, and their hybrids. Biochem Syst Ecol 33:125–131
Rehill BJ, Whitham TG, Martinsen GD, Schweitzer JA, Bailey JK, Lindroth RL (2006) Developmental trajectories in cottonwood phytochemistry. J Chem Ecol 32:2269–2285
Schweitzer JA, Bailey JK, Fischer DG, LeRoy CJ, Lonsdorf EV, Whitham TG, Hart SC (2008) Plant-soil microorganism interactions: a heritable relationship between plant genotype and associated soil microorganisms. Ecology 89:773–781
Shipley B (2007) Comparative plant ecology as a tool for integrating across scales. Ann Bot 99:965–966
Shuster SM, Lonsdorf EV, Wimp GM, Bailey JK, Whitham TG (2006) Community heritability measures the evolutionary consequences of indirect genetic effects on community structure. Evolution 60:991–1003
Simms EL, Fritz RS (1990) The ecology and evolution of host plant resistance to insects. Trends Ecol Evol 5:356–360
Sokal RR, Rohlf FJ (1995) Biometry-the principles and practice of statistics in biological research (3rd edition). Freeman, New York
Suomela J, Ayres MP (1994) Within-tree and among-tree variation in leaf characteristics of mountain birch and its implications for herbivory. Oikos 70:212–222
Tallamy DW, Raupp MJ (1991) Phytochemical induction by herbivores. Wiley, New York
Traw MB, Feeny P (2008) Glucosinolates and trichomes track tissue value in two sympatric mustards. Ecology 89:763–772
Vellend M (2006) The consequences of genetic diversity in competitive communities. Ecology 87:304–311
Waltz AM, Whitham TG (1997) Plant development affects arthropod communities: opposing impacts of species removal. Ecology 78:2133–2144
Whitham TG, Bailey JK, Schweitzer JA, Shuster SM, Bangert RK, Leroy CJ, Lonsdorf EV, Allan GJ, DiFazio SP, Potts BM, Fischer DG, Gehring CA, Lindroth RL, Marks JC, Hart SC, Wimp GM, Wooley SC (2006) A framework for community and ecosystem genetics: from genes to ecosystems. Nat Rev Genet 7:510–523
Wiltshire RJE, Potts BM, Reid JB (1998) Genetic control of reproductive and vegetative phase change in the Eucalyptus risdonii–E. tenuiramis complex. Aust J Bot 46:45–63
Wimp GM, Wooley S, Bangert RK, Young WP, Martinsen GD, Keim P, Rehill BJ, Lindroth RL, Whitham TG (2007) Plant genetics predicts intra-annual variation in the phytochemistry and arthropod community structure. Mol Ecol 16:5057–5069
Wold EN, Marquis RJ (1997) Induced defense in white oak: effects on herbivores and consequences for the plant. Ecology 78:1356–1369
Acknowledgments
Thanks to Brian Rehill, Jen Schweitzer, and Kevin Floate for their helpful advice at the beginning of this experiment. We would like to thank Kennedy Rubert-Nason, Michael Crossley, and Peter Ladwig for their help with sample processing and chemistry, and Nicholas Keuler for his assistance with calculating the effect sizes for our multivariate data. Comments from Alice Winn, Lissy Coley, and several anonymous reviewers improved this paper. Thanks to the Ogden Nature Center (Ogden, UT, USA) for providing land for our common garden; funding was provided through NSF DEB-0425908.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Phyllis Coley.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Holeski, L.M., Hillstrom, M.L., Whitham, T.G. et al. Relative importance of genetic, ontogenetic, induction, and seasonal variation in producing a multivariate defense phenotype in a foundation tree species. Oecologia 170, 695–707 (2012). https://doi.org/10.1007/s00442-012-2344-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2344-6